Abstract
Translocation and reintroduction have become major conservation actions in attempts to create self-sustaining wild populations of threatened species. However, avian translocations have a high failure rate and causes for failure are poorly understood. While ‘stress’ is often cited as an important factor in translocation failure, empirical evidence of physiological stress is lacking. Here we show that experimental translocation leads to changes in the physiological stress response in chukar partridge, Alectoris chukar. We found that capture alone significantly decreased the acute glucocorticoid (corticosterone, CORT) response, but adding exposure to captivity and transport further altered the stress response axis (the hypothalamic–pituitary–adrenal axis) as evident from a decreased sensitivity of the negative feedback system. Animals that were exposed to the entire translocation procedure, in addition to the reduced acute stress response and disrupted negative feedback, had significantly lower baseline CORT concentrations and significantly reduced body weight. These data indicate that translocation alters stress physiology and that chronic stress is potentially a major factor in translocation failure. Under current practices, the restoration of threatened species through translocation may unwittingly depend on the success of chronically stressed individuals. This conclusion emphasizes the need for understanding and alleviating translocation-induced chronic stress in order to use most effectively this important conservation tool.
Keywords: translocation, reintroduction, stress, corticosterone, chronic stress, hypothalamic-pituitary-adrenal axis
1. Introduction
Despite their important role in the conservation of threatened avian species, avian translocations have a high failure rate (Wolf et al. 1996) and causes for failure are poorly understood (Fischer & Lindenmayer 2000). Since ‘success’ is defined as a creation of a self-sustaining population, survival during the period immediately following the release of birds into a novel environment, the establishment phase, strongly affects the outcome of a translocation (Armstrong & Seddon 2008). Failure during the establishment phase can result from an increase in disease (Hartup et al. 1999; Bryant et al. 2002; Larkin et al. 2003), an increase in predation (Banks et al. 2002; Van Zant & Wooten 2003), a decreased reproductive capacity (Wolf et al. 1998) and an increased rate of dispersal from the release site (Griffith et al. 1989; Miller et al. 1999; Coates et al. 2006). In addition, ‘stress’ is often cited as an important factor (Letty et al. 2000; Teixeira et al. 2007; Chipman et al. 2008), although few studies have demonstrated empirical evidence of physiological stress.
In this study, we investigate the link between potential translocation failure and stress. In natural circumstances, the acute stress response is composed of adaptive physiological and behavioural responses to a noxious stimulus, a ‘stressor’, and mounting a response to a single acute stressor is generally thought to be beneficial (Sapolsky et al. 2000). During the acute stress response, the adrenal gland secretes glucocorticoids via the hypothalamic–pituitary–adrenal (HPA) axis. In birds, the HPA axis begins with stimulation of the hypothalamus, which secretes arginine vasotocin and corticotrophin-releasing factor to stimulate the pituitary. The pituitary then secretes adrenocorticotropin hormone (ACTH) resulting in adrenal release of corticosterone (CORT). Negative feedback quickly suppresses CORT release once the stressor diminishes or ceases (Sapolsky et al. 2000; Dallman & Bhatnagar 2001).
If a stressor persists or a series of acute stressors initiate multiple consecutive stress responses, the animal becomes chronically stressed (Wingfield & Romero 2001). Here we use ‘chronic stress’ to mean a change in stress-response physiology beyond a direct response to an acute stressor and beyond the adaptive time frame of stress-induced increase in CORT concentrations. Translocation has a high potential for causing chronic stress because it consists of multiple acute stressors initiating multiple consecutive acute stress responses. Such stressors include capture (Romero & Reed 2005; Lynn & Porter 2008; Delehanty & Boonstra 2009), handling (Kenagy & Place 2000; Oers & Carere 2007), transport (Coddington & Cree 1995; Davidson et al. 1997; Groombridge et al. 2004; Nilsson et al. 2008), captivity (Calvete et al. 2005; Franceschini et al. 2008) and release into a novel territory. In a chronically stressed animal, otherwise beneficial aspects of the acute response become detrimental (McEwen 1998). These detrimental effects mirror hypothesized causes of translocation failures; as examples, chronic stress causes immune system suppression (Dhabhar & McEwen 1997) which may lead to increased disease, reproductive axis disruption (Berga 2008) which may lead to decreased reproductive capacity, and diminishment of the fight-or-flight response to a startle stressor (Dickens & Romero in press) which may increase vulnerability to predation. Chronic stress, therefore, is a potential proximate mechanism causing failure during the establishment phase of translocation.
