Abstract
The elongated tails adorning many male birds have traditionally been thought to degrade flight performance by increasing body drag. However, aerodynamic interactions between the body and tail can be substantial in some contexts, and a short tail may actually reduce rather than increase overall drag. To test how tail length affects flight performance, we manipulated the tails of Anna's hummingbirds (Calypte anna) by increasing their length with the greatly elongated tail streamers of the red-billed streamertail (Trochilus polytmus) and reducing their length by removing first the rectrices and then the entire tail (i.e. all rectrices and tail covert feathers). Flight performance was measured in a wind tunnel by measuring (i) the maximum forward speed at which the birds could fly and (ii) the metabolic cost of flight while flying at airspeeds from 0 to 14 m s−1. We found a significant interaction effect between tail treatment and airspeed: an elongated tail increased the metabolic cost of flight by up to 11 per cent, and this effect was strongest at higher flight speeds. Maximum flight speed was concomitantly reduced by 3.4 per cent. Also, removing the entire tail decreased maximum flight speed by 2 per cent, suggesting beneficial aerodynamic effects for tails of normal length. The effects of elongation are thus subtle and airspeed-specific, suggesting that diversity in avian tail morphology is associated with only modest flight costs.
Keywords: Anna's hummingbird, flight cost, flight speed, metabolism, sexual selection, tail
1. Introduction
The long, sexually dimorphic tails of many male birds are a classic example of a sexually selected ornament. Evidence from various taxa suggests that this morphology has arisen because females favour males with elongated tails (Darwin 1871; Andersson, M. 1982, 1994; Petrie et al. 1991; Andersson, S. 1992). However, although such sexually selected traits increase reproductive fitness, they may also impose performance or viability costs on the individuals bearing them (Andersson, M. 1994). As a consequence, sexually selected traits are hypothesized to evolve to a limiting size for which any fitness benefits of further elaboration are offset by inordinate costs of growth, use or maintenance (Andersson, M. 1994; Oufiero & Garland 2007).
Long tails in birds are thought to degrade flight performance by increasing body drag (Evans & Thomas 1992; Balmford et al. 1993; Thomas 1993; Norberg 1995) or by affecting foraging rates and aerial manoeuvrability (Evans 1998; Matyjasiak et al. 1999, 2000, 2004; Park et al. 2000; Rowe et al. 2001). For the purposes of quantifying how changes in tail morphology influence drag, the tail has been modelled as an isolated flat plate in free-stream flow (Evans & Thomas 1992; Balmford et al. 1993; Thomas 1993; Norberg 1995). However, Maybury & Rayner (2001) demonstrated significant aerodynamic interaction between a bird's body and its tail. Drag on the frozen body of a starling mounted in a wind tunnel increased when the tail was removed; flow visualization revealed concomitant flow separation from the body. Tail elongation might enhance this splitter plate effect in spite of a potential increase in skin friction drag caused by increased surface area.
We tested the hypothesis that an elongated tail influences aerodynamic performance of free-flying hummingbirds. Changes in tail length might have two effects on forward flight performance. First, total drag (which includes contributions from the tail) influences the energetic requirements for flight, but primarily at high airspeeds, given the dependence of power components on the cube of relative speed (Pennycuick 1969; Rayner 2001). Morphological changes that influence body drag should thus increase metabolic costs of high-speed flight, but may have no effect at low speed. Second, the maximum speed at which a bird can fly may be limited by the muscle power output necessary to overcome total drag, although kinematic limitations may also pertain (Dial et al. 1997; Rayner 1999). If a manipulation increases a bird's drag, it may decrease maximum flight speed. Here, we evaluate the effects of tail length on both aspects of flight performance by measuring metabolic rates and maximum airspeeds of Anna's hummingbirds flying in a wind tunnel.
2. Material and methods
Anna's hummingbirds (Calypte anna) were captured in Berkeley, CA. Following two days of habituation, the birds were trained to fly in a wind tunnel over a range of airspeeds (0–14 m s−1) over the course of several hours. The birds were considered fully trained when they could feed from a mask attached to a feeder at airspeeds of 12.0 m s−1. Experiments were then carried out the following day, typically with 1 day allocated per treatment per bird (see below). Body mass was measured before and after flight trials in the tunnel; the average of these values was used in the calculation of mass-specific metabolic rates. The wind tunnel (Engineering Laboratory Design, Inc., Lake City, MN) was an Eiffel style, open-circuit design with a 6.25 : 1 contraction section and a working section measuring 45.5×45.5×91.5 cm. Spatial variation in velocity was less than 1 per cent of the mean value. After the experiments, all the birds were released back into the wild.
