Abstract
Birds in which both sexes produce complex songs are thought to be more common in the tropics than in temperate areas, where typically only males sing. Yet the role of phylogeny in this apparent relationship between female song and latitude has never been examined. Here, we reconstruct evolutionary changes in female song and breeding latitude in the New World blackbirds (Icteridae), a family with both temperate and tropical representatives. We provide strong evidence that members of this group have moved repeatedly from tropical to temperate breeding ranges and, furthermore, that these range shifts were associated with losses of female song more often than expected by chance. This historical perspective suggests that male-biased song production in many temperate species is the result not of sexual selection for complex song in males but of selection against such songs in females. Our results provide new insights into the differences we see today between tropical and temperate songbirds, and suggest that the role of sexual selection in the evolution of bird song might not be as simple as we think.
Keywords: ancestral state reconstruction, comparative methods, duetting, female bird song, phylogeny, sexual selection
1. Introduction
Most research on the evolution of bird song has focused on north temperate passerines in which songs are produced almost exclusively by males (Marler & Slabbekoorn 2004; Catchpole & Slater 2008). Female song is rare in temperate species (Riebel 2003), and where it does occur it is produced less frequently than male song and often with less complexity (e.g. Beletsky 1982, 1983; Arcese et al. 1988). In the tropics, however, females of many species sing (Kroodsma et al. 1996; Morton 1996; Langmore 1998; Slater & Mann 2004), either coordinating their vocalizations with males to perform complicated duets (Farabaugh 1982; Hall 2004), or producing solo songs similar to those of males (Whittingham et al. 1992, 1997; Price et al. 2008). Female bird song may even be the norm rather than the exception in tropical environments (Morton 1996), yet we know surprisingly little about the origins of this latitudinal difference (Slater & Mann 2004). Furthermore, the apparent correlation between tropical breeding and female song has never been tested using methods that correct for phylogeny. Without controlling for phylogeny, one or a few species-rich tropical clades with female song could strongly bias our interpretations and create an apparent trend where none actually exists (Felsenstein 1985; Harvey & Pagel 1991).
Hypotheses to explain the greater prevalence of female song in the tropics have generally focused on factors selecting for tropical females to sing as males, such as mate attraction, shared territorial defence or other forms of behavioural coordination by long-term pairs (Langmore 1998, 2000; Hall 2004; Slater & Mann 2004). This focus may lead some researchers to assume that the ancestors of these birds resembled temperate species in lacking female song, but it is equally plausible that both sexes sang in ancestors and that female singing has been repeatedly lost during the evolution of temperate taxa (Riebel et al. 2005; Garamszegi et al. 2007). Indeed, such losses of female song seem especially probable in temperate species that have recent tropical ancestors (Kondo & Omland 2007; Barker et al. 2008). Rather than asking why females sing in the tropics (e.g. Slater & Mann 2004), it may be more appropriate in many cases to ask why females do not sing in temperate-breeding species. Yet, the idea that selection could favour losses of female song during vocal evolution has received comparatively little attention.
Here we reconstruct the evolution of female song and compare it to breeding latitude in the New World blackbirds (Icteridae), a songbird family with both temperate and tropical representatives (Jaramillo & Burke 1999). The behavioural and ecological traits of many members of this clade have been intensively studied (Bent 1958; Orians 1985; Robinson 1986; Skutch 1996; Jaramillo & Burke 1999; Searcy et al. 1999; Fraga 2008), and some are models for investigating the evolution of bird song by sexual selection (e.g. Agelaius phoeniceus: Searcy & Yasukawa 1995). Moreover, female song rates vary substantially across the family, from species in which both sexes regularly produce similar songs (e.g. Agelaius assimilis: Whittingham et al. 1992, 1997; Icterus pustulatus: Price et al. 2008) or interpose their notes to produce complicated duets (e.g. Dives dives: Orians 1983), to taxa in which female song is relatively rare or absent (e.g. Icterus galbula: Beletsky 1982; Sturnella neglecta: Lanyon 1994). Substantial differences in female song have even been reported between closely related sister taxa (A. phoeniceus and A. assimilis: Whittingham et al. 1992; Barker et al. 2008). The New World blackbirds therefore provide an ideal model clade for addressing questions about the evolution of female bird song, including changes that have occurred over relatively short time scales.