To quantify the effects of translocation on stress physiology, we determined whether CORT release is altered in translocated birds and evaluated HPA axis performance as an underlying mechanism since its role has been shown by prior research in laboratory (Dallman & Bhatnagar 2001) and wild-caught animals (Rich & Romero 2005). We used wild and free-ranging chukar, Alectoris chukar, as a study species to run large-scale physiological experiments in the wild with simulated translocations. We partitioned major components of translocation (capture, transport, captivity and release to novel area) in order to establish how each step of the translocation process affected CORT release. We measured changes in baseline and stress-induced CORT as well as changes in adrenal sensitivity and efficacy of the negative feedback system (Rich & Romero 2005) in addition to changes in chukar mass. We hypothesized that if translocation procedures cause chronic stress, then subjecting individuals to a progressive series of translocation components will result in increasingly disrupted stress physiology.
2. Material and methods
(a) Study species
Chukars are non-migratory desert-adapted ground birds. Introduced to North America as game birds in the early 1900s, they now occupy most of the arid mountains of the western United States. We captured wild chukar from a remote region of the Mojave Desert within the United States Navy China Lake Naval Air Weapons Station (CLNAWS) near Ridgecrest, CA (117°37′ W, 36°3′ N). Limited human access into CLNAWS assured minimal, non-experimental human interaction with our population. We chose chukar for this study because they presented an opportunity for large-scale physiological experimentation in the wild, not available with small populations or sensitive species.
We trapped chukar (n=346) at human-made rainwater catchments known as ‘guzzlers’ from mid-July, August 2006–2007. The guzzler system and trapping technique at CLNAWS has been described (Delehanty et al. 2004). Trapped animals were provided with water ad libitum and shade and were removed from the trapping cage within three hours of entering.
(b) Translocation protocol
We banded and weighed chukar immediately upon capture, then used stratified random assignment to divide individuals into three groups to evaluate the different components of translocation (illustrated conceptually in figure 1a): effects of initial capture; effects of holding animals in captivity and effects of release of animals to an unfamiliar area. As the control, we established a population-level ‘pre-capture’ baseline. We captured and immediately sampled this large subset of chukar exclusively to establish the overall naive endocrine response within the population. So as not to confound actual experimental treatment measurements with pre-treatment blood-sampling experience by individual chukar, we then excluded these control individuals from entry into treatment groups. Therefore, experimental chukars were first sampled upon recapture, at the end of their treatment protocol. Allocation of birds to control and treatment groups was randomized across time.
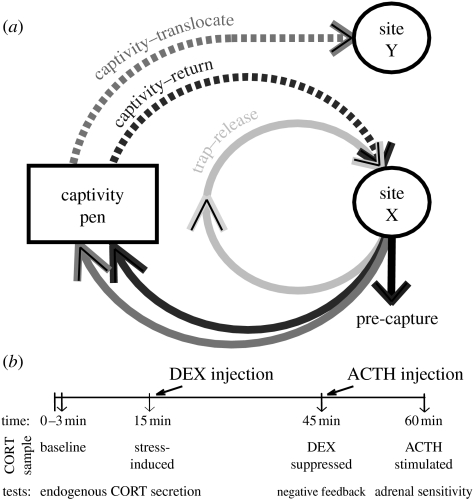
Figure 1.
(a) Experimental design schematic. The control group, pre-capture, represents the naive endocrine response of the population. All treatment groups were sampled upon the first recapture, post-release. The trap–release treatment tested the effect of single human capture interaction. The captivity–return treatment tested the effect of time spent in captivity as well as transport. The captivity–translocate treatment represents a full translocation. This figure is simplified to indicate site X as the home site and site Y as the novel, translocation site, but note that we used several sites in this experiment and all sites served as a capture and recapture sites for treatments and controls. (b) HPA challenge injection protocol. We commenced sampling within 3 min to first acquire a baseline CORT sample. Birds were then placed in an opaque bag and sampled at 15 min for a stress-induced CORT sample, immediately injected with DEX and sampled 30 min later for a DEX suppressed CORT sample, and then immediately injected with ACTH and sampled 15 min later for an adrenal-stimulated CORT sample.