(a) Tail manipulations
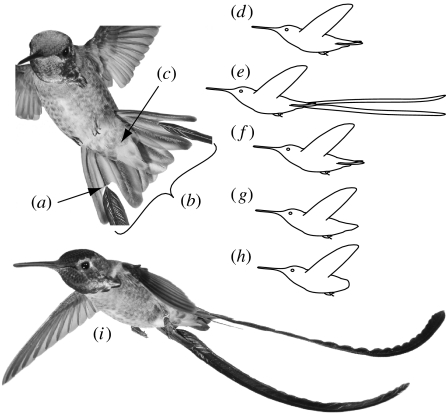
Tail length was increased by altering the fourth rectrix, which is the second-to-outermost tail feather (figure 1a,b). The outermost tail feather was not used as it serves in sound production (Clark & Feo 2008), and the fourth rectrix is also the elongated feather in the red-billed streamertail (Trochilus polytmus) that was used in our experimental manipulations. Five consecutive treatments were employed, always in the same order (figure 1d–i): (i) no manipulation (control 1), (ii) tail elongation with red-billed streamertail rectrices, (iii) reinsertion of the bird's own fourth rectrices (sham manipulation; control 2), (iv) plucking of all rectrices (no-rectrices treatment), and (v) plucking of all tail coverts and rectrices (no-tail treatment). All the birds underwent treatment 1, but not all subsequent treatments were performed on each bird.
Figure 1.
Tail morphology and manipulations of tail length in Anna's hummingbirds: (a) the arrow indicates where two feathers have been spliced together (see text for more details), (b) rectrices and (c) tail coverts; (d–h) five consecutive experimental manipulations: (d) no manipulation (control 1), (e) long-tail treatment, (f) sham manipulation (control 2), (g) no-rectrices treatment and (h) no-tail treatment (rectrices and coverts removed); and (i) photo of a male Anna's hummingbird flying with attached tail streamers.
Manipulations were performed by restraining a hummingbird and then cutting off the distal portion of its fourth rectrices at a point 10–15 mm from the base. An insect pin (0.1 mm diameter×6 mm) was dipped in glue (cyanoacrylate) and inserted approximately 3 mm into the hollow shaft of the feather to be attached, such that the remaining 3 mm of the pin remained exposed. This free end was also dipped in glue and then inserted into the hollow shaft of the bird's fourth tail feather. The newly attached feather was positioned such that its vane was aligned with that of the bird's original feather (figure 1a). Often this procedure necessitated clipping a small (roughly 1 mm) piece of feather, thus the sham manipulation treatment (in which the bird's own fourth rectrices were cut and then reattached) reduced its length by an average of 1 mm. The long-tail treatment increased body mass by approximately 0.02 g, representing 0.4 per cent of body mass on average. Tail length initially averaged 31 mm; the elongated treatment corresponded to an average tail length of 190 mm.
(b) Metabolic measurements
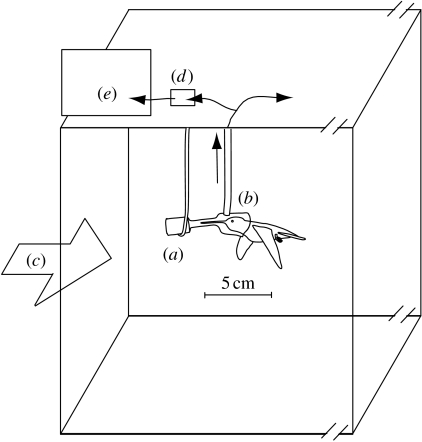
A respirometry mask made of plastic, glue and clear acetate was suspended 15 cm from the ceiling of the working section of the wind tunnel (figure 2). The mask was oriented such that feeding birds would fly directly and symmetrically into the airflow. Mask size was minimized to reduce its aerodynamic profile, but the profile was still substantial given the need to frequently refill the 2 ml food reservoir and to encompass the entire head and bill of a feeding bird (see the electronic supplementary material for particle image velocimetry of the flow field around the mask). Food (sugar solution) was available ad libitum during measurements, and the birds fed regularly, ensuring respiratory quotients near 1 (Welch et al. 2007). A perch was provided in the working section, well downstream from the mask.
Figure 2.
Metabolic measurements within the working section of a wind tunnel. (a) Food reservoir, (b) mask and hummingbird were traced from a photograph and thus accurately portray the bird's dimensions relative to the mask. The mask is approximately 2 cm in diameter (see figure 1 in the electronic supplementary material). The reservoir was located directly upstream of the mask. Within the working section of a wind tunnel (wind direction indicated by (c)), hummingbirds voluntarily inserted their heads into the mask to obtain food. Air was drawn through the mask at a rate of 3.9 l min−1. A 0.3 l min−1 subsample was drawn through (d) desiccant and into (e) an oxygen sensor, while the remaining air passed through a flow meter.