Our phylogenetic analysis focused on 65 tropical and temperate blackbird species with well documented vocal behaviours and well established molecular relationships. Relationships among these taxa were based on a larger phylogeny of the entire family Icteridae (Lanyon & Barker 2007), which uses DNA sequence data from multiple mitochondrial and nuclear loci to resolve relationships among the family's four previously defined subclades (Lanyon & Omland 1999): the oropendolas and caciques, the orioles, the grackles and allies, and the meadowlarks and allies. Our main objectives were to examine: (i) whether current differences among taxa in female song are the result of historical gains or losses, (ii) whether or not these changes were associated with changes in breeding latitude, and (iii) whether similar evolutionary patterns have occurred in each of the four major subclades. We hope that the historical patterns revealed in this study will provide a useful starting point for future investigations into the ecological and social pressures selecting for differences in female song.
2. Material and methods
(a) Scoring female song and breeding latitude
We gathered information on female singing behaviour from species accounts in identification guides and from published studies (listed in appendix A). We defined ‘songs’ as vocalizations used in defending resources or advertising for mates, following the definitions described by authors of species account descriptions (Howell & Webb 1995; Jaramillo & Burke 1999). Songs of most New World blackbird species are regularly given in association with a visual display, such as the ‘bow display’ of oropendolas and the ‘song spread’ of many other icterids (Jaramillo & Burke 1999), so it was presumably straightforward for previous authors to identify male and female vocalizations as songs rather than other types of sounds in these taxa. We did not include species in which song was not clearly described or in which singing differences between the sexes are not known (all excluded taxa were tropical or south temperate and were distributed across 15 genera). Altogether, we scored singing behaviour in 65 of the approximately 101 named species in the family Icteridae (Clements 2007).
Female song can differ from male song in a variety of ways, including rates of production and general acoustic features. In our analysis, we focused solely on the reported rates of female song relative to male song and ignored comparisons of song structure, since most descriptions did not include sound spectrograms and we could not quantify acoustic differences between songs based on verbal descriptions alone. It was also difficult to reliably differentiate between females that sing relatively infrequently and those that never sing, since infrequent female song in some species could have gone unnoticed by researchers (Garamszegi et al. 2007). Therefore, we combined these cases (i.e. species reported to have infrequent female song and those in which females are not known to sing) into one discrete character state in our analysis and scored each species as having either: (i) similar song rates in either sex or (ii) females that sing less often than males. Scoring female song rates as a binary character ignored much of the variation in female singing behaviour across species but this approach also allowed us to reconstruct changes in a way that was biased against identifying ancestors as having female song, since taxa were given this score only if females sing as often as males.
Species scored as having similar male–female song rates included taxa in which both sexes regularly produce solo songs or combine their songs into antiphonal duets. In at least one species (I. pustulatus), females sing more often than males during nest-building and following the breeding season (Price et al. 2008); we scored this taxon as having similar song rates in the sexes based on reports that males may sing more often during other parts of the year (Jaramillo & Burke 1999). When only male song was described in detail, we assumed that female song was either rare or absent and scored these species as ‘females sing less often than males’. An independent observer unfamiliar with the taxa and our hypotheses also scored female song rates based on our compiled literature descriptions to verify that our scoring was accurate.
We determined breeding ranges using range maps in Jaramillo & Burke (1999) and scored each species as having either a (i) tropical, (ii) north temperate or (iii) south temperate breeding ranges. A species was scored as tropical if approximately 80 per cent or more of its breeding range fell between 23.5° latitude north and south and was scored as north or south temperate if at least 80 per cent of its breeding range fell north or south of these latitudes, respectively. If two zones each contained more than 20 per cent of a breeding range's area (north temperate and tropical: five species; south temperate and tropical: six species; see appendix A), we scored the species based on the location in which most previous studies or observations of singing behaviour had been conducted.
(b) Ancestral state reconstructions and comparative analyses
We determined relationships among our 65 taxa using a molecular phylogeny for the entire family Icteridae (Lanyon & Barker 2007). This phylogeny is based on DNA sequence data from two mitochondrial gene regions (cytochrome b and ND2) and four nuclear regions (RAG1, beta fibrinogen intron 5, aconitase 1 intron 10 and myoglobin intron 2). Mitochondrial data were obtained for all taxa, whereas nuclear data were obtained from selected divergent taxa (i.e. at least one sample from every genus and multiple samples from within genera with mitochondrial DNA divergences exceeding 7%).
We reconstructed ancestral character states for female song and breeding latitude on the rooted phylogeny using unordered parsimony in MacClade v. 4.06 (Maddison & Maddison 2003) and using maximum-likelihood methods in Mesquite v. 2.5 (Maddison & Maddison 2008). Our maximum-likelihood analyses used the Markov k-state one-parameter model, which assumes equal rates of change between character states. Maximum likelihood has advantages over parsimony in that it uses information about amounts of genetic divergence between taxa (i.e. branch lengths) and indicates degree of uncertainty in possible ancestral states. In all of these reconstructions, we refer to decreases in the occurrence of female song relating to male song as ‘losses’ and increases in female song as ‘gains’.