The trap–release treatment consisted of animals captured, handled for a brief period of time (<15 min to band and weigh) and then immediately released back at the site of capture (‘home site’). This treatment represented the handling phase of translocation. The second group, captivity–return treatment, consisted of chukar captured, handled, transported to a captive holding facility for 10 days, and then returned to their home site. These chukars experienced handling, shipping and holding phases of translocation but were not exposed to a novel area. The third treatment, captivity–translocate, consisted of chukar captured, handled, transported, in captivity for 10 days, and translocated to an unfamiliar, new ‘translocation site’. These birds experienced all phases of translocation.
All home sites and translocation sites used were within a 16.5 km radius, at the same elevation, in the same habitat type, and all sites had a resident chukar population using the water source. Both the translocation sites (represented as site Y in figure 1a) and home sites (represented as site X in figure 1a) used for the captivity–translocate birds also served as home sites for the pre-capture, trap–release and captivity–return individuals to ensure that differences in treatment were not confounded by potential differences among sites. As with the co-occurring mountain quail, Oreortyx pictus (Delehanty et al. 2004), chukar are very site-faithful to water sources and capture data from this study demonstrated this fidelity (Dickens et al. in press).
Chukar brought into captivity were housed in 120×240×140 cm pens with no more than 20 birds occupying each pen for 10 days. Time in captivity decreased CORT responsiveness and damped negative feedback but these endocrine responses began to recover by the end of the 10 days of captivity and prior to release (Dickens et al. 2009).
(c) Stress responsiveness and HPA challenge
Except for the pre-capture controls, we sampled all individuals after they were released and then recaptured from the wild. All sampling series commenced within 3 min of our approaching the trap and began with a baseline CORT sample (Romero & Reed 2005) for each individual. In 2006, we recaptured individuals 1–18 days after release. After acquiring the baseline sample, birds were placed in an opaque bag and sampled at 15, 30 and 60 min to measure capacity to mount an endocrine stress response. In 2007, we recaptured individuals 1–31 days after release and followed a stress–injection protocol to test multiple HPA parameters within each individual (figure 1b). First, we obtained the baseline CORT sample then placed the chukar in an opaque bag and took a blood sample at 15 min to measure capacity for the endocrine stress response. Immediately following the 15-min sample, we injected intramuscularly 1000 μg ml−1 of dexamethasone (DEX), a synthetic glucocorticoid (Dickens et al. 2009). We then re-sampled the individual at 45 min (30 min after DEX injection) to measure strength of the negative feedback system to suppress endogenous stress-induced CORT release. DEX suppression is a commonly used tool for determining the efficacy of the CORT negative feedback response system (Sapolsky & Altmann 1991; Romero 2004) and has been previously shown to be physiologically relevant for chukar (Dickens et al. 2009). The DEX-suppressed sample was followed by a 100 IU kg−1 ACTH injection (Dickens et al. 2009) to stimulate the adrenal gland. We then resampled the individual at 60 min (15 min after ACTH injection) to measure adrenal sensitivity.
Dickens et al. (2009) previously validated the injection controls used here. These controls consisted of naive, wild-caught individuals injected in a similar series but with saline replacing one or both of the drug injections (i.e. DEX→saline, saline→ACTH and saline→saline) to validate the effect of the drug versus the vehicle. CORT was measured using a direct Radioimmunoassay (described in Wingfield et al. 1992) and intra- and inter-assay variabilities were less than 9 and 18 per cent, respectively.
(d) Data analysis
We evaluated changes in CORT concentrations over time of stimulation or suppression by calculating the integrated CORT value for each individual. The use of this approach has been previously described in detail (Romero 2004). For the injection protocol we analysed individual integrated responses for each protocol stage (0–15: stress, 15–45: negative feedback and 45–60: adrenal stimulation). We compared 2006 and 2007 15 min stress-induced CORT data using MANOVA to evaluate a group×year effect. We also calculated per cent weight loss as the difference between an individual's weight at time of capture and weight at recapture per original capture weight. Means for integrated CORT values and weight changes were analysed using ANOVA and a post hoc Tukey's test if the p-value was <0.05. As day of recapture varied between 1 and 31 days post-release, we added ‘days elapsed prior to recapture’ as a variable in the analysis and analysed group×days-elapsed using a least squares model. If an unequal variance was determined by a Levene's test, we transformed the data according to Tukey's Ladder and reran the analysis. Every individual on each day represented an independent measurement.
3. Results
Recapture of individuals was evenly distributed across the first 20 days post-release and only one individual was recaptured and sampled at 31 days. We found no effect of days elapsed prior to recapture in our analysis (stress: F3,88=0.58, r2=0.03, p=0.5620; DEX response: F3,67=1.27, r2=0.07, p=0.2897; ACTH response: F3,44=2.91, r2=0.15, p=0.063; weight change: F2,60=0.21, r2 =0.28, p=0.65) and we removed this variable from further analysis.