Our respirometry set-up was similar to that described for hovering hummingbird energetics in Bartholomew & Lighton (1986), Wells (1993) and Chai & Dudley (1996; figure 2). At high airspeeds, potential loss of expired respiratory gases from the mask necessitated flow rate calibration via the following procedure. At an airspeed of 10 m s−1, flow rate of air through the mask was varied from 1 to 4.5 l min−1. Metabolic rates of birds feeding while flying at this speed converged asymptotically at flow rates greater than 3.0 l min−1, so for all experiments air was drawn through the mask at 3.9 l min−1. Owing to the low and relatively invariant laboratory humidity, this gas stream was not dried. A 0.3 l min−1 subsample of this air was dried in a 1.5 ml desiccation chamber filled with indicating Drierite, and then passed through an oxygen analyser (Applied Electrochemistry S-3A/II). The Drierite was replaced as needed, approximately every 10 min. The oxygen analyser output was sampled at 240 Hz using a graphical user interface in Labview (National Instruments), and sets of 12 samples were averaged to reduce electrical noise, yielding an effective sampling rate of 20 Hz. Equilibrium (minimum) values of oxygen partial pressure were typically reached less than 2 s from the onset of a feeding bout. The change in oxygen partial pressure from baseline to equilibrium was multiplied by the flow rate (adjusted by 1% to account for ambient humidity) to estimate metabolic rate. Oxygen depletion was measured for multiple feeding bouts at each airspeed for a given individual, and then an objective set of criteria was used to eliminate curves associated with (i) excessively short feeding duration, (ii) head displacement inside the mask during a feeding bout, and (iii) obstruction of air flow by the bird's head within the mask. Traces were analysed blind relative to their corresponding airspeed.
At the onset of any given experimental treatment, a bird was restrained for approximately 15 min while its tail feathers were altered, and was then released into the tunnel's working section. Restraint tended to disconnect neighbouring barbs of the wing and tail feathers (potentially influencing flight performance), so the bird was first allowed 30 min in still air to preen and to acclimatize to the working section. Measurements of metabolic rates were then made while the bird was flying at airspeeds of 0.0 (i.e. hovering), 2.0, 4.0, 6.0, 8.0, 10, 12, 13 and 14 m s−1. In the first experimental treatment, wind tunnel speed order was selected randomly except that 13 and 14 m s−1 were always last, because many of the birds could not feed at these two speeds. Birds were not flown at speeds of 13 and 14 m s−1 for more than 5 min to ensure that, if unable to feed, their respiratory quotients remained near 1. For subsequent experimental treatments, the same order of tunnel speeds was used. Up to six feeding bouts per bird per treatment were obtained at each airspeed. Metabolic measurements for a single experimental treatment over the full range of speeds were typically collected over the course of several hours.
The power curve relating metabolic costs of flight to airspeed are hypothesized to be U-shaped, with an ascending right side that increases in proportion to the cube of airspeed and a minimum at intermediate airspeeds (Rayner 2001). We used the following underlying model to test this relationship:
| (2.1) |
where VO2 is the mass-specific metabolic rate; v is airspeed; and β0, β1 and β2 are statistically estimated parameters. Equation (2.1) has a theoretical basis (Rayner 2001); however, it is undefined at v=0 (hovering flight). So, by necessity, we excluded metabolic values for hovering from the main statistical analysis. Effects of experimental treatment on hovering metabolic rates were therefore tested in a separate ANOVA, with bird ID included as a random factor. We are aware that use of equation (2.1) in the analysis de facto assumes that the hummingbird metabolic power curve is U-shaped, whereas this has been disputed (Berger 1985; Ellington 1991). However, alternative models (such as a parabola, v+v3) suffered from greater multicollinearity between the velocity terms than did equation (2.1). Because our purpose here was to test whether tail length had significant interactions with the v3 portion of the curve in particular (and therefore reducing multicollinearity was of primary importance), equation (2.1) offered the best alternative for a test of this hypothesis.
Effects of experimental treatments were analysed statistically using a general linear model (GLM) with experimental treatment as a fixed factor, bird identity as a random factor and v−1, v3, treatment×v−1 interaction and treatment×v3 interaction as covariates. The interaction terms were included to explicitly test the hypothesis that the effects of experimental treatment varied with v3, and not v−1.
(c) Maximum flight speeds
We measured maximum forward flight speeds using an assay similar to that of Chai et al. (1999). The birds naturally preferred to fly towards the front of the test section (i.e. upwind), and this tendency was reinforced by the presence of the experimenter standing near the back of the test section (i.e. downwind). The tunnel was set initially to an airspeed of 12.6 m s−1, which was then slowly increased. When a flying bird was unable to maintain position at the front of the working section, it slowly drifted backwards past a predetermined plane at the midpoint of the working section. The wind tunnel was turned off, and only then did the experimenter note and record the prior wind speed at maximal flight. The small number of trials in which a bird rapidly flew to the back of the working section, such as when it appeared to lose control, were excluded. After each measurement, the bird was allowed to rest and preen for at least 10 min in still air. Maximum airspeed was measured three times in succession for each bird under a given treatment; these measurements typically showed low variation (average s.d.: 0.3 m s−1, n=56 sets of measurements). In several cases, the bird could fly at the tunnel's top speed of 15.6 m s−1, in which case this value was considered to be the bird's maximum airspeed. For statistical analyses, the three measurements of maximum speed were averaged, and each experimental treatment was compared with control treatments using a repeated-measures ANOVA.