We tested for an association between changes in female song and breeding latitude using the concentrated changes test in MacClade (Maddison 1990). This parsimony-based approach requires each character under comparison to have two states, so we converted breeding latitude from a multi-state to a binary character by combining north temperate and south temperate ranges into one character state. We felt justified in combining these states because only 1 out of the 18 changes in our multi-state parsimony reconstruction of breeding range was between north and south temperate ranges (see below). We used breeding latitude as our independent character in this analysis to test the hypothesis that losses of female song have been concentrated in temperate lineages. When a character had more than one most parsimonious reconstruction, we performed multiple tests using either DELTRAN (that favours convergent changes in character states over reversals) or ACCTRAN (that favours reversals over convergent changes) assumptions for each character. Each test included 10 000 simulations.
We further examined the relationship between changes in female song and breeding latitude using Pagel's (1994) discrete likelihood correlation method, as implemented in Mesquite (Maddison & Maddison 2008). This likelihood-based method tests for an association between discrete character state changes in two binary characters by comparing a dependent model of correlated trait evolution to an independent model of uncorrelated character state changes. We calculated the likelihood of each model and relative transition rate parameters between each possible pair of character states using 10 000 simulation replicates.
3. Results
(a) Reconstructing ancestral states
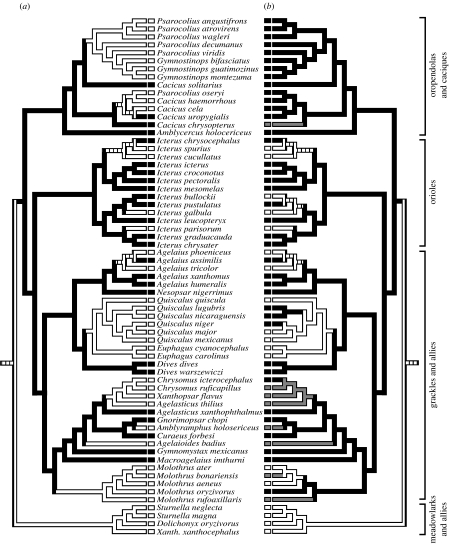
Parsimony reconstructions of female song evolution (figure 1a) suggest that female song rates have decreased repeatedly in comparison to male song in the New World blackbirds. Sixteen most parsimonious reconstructions were possible on the tree, and all showed multiple independent losses of female song (14 losses and no gains in DELTRAN reconstruction; 11 losses and three gains in ACCTRAN reconstruction). All reconstructions indicate that males and females had similar song rates in the ancestors of the oropendolas and caciques, the orioles and the grackles and allies, and that females sang less than males in the ancestor of the meadowlarks and allies.
Figure 1.
Ancestral state reconstructions of (a) female song rates and (b) breeding range latitudes calculated using unordered parsimony on a molecular phylogeny of the New World blackbird family. Female song rates were scored as a binary character relative to male song rates and breeding ranges were assessed relative to 23.5° latitude north and south. Four major subclades within the family are indicated on the right. These reconstructions indicate that female song rates have decreased repeatedly with movements from tropical to temperate breeding ranges. (a) Black bars, similar song rates in both sexes; white bars, females sing less than males; striped bars, equivocal. (b) Black bars, tropical breeding; white bars, north temperate breeding; grey bars, south temperate breeding; striped bars, equivocal.
Parsimony reconstructions of breeding latitude (figure 1b) showed that taxa have moved from tropical to temperate breeding areas repeatedly, much more often than the reverse. All 16 possible reconstructions agree that ancestors of the oropendolas and caciques, orioles and grackles and allies had tropical breeding ranges, and that the ancestor of the meadowlarks and allies was north temperate. More movements have occurred to and from north temperate areas (11) than south temperate areas (6), using either DELTRAN or ACCTRAN assumptions, which makes sense given the greater land area in the north. We reconstructed only one direct exchange between north and south temperate ranges, and this may be an artefact of scoring a species that is widely distributed in the tropics (Molothrus bonariensis) as a south temperate breeder because most observations of its vocal behaviour have been in the south temperate zone (Jaramillo & Burke 1999; Lowther & Post 1999). Neither breeding range nor female song frequency was resolved for the ancestor of the family.