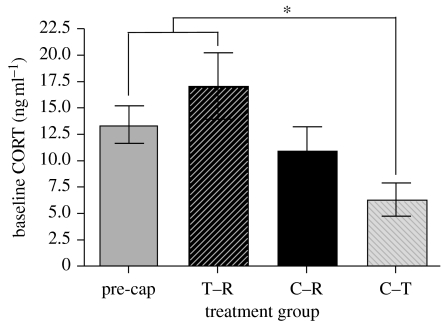
The captivity–translocate (n=12) group had a significantly lower baseline CORT concentration (F3,83=3.53; p<0.02; figure 2) compared with the pre-capture (n=35) and trap–release groups (n=20).
Figure 2.
Mean±s.e. of baseline CORT concentrations (sampled within 3 min) for initial capture of individuals in the pre-capture group (pre-cap, n=35), and upon first recapture of individuals in the trap–release (T–R, n=20), captivity–return (C–R, n=21) and captivity–translocate (C–T, n=12) groups. The asterisk indicates statistical significance between the C–T and pre-cap and T–R groups.
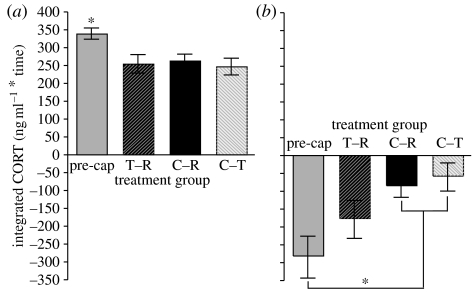
There was a significant group×year interaction (F2,94=3.99; p<0.03) when we compared the 15 min integrated stress-induced CORT in 2006 and 2007 data and we ran t-tests a posteriori to compare these values. Pre-capture values were constant between years (2006: 341.94±23.34, n=16 versus 2007: 340.14±15.5 ng ml−1, n=34; F1,49=0.23, p=0.63) as were the trap–release values (2006: 260±27.03, n=22 versus 2007: 255.52±28.35 ng ml−1, n=20; F1,40=0.016, p=0.90). The captivity–return values were significantly lower in 2006 (194.79±17.25, n=24 versus 2007: 264.46±16.58 ng ml−1, n=26; F1,48=8.48, p<0.006). We did not compare the captivity–translocate group since the 2006 season did not contain this group (Dickens et al. in press).
In 2007, captivity–translocate, trap–release and captivity–return treatments had significantly lower 15-min integrated stress-induced CORT compared with the pre-capture group (F3,88=5.48; p<0.002; figure 3a). We also observed a significant effect of treatment on DEX responsiveness. The captivity–return (n=25) and captivity–translocate treatment (n=12) had a significantly reduced response to DEX negative feedback (F3,67=3.99; p<0.02; figure 3b) relative to pre-capture (n=18), but the trap–release treatment (n=20) was not statistically different from any other group. We did not observe a treatment effect on responsiveness to ACTH stimulation although the CORT response to exogenous ACTH was very low in all treatments (F3,44=1.26; p=0.29).
Figure 3.
Mean integrated CORT concentrations±s.e. during the following protocol in which animals were restrained in an opaque bag: (a) 15 min post restraint (stress-induced CORT), and (b) 30 min post-injection of DEX (negative feedback, CORT suppression). The pre-capture control (pre-cap, n=34 for (a); n=18 for (b)) represents responses of naïve birds upon initial capture. Sampling of individuals in the trap–release (T–R, n=20), captivity–return (C–R, n=25) and captivity–translocate (C–T, n=12) treatments occurred upon recapture. The asterisk indicates statistical significance for (a) between the pre-cap and other groups and for (b) between the C–R and C–T as compared to the pre-cap. There were no significant changes for ACTH stimulated CORT concentrations.
All individuals in the captivity–translocate treatment (n=16) lost weight and, as a group, they lost significantly more weight (87.5%±2.2% of original capture weight) than the trap–release (100.6%±1.9%; n=22) and captivity–return (99.4%±1.8%; n=25) chukar (F2,60=11.99; p<0.0001).