3. Results
(a) Metabolic measurements
We measured metabolic rates during flight of five male and one female Anna's hummingbird across five experimental treatments, and across speeds of 0.0, 2.0, 4.0, 6.0, 8.0, 10, 12, 13 and 14 m s−1. However, values of 0.0 m s−1 were analysed separately from the remaining speeds (because equation (2.1) is undefined at v=0). Few of the birds were able to feed at speeds of 13 and 14 m s−1.
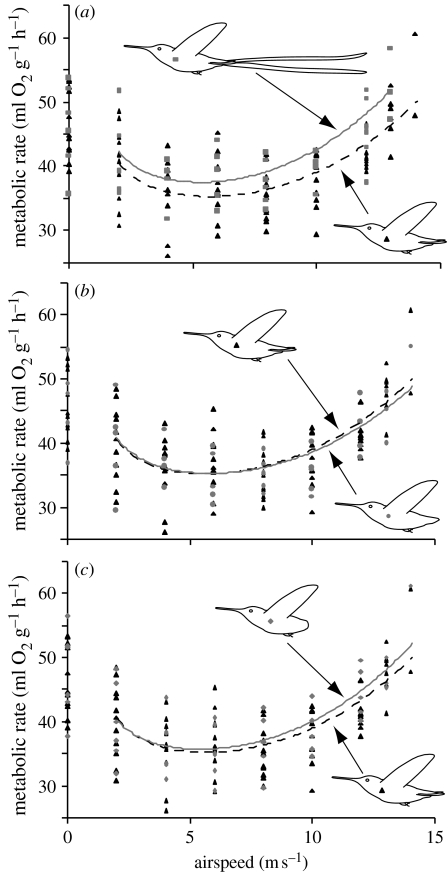
Metabolic rate as a function of flight speed is plotted for all of the experimental treatments in figure 3. In all, 864 samples were included in the GLM analysis of the relationship between metabolic rate and flight speed. The main independent variables in the model—v−1, v3 and bird identity—were highly statistically significant (v−1: F1,844=197, p<0.001; v3: F1,844=640, p<0.001; bird ID: F5,844=112, p<0.001). The treatment×v−1 interaction term was not statistically significant (F4,844=1.27, p=0.28), whereas the treatment×v3 interaction was marginally statistically significant (F4,844=2.07, p=0.0825).
Figure 3.
Metabolic rate of six hummingbirds as a function of airspeed and experimental treatment. Each point represents an average for one bird at each speed and treatment. The two control treatments are both symbolized with black triangles, and the same control points are replotted in each figure. Curves from the GLM (based on equation (2.1)) have been fitted to each treatment, not including hovering metabolic data. Black dashed line indicates control treatments (equation: VO2=30.6+20.0v−1+0.0065v3); grey solid line indicates experimental treatments. (a) Long-tail treatment (grey squares) relative to the controls (VO2=32.7+19.0v−1+0.008v3), (b) the no-rectrices treatment (grey circles) relative to the controls (VO2=30.6+20.8v−1+0.006v3) and (c) the no-tail treatment (grey diamonds) relative to the control treatments (VO2=31.3+17.9v−1+0.007v3). See text for statistics.
Within the GLM, the parameters fitted to v−1 and v3 for each individual treatment (i.e. representing individual treatment×velocity interactions) were compared with the parameters for the first control treatment (no manipulation). The v3 parameter was significantly higher for the long-tail treatment (t=2.47, p=0.014; figure 3a), whereas none of the other parameters were significantly different (control 2: t=1.46, p=0.144; no-rectrix treatment: t=0.203, p=0.84; no-tail treatment, t=1.64, p=0.101; figure 3b,c). With respect to v−1, there was a significant difference in parameter value between the two control treatments (t=−2.088, p=0.037). None of the other v−1 parameters for other treatments were significantly different from the first treatment (|t|<1.62, p>0.10). The difference between the controls (despite a non-significant treatment×v−1 interaction; previous paragraph) caused concern that there may have been differences between the control treatments at low speeds. However, in a separate analysis of the data for 0 m s−1, there was no significant difference between the experimental treatments (ANOVA, F4,129=1.30, p=0.27), whereas bird ID (which was included as a random factor) was statistically significant (ANOVA, F5,129=75.9, p<0.001).
In the no-tail treatment, perching at high airspeeds (between feeding bouts) seemed to pose a greater challenge, and the birds appeared to have increased difficulty feeding at airspeeds greater than 10 m s−1. Neither of these qualitative effects were evident in the long-tail or no-rectrix treatments. No bird ever voluntarily flew in the wake of the mask except when attempting to feed or actually feeding.
(b) Maximum flight speeds
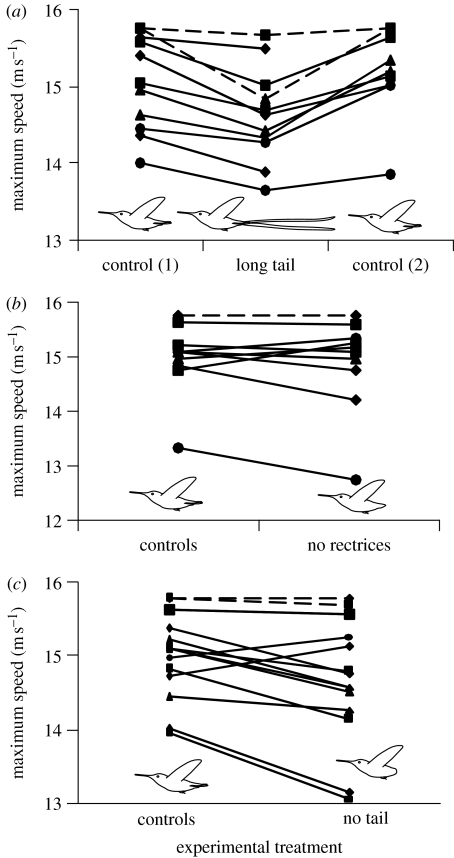
Maximum forward airspeeds did not differ between the two control treatments (paired t-test, p=0.3, n=9 birds), whereas adding the elongated fourth rectrices significantly reduced maximum forward airspeed from 15.1 to 14.6 m s−1, a 3.4 per cent decrease (repeated-measures ANOVA, p<0.0001, n=11 birds; figure 4a). The no-rectrix treatment did not significantly differ from the control treatments (repeated-measures ANOVA, p=0.7, n=10 birds; figure 4b), whereas the no-tail treatment significantly decreased maximum airspeed by 2 per cent (repeated-measures ANOVA, p=0.02, n=15 birds; figure 4c). Several birds exhibited apparent maximum speeds of 15.6 m s−1 in one or more trials, and one bird was able to match the tunnel's top speed of 15.6 m s−1 for up to 2 min in almost every trial for which it was measured (dashed lines, figure 4). This results in an underestimate of maximum airspeed for some trials (mostly controls), but because we hypothesized a reduction in maximum airspeed for both long- and no-tail treatments relative to the controls, this bias is conservative relative to the hypotheses we tested.
Figure 4.
Maximum speed attained by Anna's hummingbirds flying in a wind tunnel under five experimental treatments. Each line represents one hummingbird. The dashed lines represent birds that could match the tunnel's top speed. (a) The hummingbirds exhibited significantly lower maximum speeds in the long-tail treatment than they did when unmanipulated (control 1), or with a sham manipulation (control 2; repeated-measures ANOVA, p<0.0001, n=11 birds). (b) Maximum speed showed no change in the no-rectrix treatment, relative to the control values (n=10 birds, repeated-measures ANOVA, p=0.7). (c) The no-tail treatment exhibited a significant decrease in maximum speed relative to the controls (n=15 birds, repeated-measures ANOVA, p=0.02). The control values have been averaged in (b,c) because both controls were measured prior to these experimental manipulations.
4. Discussion
These results provide two independent lines of evidence that elongated, ornamental tails of birds can increase the costs of flight. Adding a long tail to Anna's hummingbirds shifted the high-speed portion of the metabolic power curve upwards (figure 3a), as shown by the significant interaction between the long-tail treatment and v3, and lack of a significant interaction between the long-tail treatment and v−1. This upwards shift corresponds to an increase in the metabolic cost of flight at high speeds of approximately 11 per cent (figure 3a). Assuming that maximum airspeeds are limited physiologically by muscle power output, an 11 per cent increase in metabolic power requirements would predict a decrease in top speed of 3.6 per cent, assuming that flight power requirements rise as the cube of airspeed (Pennycuick 1969; Rayner 2001). Remarkably, our behavioural assay of maximum flight speed demonstrated an average decrease of 3.4 per cent following addition of long tail feathers (figure 4a).
Removing only the rectrices did not appear to have a significant impact on forward flight. In the metabolic experiments, this experimental manipulation (including the interaction terms) was not significantly different from the controls (figure 3b). Moreover, this treatment had no effect on maximum airspeed (figure 4b), suggesting that drag and stability during fast forward flight were not changed. By contrast, when all coverts and rectrices were removed, the hummingbirds exhibited a significant 2 per cent decrease in maximum speed relative to the control treatments (figure 4c), suggesting that the entire lack of a tail (including coverts) caused an increase in body drag, a change in trim, reduction in stability or other such adverse aerodynamic effects that might compromise performance. As argued previously, a decrease in maximum speed deriving from enhanced drag would also predict an increase in metabolic expenditure at high speeds, in this case by 6 per cent. Measured metabolic rates at high speeds were an average of 6.1 per cent higher at 12 m s−1 in the no-tail treatments relative to control treatments (figure 3c), although the no tail×v3 interaction was not statistically significant (p=0.101). Because some individuals that were missing their entire tails were unable to feed at airspeeds greater than 10 m s−1, this lack of significance may in part derive from reduced statistical power. We therefore interpret our data as consistent with the findings of Maybury & Rayner (2001), who found that the presence of a short tail can decrease drag on a bird's body.