4. Discussion
This study demonstrates, with empirical evidence that experimental translocation leads to changes in the stress physiology of birds handled in a manner typical of many conservation activities. Furthermore, the effects of the progressive sequence of capture, handling, transport, captivity and release to a new location seem to be additive, meaning that the sequence of acute stressors typically associated with the process of translocation causes meaningful physiological stress in birds. Translocated chukar had decreased baseline CORT concentrations, a reduced capacity to mount a CORT response to an acute stressor, a decreased sensitivity to negative feedback and significant weight loss.
Interestingly, ‘days elapsed prior to recapture’ was not associated with the magnitude of these changes, indicating that the changes to HPA function occurred quickly and persisted beyond the cessation of exposure to translocation-associated acute stressors. Unfortunately, our study did not allow follow-up beyond the 31 days of recapture and, as some studies suggest, animals may eventually recover from the stress of translocation (Franceschini et al. 2008). However, chronic stress effects lasting even a few weeks after release may correspond to the increased mortality that would hinder the establishment of the translocated population (Armstrong & Seddon 2008). Regardless, our study is the first to demonstrate that these alterations in the adaptive endocrine response of wild birds persist even after they are returned to their natural habitat.
Partitioning translocation into its various components resulted in the surprising finding that a single exposure to a capture and handling event was sufficient to cause a long-term decrease in endocrine responsiveness. This decreased responsiveness was observed in all the three treatment groups, including the group that was handled only briefly and was not transported nor held in captivity nor translocated (figure 3a). We saw even further attenuation in CORT responses with captivity–return in 2006, although not in 2007, indicating that additive effects are possible. In another portion of our study, we observed a behavioural difference between years in the propensity of chukar to move across the landscape (Dickens et al. in press), and it is interesting to consider that increased chukar movement occurred in association with greater endocrine change as well as with inter-year environmental variation.
Captivity appears to be the critical step for altering the HPA axis and causing inadequate termination of CORT secretion. We observed that the negative feedback response was attenuated to an equivalent degree after captivity, regardless of further treatment steps (figure 3b). The lack of response to DEX by chukar recaptured from the wild, points to a serious endocrinological outcome of translocation. The total period of elevated CORT is a critically important facet of the stress response because high, stress-induced concentrations of CORT can become damaging if secretion is not terminated quickly following the acute stressor (Sapolsky et al. 2000). Inadequate negative feedback responsiveness, as expressed in recaptured chukar in this study, implies these individuals will endure elevated CORT for a longer period of time when faced with a stressor, and, therefore, inappropriately cope with acute stressors in the wild (Romero 2004). In wild, free-ranging baboons (Papio cynocephalus), disrupted negative feedback in cortisol has been implicated in cardiac disease (Sapolsky & Altmann 1991). In humans, such a disruption in the DEX suppression response as a result of HPA dysregulation is often used as a diagnostic tool for severe depression (Carroll et al. 1981; Bostwick 2005). Here we demonstrate a similar result of chronic stress in wild birds. In essence, translocated animals may be suffering from a stress syndrome similar to or parallel to human disease. The evidence in our study points to captivity as a main factor for this stress syndrome. In conservation practice, a captivity period often is used for disease screening and quarantine. Our results indicate that captivity and additional anthropogenic stressors while in captivity may exacerbate chronic stress with the potential to compromise the health of animals and their ability to cope with stressors following release.
The decrease in baseline CORT concentration that we observed in chukar following their release parallels other recent data on chronic stress in wild, free-living and wild-caught captive birds (Rich & Romero 2005; Cyr & Romero 2007). The captivity–translocate chukar also experienced greater weight loss compared with the other groups. Chronic stress often results in weight loss in laboratory animals (Konkle et al. 2003; Bartolomucci et al. 2004) and captive wild species (Rich & Romero 2005). Weight loss did not correlate to the time elapsed since translocation. We recognize that many social and ecological effects probably influence energy balance following translocation. But these challenges following translocation can be reasonably viewed as potential additional stressors and it is intriguing to observe that chukar with the most compromised stress physiology demonstrated the sustained weight loss. Therefore, the key aspect of translocation, the novel environment, resulted in a full spectrum of chronic stress indicators: decreased baseline CORT; decreased CORT response to an acute stressor; desensitized HPA negative feedback; and weight loss.