During the measurement of flight metabolic rates, the birds were flying in the turbulent wake of the large and stationary mask, and airflow over the body was necessarily altered, relative to the free stream (see fig. 1 in the electronic supplementary material). The aerodynamic consequences of the mask on patterns of dorsal boundary-layer separation (Maybury & Rayner 2001), and other mechanisms by which tail presence might influence body drag and flight stability, are unknown. Nonetheless, metabolic comparisons among experimental treatments demonstrated differences relative to controls that were both qualitatively and quantitatively consistent with the independent results for maximum airspeeds during free flight (for which there was no mask influence). Also, any time lag between measured respiration rate and actual expenditure of mechanical power would bias metabolic samples towards free-flight rates as the birds approached the feeder. The mask diameter was comparable to the width of the bird's body, and aerodynamic interactions between mask and wings were likely to be negligible given the wing length of approximately 6 cm.
(a) Performance consequences of tail elongation
These experiments suggest that tail length generally exerts subtle effects on hummingbird flight performance. Time-budget analyses suggest that hummingbirds spend from 1 to 7 per cent of the day engaged in fast forward flight, compared with 7 to 15 per cent of the day either hovering or flying slowly (Pearson 1954; Stiles 1971; Wolf & Hainsworth 1971; Carpenter et al. 1983; Temeles et al. 2005). Because the metabolic costs of a long tail are most evident at the highest flight speeds, the changes demonstrated here would only influence a small fraction of a hummingbird's daily expenditure. These costs also appear minor when compared with other important aspects of avian life history, such as migration or moult. Moult elevates mass-specific hovering metabolic rates in hummingbirds by up to 37 per cent (Chai 1997), and preparing for migration increases daily energetic intake considerably above subsistence levels (compare Weathers & Stiles 1989; Carpenter et al. 1993). Most theoretical and behavioural studies of the role of the avian tail in flight have focused on aerial insectivores, such as swallows (Norberg 1994; Evans 1998; Matyjasiak et al. 1999, 2000, 2004; Buchanan & Evans 2000; Park et al. 2001; Rowe et al. 2001). These taxa may be particularly sensitive to small tail-associated changes in body drag, given their high flight speeds maintained over a substantial portion of the day.
In our experiments, we used the elongated tail feathers of the red-billed streamertail, which are among the longest tail feathers of any hummingbird, and which exhibit a relative length comparable to the extreme tails that have arisen in numerous other highly ornamented avian taxa (Winquist & Lemon 1994). More modestly elongated tail feathers should incur even lower costs than those reported here. Likewise, the intraspecific variation in the length of males' elongated tail feathers is typically far less than the extreme manipulation we performed here, often of the order of a few millimetres (Alatalo et al. 1988; Fitzpatrick 1997). These small differences are hypothesized to serve as honest (costly) signals, but our data suggest that differences of only a couple of millimetres would have virtually imperceptible effects on top flight speed or the metabolic power curve, and thus the cost differential would likewise be quite small. Whether this small difference could nonetheless be important to females is unclear.
Elongated tails in birds have evolved dozens if not hundreds of times; by comparison, very few taxa appear to have evolved elongated wing feathers (e.g. standard-winged bird of paradise, Semioptera wallaceii; argus pheasant, Argusianus argus; and members of the nightjar genus Macrodipteryx), perhaps because wing hypertrophy imposes greater and more varied mechanical costs during flight. Similarly, morphological diversity in dung beetle (Onthophagus spp.) horns, which are sexually selected weapons, favours production of structures that are relatively cost-free (Emlen 2001). Overall, the specific costs of elaborated tails are likely to be taxon- and context-specific. We propose, however, that sexual selection has generated enormous diversity in avian tail morphology because, by ‘hiding’ in the wake of the body, such modifications can be relatively cost-free.
Acknowledgements
This research was approved by the UC-Berkeley Animal Care and Use Committee, and was performed under requisite state and federal permits for hummingbird capture. Feathers from the red-billed streamertail were collected and exported under CITES permits from the National Environmental Protection Agency of Jamaica.
We thank Bo Yu, Lisa Nguyen and Ronald Yeh for their assistance with data collection; the UC-Berkeley Biomechanics group, Steve Beissinger, Don Powers and members of the Dudley Laboratory for discussion; anonymous reviewers for their valuable statistical advice and constructive comments; and the USFWS, CA Department of Fish and Game, and the National Environmental Protection Agency of Jamaica for permits. This paper is a portion of C.J.C.'s PhD.
Supplementary Material
A summary of PIV data collected that show the potential for ‘mask effects’ at airspeeds of 10 m s−1
References
- Alatalo R.V., Hoglund J., Lundberg A. Patterns of variation in tail ornament size in birds. Biol. J. Linn. Soc. 1988;34:363–374. doi:10.1111/j.1095-8312.1988.tb01969.x [Google Scholar]
- Andersson M. Female choice selects for extreme tail length in a widowbird. Nature. 1982;299:818–820. doi:10.1038/299818a0 [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Andersson S. Female preference for long tails in lekking Jackson's widowbirds: experimental evidence. Anim. Behav. 1992;43:379–388. doi:10.1016/S0003-3472(05)80098-3 [Google Scholar]
- Balmford A., Thomas A.L.R., Jones I.L. Aerodynamics and the evolution of long tails in birds. Nature. 1993;361:628–631. doi:10.1038/361628a0 [Google Scholar]
- Bartholomew G.A., Lighton J.R.B. Oxygen consumption during hover-feeding in free-ranging Anna hummingbirds Calypte anna. J. Exp. Biol. 1986;123:191–199. doi: 10.1242/jeb.123.1.191. [DOI] [PubMed] [Google Scholar]
- Berger, M. 1985 Sauerstoffverbrauch von Kolibris (Colibri coruscans und C. thalassinus) beim Horizontalflug. In BIONA report 3 (ed. W. Natchtigall). Stuttgart, Germany: G. Fischer.
- Buchanan K.L., Evans M.R. The effect of tail streamer length on aerodynamic performance in the barn swallow. Behav. Ecol. 2000;11:228–238. doi:10.1093/beheco/11.2.228 [Google Scholar]
- Carpenter F.L., Paton D.C., Hixon M.A. Weight gain and adjustment of feeding territory size in migrant hummingbirds. Proc. Natl Acad. Sci. USA. 1983;80:7259–7263. doi: 10.1073/pnas.80.23.7259. doi:10.1073/pnas.80.23.7259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter F.L., Hixon M.A., Beuchat C.A., Russell R.W., Paton D.C. Biphasic mass gain in migrant hummingbirds: body composition changes, torpor, and ecological significance. Ecology. 1993;74:1174–1182. doi:10.2307/1940487 [Google Scholar]
- Chai P. Hummingbird hovering energetics during moult of primary flight feathers. J. Exp. Biol. 1997;200:1527–1536. doi: 10.1242/jeb.200.10.1527. [DOI] [PubMed] [Google Scholar]
- Chai P., Dudley R. Limits to flight energetics of hummingbirds hovering in hypodense and hypoxic gas mixtures. J. Exp. Biol. 1996;199:2285–2295. doi: 10.1242/jeb.199.10.2285. [DOI] [PubMed] [Google Scholar]
- Chai P., Altshuler D.L., Stephens D.B., Dillon M.E. Maximal horizontal flight performance of hummingbirds: effects of body mass and molt. Physiol. Biochem. Zool. 1999;72:145–155. doi: 10.1086/316652. doi:10.1086/316652 [DOI] [PubMed] [Google Scholar]
- Clark C.J., Feo T.J. The Anna's hummingbird chirps with its tail: a new mechanism of sonation in birds. Proc. R. Soc. B. 2008;275:955–962. doi: 10.1098/rspb.2007.1619. doi:10.1098/rspb.2007.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. Princeton University Press; Princeton, NJ: 1871. The descent of man, and selection in relation to sex. [Google Scholar]
- Dial K.P., Biewener A.A., Tobalske B.W., Warrick D.R. Mechanical power output of bird flight. Nature. 1997;390:67–70. doi:10.1038/36330 [Google Scholar]
- Ellington C.P. Limitations on animal flight performance. J. Exp. Biol. 1991;160:71–91. [Google Scholar]
- Emlen D.J. Costs and the diversification of exaggerated animal structures. Science. 2001;291:1534–1536. doi: 10.1126/science.1056607. doi:10.1126/science.1056607 [DOI] [PubMed] [Google Scholar]
- Evans M.R. Selection on swallow tail streamers. Nature. 1998;394:233–234. doi:10.1038/28297 [Google Scholar]
- Evans M.R., Thomas A.L.R. The aerodynamic and mechanical effects of elongated tails in the scarlet-tufted malachite sunbird: measuring the cost of a handicap. Anim. Behav. 1992;43:337–347. doi:10.1016/S0003-3472(05)80229-5 [Google Scholar]
- Fitzpatrick S. Patterns of morphometric variation in birds' tails: length, shape and variability. Biol. J. Linn. Soc. 1997;62:145–162. doi:10.1111/j.1095-8312.1997.tb01619.x [Google Scholar]
- Matyjasiak P., Jablonski P.G., Olejniczak I., Boniecki P., Lee S.-D. Foraging cost of a long tail ornament: an experiment with sand martin females. Ethology. 1999;105:521–530. doi:10.1046/j.1439-0310.1999.00422.x [Google Scholar]
- Matyjasiak P., Jablonski P.G., Olejniczak I., Boniecki P. Imitating the initial evolutionary stage of a tail ornament. Evolution. 2000;54:704–711. doi: 10.1111/j.0014-3820.2000.tb00072.x. doi:10.1111/j.0014-3820.2000.tb00072.x [DOI] [PubMed] [Google Scholar]