Our results provide a new, empirical perspective into the stress physiology of avian translocations and reveal important issues for designing translocations for avian conservation. In particular, these results may help to identify and reduce, where possible, the number of stressors translocated birds are subjected to in order to reduce chronic stress effects during and following translocation. Even the most basic translocation procedures such as capture and handling can induce some form of chronic stress and our study is the first to indicate that birds can retain the effects of these stressors even after quickly being returned to their natural habitat. Our results point to an unfortunate dilemma for conservation action: the restoration of threatened species through translocation may depend on the success in the wild of chronically stressed individuals. Understanding and alleviating translocation-induced chronic stress is an important challenge in the restoration of threatened wildlife populations through translocation.
Acknowledgments
All animal use complied with AAALAC guidelines and was approved by the Tufts Institutional Animal Care and Use Committee.
We thank the China Lake Naval Air Weapons Station for the opportunity to conduct these studies, T. G. Campbell for his support on the base, and K. Earle and G. Kleinman for their help in the field. Funding was provided by NSF (#IOB-0542099 to LMR), and AOU, Sigma Xi, and the SICB Grants-in-Aid of Research (to M.J.D.).
References
- Armstrong D.P., Seddon P.J. Directions in reintroduction biology. Trends Ecol. Evol. 2008;23:20–25. doi: 10.1016/j.tree.2007.10.003. doi:10.1016/j.tree.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Banks P.B., Norrdahl K., Korpimaki E. Mobility decisions and the predation risks of reintroduction. Biol. Conserv. 2002;103:133–138. doi:10.1016/S0006-3207(01)00110-0 [Google Scholar]
- Bartolomucci A., Pederzani T., Sacerdote P., Panerai A.E., Parmigiani S., Palanza P. Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology. 2004;29:899–910. doi: 10.1016/j.psyneuen.2003.08.003. doi:10.1016/j.psyneuen.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Berga S.L. Stress and reprodution: a tale of false dichotomy? Endocrinology. 2008;149:867–868. doi: 10.1210/en.2008-0004. doi:10.1210/en.2008-0004 [DOI] [PubMed] [Google Scholar]
- Bostwick J.M. The stress axis gone awry: a possible neuroendocrine explanation for increased risk of completed suicide. Prim. Psychiatry. 2005;12:49–52. [Google Scholar]
- Bryant A.A., Schwantje H.M., de With N.I. ABF Publishing House; Moscow, Russia: 2002. Disease and unsuccessful reintroduction of Vancouver Island marmots (Marmota vancouverensis) [Google Scholar]
- Calvete C., Angulo E., Estrada R., Moreno S., Villafuerte R. Quarantine length and survival of translocated European wild rabbits. J. Wildl. Manage. 2005;69:1063–1072. doi:10.2193/0022-541X(2005)069[1063:QLASOT]2.0.CO;2 [Google Scholar]
- Carroll B.J., Feinberg M., Greden J.F. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch. Gen. Psychiatry. 1981;38:15–22. doi: 10.1001/archpsyc.1981.01780260017001. [DOI] [PubMed] [Google Scholar]
- Chipman R., Slate D., Rupprecht C., Mendoza M. Downside risk of wildlife translocation. Dev. Biol. 2008;131:223–232. [PubMed] [Google Scholar]
- Coates P.S., Stiver S.J., Delehanty D.J. Using sharp-tailed grouse movement patterns to guide release-site selection. Wildl. Soc. Bull. 2006;34:1376–1382. doi:10.2193/0091-7648(2006)34[1376:USGMPT]2.0.CO;2 [Google Scholar]
- Coddington E.J., Cree A. Effect of acute captivity stress on plasma concentrations of corticosterone and sex steroids in female whistling frogs, Litoria ewingi. Gen. Comp. Endocrinol. 1995;100:33–38. doi: 10.1006/gcen.1995.1129. doi:10.1006/gcen.1995.1129 [DOI] [PubMed] [Google Scholar]
- Cyr N.E., Romero L.M. Chronic stress in free-living European starlings reduces corticosterone concentrations and reproductive success. Gen. Comp. Endocrinol. 2007;151:82–89. doi: 10.1016/j.ygcen.2006.12.003. doi:10.1016/j.ygcen.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Dallman M.F., Bhatnagar S. Chronic stress and energy balance: role of the hypothalamo–pituitary–adrenal axis. In: McEwen B.S., Goodman H.M., editors. Handbook of physiology; section 7: the endocrine system; volume iv: coping with the environment: neural and endocrine mechanisms. Oxford University Press; New York, NY: 2001. pp. 179–210. [Google Scholar]
- Davidson G.W., Thorarensen H.T., Lokman M., Davie P.S. Stress of capture and captivity in kahawai Arripis trutta. Comp. Biochem. Physiol. A Physiol. 1997;118:1405–1410. doi:10.1016/S0300-9629(97)86806-5 [Google Scholar]
- Delehanty B., Boonstra R. Impact of live trapping on stress profiles of Richardson's ground squirrel (Spermophilus richardsonii) Gen. Comp. Endocrinol. 2009;160:176–182. doi: 10.1016/j.ygcen.2008.11.011. doi:10.1016/j.ygcen.2008.11.011 [DOI] [PubMed] [Google Scholar]
- Delehanty D.J., Eaton S.S., Campbell T.G. From the field: mountain quail fidelity to guzzlers in the Mojave Desert. Wildl. Soc. Bull. 2004;32:588–593. doi:10.2193/0091-7648(2004)32[588:FTFMQF]2.0.CO;2 [Google Scholar]
- Dhabhar F.S., McEwen B.S. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav. Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. doi:10.1006/brbi.1997.0508 [DOI] [PubMed] [Google Scholar]
- Dickens, M. J. & Romero, L. M. In press. Wild European starlings (Sturnus vulgaris) adjust to captivity with sustained sympathetic nervous system drive and a reduced fight-or-flight response. Physiol. Biochem. Zool [DOI] [PubMed]
- Dickens M.J., Earle K.A., Romero L.M. Initial transference of wild birds to captivity alters stress physiology. Gen. Comp. Endocrinol. 2009;160:76–83. doi: 10.1016/j.ygcen.2008.10.023. doi:10.1016/j.ygcen.2008.10.023 [DOI] [PubMed] [Google Scholar]
- Dickens, M. J., Delehanty, D. J. & Romero, L. M. In press. What happens to translocated game birds that ‘disappear’? Anim. Conserv
- Fischer J., Lindenmayer D.B. An assessment of the published results of animal relocations. Biol. Conserv. 2000;96:1–11. doi:10.1016/S0006-3207(00)00048-3 [Google Scholar]
- Franceschini M.D., Rubenstein D.I., Low B., Romero L.M. Fecal glucocorticoid metabolite analysis as an indicator of stress during translocation and acclimation in an endangered large mammal, the Grevy's zebra. Anim. Conserv. 2008;11:263–269. doi:10.1111/j.1469-1795.2008.00175.x [Google Scholar]
- Griffith B., Scott J.M., Carpenter J.W., Reed C. Translocation as a species conservation tool—status and strategy. Science. 1989;245:477–480. doi: 10.1126/science.245.4917.477. doi:10.1126/science.245.4917.477 [DOI] [PubMed] [Google Scholar]
- Groombridge J.J., Massey J.G., Bruch J.C., Malcolm T.R., Brosius C.N., Okada M.M., Sparklin B. Evaluating stress in a Hawaiian honeycreeper, Paroreomyza montana, following translocation. J. Field Ornithol. 2004;75:183–187. [Google Scholar]
- Hartup B.K., Kollias G.V., Jacobsen M.C., Valentine B.A., Kimber K.R. Exertional myopathy in translocated river otters from New York. J. Wildl. Dis. 1999;35:542–547. doi: 10.7589/0090-3558-35.3.542. [DOI] [PubMed] [Google Scholar]
- Kenagy G.J., Place N.J. Seasonal changes in plasma glucocorticosteroids of free-living female yellow-pine chipmunks: effects of reproduction and capture and handling. Gen. Comp. Endocrinol. 2000;117:189–199. doi: 10.1006/gcen.1999.7397. doi:10.1006/gcen.1999.7397 [DOI] [PubMed] [Google Scholar]
- Konkle A.T.M., Baker S.L., Kentner A.C., Barbagallo L.S.-M., Merali Z., Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. doi:10.1016/j.brainres.2003.08.047 [DOI] [PubMed] [Google Scholar]
- Larkin J.L., Alexy K.J., Bolin D.C., Maehr D.S., Cox J.J., Wichrowski M.W., Seward N.W. Meningeal worm in a reintroduced elk population in Kentucky. J. Wildl. Dis. 2003;39:588–592. doi: 10.7589/0090-3558-39.3.588. [DOI] [PubMed] [Google Scholar]
- Letty J., Marchandeau S., Clobert J., Aubineau J. Improving translocation success: an experimental study of anti-stress treatment and release method for wild rabbits. Anim. Conserv. 2000;3:211–219. doi:10.1111/j.1469-1795.2000.tb00105.x [Google Scholar]