- Matyjasiak P., Matyjasiak J., de Lope F., Møller A.P. Vane emargination of outer tail feathers improves flight manoeuvrability in streamerless hirundines, Hirundinidae. Proc. R. Soc. Lond. B. 2004;271:1831–1838. doi: 10.1098/rspb.2004.2812. doi:10.1098/rspb.2004.2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maybury W.J., Rayner J.M.V. The avian tail reduces body parasite drag by controlling flow separation and vortex shedding. Proc. R. Soc. Lond. B. 2001;268:1405–1410. doi: 10.1098/rspb.2001.1635. doi:10.1098/rspb.2001.1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg R.Å. Swallow tail streamer is a mechanical device for self-deflection of tail leading edge, enhancing aerodynamic efficiency and flight manoeuvrability. Proc. R. Soc. Lond. B. 1994;257:227–233. doi:10.1098/rspb.1994.0119 [Google Scholar]
- Norberg U.M. How a long tail and changes in mass and wing shape affect the cost for flight in animals. Funct. Ecol. 1995;9:48–54. doi:10.2307/2390089 [Google Scholar]
- Oufiero C.E., Garland T., Jr Evaluating performance costs of sexually selected traits. Funct. Ecol. 2007;21:676–689. doi:10.1111/j.1365-2435.2007.01259.x [Google Scholar]
- Park K.J., Evans M.R., Buchanan K.L. Assessing the aerodynamic effects of tail elongations in the house martin (Delichon urbica): implications for the initial selection pressures in hirundines. Behav. Ecol. Sociobiol. 2000;48:364–372. doi:10.1007/s002650000250 [Google Scholar]
- Park K.J., Buchanan K.L., Evans M.R. Sexy streamers? The role of natural and sexual selection in the evolution of hirundine tail streamers. Evolution. 2001;55:445–446. doi: 10.1111/j.0014-3820.2001.tb01307.x. doi:10.1111/j.0014-3820.2001.tb01307.x [DOI] [PubMed] [Google Scholar]
- Pearson O.P. The daily energy requirements of a wild Anna hummingbird. The Condor. 1954;56:317–322. doi:10.2307/1365017 [Google Scholar]
- Pennycuick C.J. The mechanics of bird migration. Ibis. 1969;111:525–556. doi:10.1111/j.1474-919X.1969.tb02566.x [Google Scholar]
- Petrie M., Halliday T., Sanders C. Peahens prefer peacocks with elaborate trains. Anim. Behav. 1991;41:323–331. doi:10.1016/S0003-3472(05)80484-1 [Google Scholar]
- Rayner J.M.V. Estimating power curves of flying vertebrates. J. Exp. Biol. 1999;202:3449–3461. doi: 10.1242/jeb.202.23.3449. [DOI] [PubMed] [Google Scholar]
- Rayner J.M.V. Mathematical modelling of the avian flight power curve. Math. Methods Appl. Sci. 2001;24:1485–1514. doi:10.1002/mma.196 [Google Scholar]
- Rowe L.V., Evans M.R., Buchanan K.L. The function and evolution of the tail streamer in hirundines. Behav. Ecol. 2001;12:157–163. doi:10.1093/beheco/12.2.157 [Google Scholar]
- Stiles F.G. Time, energy, and territoriality of the Anna Hummingbird (Calypte anna) Science. 1971;173:818–821. doi: 10.1126/science.173.3999.818. doi:10.1126/science.173.3999.818 [DOI] [PubMed] [Google Scholar]
- Temeles E.J., Goldman R.S., Kudla A.U. Foraging and territory economics of sexually dimorphic purple-throated caribs (Eulampis jugularis) on three Heliconia morphs. Auk. 2005;122:187–204. doi:10.1642/0004-8038(2005)122[0187:FATEOS]2.0.CO;2 [Google Scholar]
- Thomas A.L.R. On the aerodynamics of birds' tails. Phil. Trans. R. Soc. Lond. B. 1993;340:361–380. doi:10.1098/rstb.1993.0079 [Google Scholar]
- Weathers W.W., Stiles F.G. Energetics and water balance in free-living tropical hummingbirds. The Condor. 1989;91:324–331. doi:10.2307/1368310 [Google Scholar]
- Welch K.C., Jr, Altshuler D.L., Suarez R.K. Oxygen consumption rates in hovering hummingbirds reflect substrate-dependent differences in P/O ratios: carbohydrate as a ‘premium fuel’. J. Exp. Biol. 2007;210:2146–2153. doi: 10.1242/jeb.005389. doi:10.1242/jeb.005389 [DOI] [PubMed] [Google Scholar]
- Wells D.J. Muscle performance in hovering hummingbirds. J. Exp. Biol. 1993;178:39–57. [Google Scholar]
- Winquist T., Lemon R.E. Sexual selection and exaggerated male tail length in birds. Am. Nat. 1994;143:95–116. doi:10.1086/285597 [Google Scholar]
- Wolf L.L., Hainsworth F.R. Time and energy budgets of territorial hummingbirds. Ecology. 1971;52:980–988. doi:10.2307/1933803 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A summary of PIV data collected that show the potential for ‘mask effects’ at airspeeds of 10 m s−1