- Lynn S.E., Porter A.J. Trapping initiates stress response in breeding and non-breeding house sparrows Passer domesticus: implications for using unmonitored traps in field studies. J. Avian Biol. 2008;39:87–94. doi:10.1111/j.0908-8857.2008.04204.x [Google Scholar]
- McEwen B.S. Seminars in medicine of the Beth Israel Deaconess Medical Center: protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. doi:10.1056/NEJM199801153380307 [DOI] [PubMed] [Google Scholar]
- Miller B., Ralls K., Reading R.P., Scott J.M., Estes J. Biological and technical considerations of carnivore translocation: a review. Anim. Conserv. 1999;2:59–68. doi:10.1111/j.1469-1795.1999.tb00049.x [Google Scholar]
- Nilsson P.B., Hollmén T.E., Atkinson S., Mashburn K.L., Tuomi P.A., Esler D., Mulcahy D.M., Rizzolo D.J. Effects of ACTH, capture, and short term confinement on glucocorticoid concentrations in harlequin ducks (Histrionicus histrionicus) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008;149:275–283. doi: 10.1016/j.cbpa.2008.01.002. doi:10.1016/j.cbpa.2008.01.002 [DOI] [PubMed] [Google Scholar]
- Oers K., Carere C. Long-term effects of repeated handling and bleeding in wild caught great tits Parus major. J. Ornithol. 2007;148:S185–S190. doi:10.1007/s10336-007-0200-y [Google Scholar]
- Rich E.L., Romero L.M. Exposure to chronic stress downregulates corticosterone responses to acute stressors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R1628–R1636. doi: 10.1152/ajpregu.00484.2004. doi:10.1152/ajpregu.00484.2004 [DOI] [PubMed] [Google Scholar]
- Romero L.M. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 2004;19:249–255. doi: 10.1016/j.tree.2004.03.008. doi:10.1016/j.tree.2004.03.008 [DOI] [PubMed] [Google Scholar]
- Romero L.M., Reed J.M. Collecting baseline corticosterone samples in the field: is under three minutes good enough? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005;140:73–79. doi: 10.1016/j.cbpb.2004.11.004. doi:10.1016/j.cbpb.2004.11.004 [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M., Altmann J. Incidence of hypercortisolism and dexamethasone resistance increases with age among wild baboons. Biol. Psychiatry. 1991;30:1008–1016. doi: 10.1016/0006-3223(91)90121-2. doi:10.1016/0006-3223(91)90121-2 [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M., Romero L.M., Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. doi:10.1210/er.21.1.55 [DOI] [PubMed] [Google Scholar]
- Teixeira C.P., de Azevedo C.S., Mendl M., Cipreste C.F., Young R.J. Revisiting translocation and reintroduction programmes: the importance of considering stress. Anim. Behav. 2007;73:1–13. doi:10.1016/j.anbehav.2006.06.002 [Google Scholar]
- Van Zant J.L., Wooten M.C. Translocation of Choctawhatchee beach mice (Peromyscus polionotus allophrys): hard lessons learned. Biol. Conserv. 2003;112:405–413. doi:10.1016/S0006-3207(02)00338-5 [Google Scholar]
- Wingfield J.C., Romero L.M. Adrenocortical responses to stress and their modulation in free-living vertebrates. In: McEwen B.S., Goodman H.M., editors. Handbook of physiology; section 7: the endocrine system; volume iv: coping with the environment: neural and endocrine mechanisms. Oxford University Press; New York, NY: 2001. pp. 211–234. [Google Scholar]
- Wingfield J.C., Vleck C.M., Moore M.C. Seasonal changes of the adrenocortical response to stress in birds of the Sonoran Desert. J. Exp. Zool. 1992;264:419–428. doi: 10.1002/jez.1402640407. doi:10.1002/jez.1402640407 [DOI] [PubMed] [Google Scholar]
- Wolf C.M., Griffith B., Reed C., Temple S.A. Avian and mammalian translocations: update and reanalysis of 1987 survey data. Conserv. Biol. 1996;10:1142–1154. doi:10.1046/j.1523-1739.1996.10041142.x [Google Scholar]
- Wolf C.M., Garland T., Jr, Griffith B. Predictors of avian and mammalian translocation success: reanalysis with phlogenetically independent contrasts. Biol. Conserv. 1998;86:243–255. doi:10.1016/S0006-3207(97)00179-1 [Google Scholar]