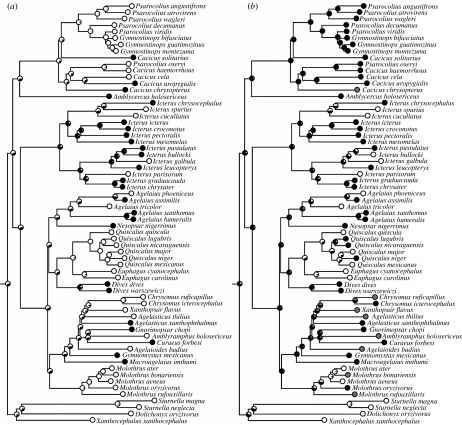
Maximum-likelihood reconstructions of female song (figure 2a) provided mixed support for the patterns suggested by parsimony reconstructions. Likelihood values agreed with parsimony in strongly indicating that males and females had similar song rates in the ancestor of the orioles (likelihood=0.80) and that females sang less than males in the ancestor of the meadowlarks and allies (0.70). However, unlike in parsimony reconstructions, likelihood values for the ancestors of the oropendolas and caciques, and the grackles and allies provided little support for the idea that males and females in these taxa had similar song rates (0.36 and 0.34, respectively). The only changes in female song that were clearly supported by both reconstruction methods were in the oriole clade, in which losses have occurred at least three times independently.
Figure 2.
Maximum-likelihood reconstructions of (a) female song rates and (b) breeding range latitudes, assuming equal rates of change between characters states (Markov k-state one parameter model). Black and white fill in circles indicate the proportional likelihoods of ancestral states. (a) Black dots, similar song rates in both sexes; white dots, females sing less than males. (b) Black dots, tropical breeding; white dots, north temperate breeding; grey dots, south temperate breeding.
By contrast, maximum-likelihood reconstructions of breeding latitude (figure 2b) largely agreed with parsimony reconstructions by indicating tropical breeding ranges for ancestors of the oropendolas and caciques (likelihood=0.91), orioles (0.93) and grackles and allies (0.91). Tropical breeding was also suggested for the ancestor of the meadowlarks and allies (likelihood=0.60) and the ancestor of the Icteridae (0.78). Thus, both reconstruction methods showed repeated historical movements by icterids from tropical to temperate breeding ranges.
(b) Correlated evolution between female song and breeding latitude
Concentrated change tests using all possible combinations of DELTRAN and ACCTRAN assumptions supported a strong and significant association between temperate breeding and decreases in the occurrence of female song, with all tests producing p-values of less than 0.01 (DELTRAN song versus DELTRAN latitude: p=0.0006; ACCTRAN song versus DELTRAN latitude: p=0.0091; DELTRAN song versus ACCTRAN latitude: p=0.0005; ACCTRAN song versus ACCTRAN latitude: p=0.0001). Of the 11 or more independent losses of female song shown in parsimony reconstructions, at least seven have occurred on branches of the tree in which temperate breeding is the reconstructed state (figure 1). Furthermore, these losses have occurred in both north temperate and south temperate taxa. Only two temperate-breeding icterid species are reported to have frequent female song: Icterus bullockii in the north (Miller 1931; Rising & Williams 1999) and Cacicus chrysopterus in the south (Jaramillo & Burke 1999).
A relationship between changes in female song and breeding latitude was also supported using Pagel's (1994) test for correlated evolution. Comparisons of maximum-likelihood models indicated that the dependent model of correlated trait evolution (log likelihood=−67.9) was significantly more likely than the independent model (log likelihood=−76.2), with a likelihood ratio of 8.3 (p<0.05). Estimated transition rates (q) between each combination of character states in the dependent model showed that losses of female song were far more likely to occur in temperate breeders (q31=319.1) than tropical breeders (q42=0.00), while gains in the occurrence of female song were relatively unlikely in all lineages (q13=0.00, q24=0.13).
Notable exceptions to the association between female song and latitude can be seen in the oropendolas and caciques, in which female song has changed multiple times among tropical taxa, and in the cowbird clade (genus Molothrus), in which parsimony suggests that female song was lost in a tropical ancestor (figure 1). Likewise, where reversals from temperate to tropical breeding ranges have occurred in this family, as appears to have happened in Chrysomus icterocephalus and twice in the genus Quiscalus, these movements have not been accompanied by apparent changes in female song.
4. Discussion
The view that female song is more common in tropical than in temperate songbirds has been widely held among researchers (Kroodsma et al. 1996; Morton 1996; Langmore 1998; Slater & Mann 2004). To our knowledge, however, our study is the first to test this association using a phylogenetic approach. By controlling for phylogeny, we show that differences in female song between tropical and temperate New World blackbird species are not explained by just a few species-rich clades (Felsenstein 1985) but rather are the result of repeated, correlated historical changes in female song and breeding latitude. Frequent singing by females is significantly associated with tropical breeding in this family; a relationship supported using multiple phylogenetic comparative methods. Females of temperate breeding species almost invariably sing less often than males, if at all, and evolutionary reconstructions show that most of these temperate species had tropical ancestors. Our results therefore suggest that female song has been lost repeatedly in this group in association with repeated evolutionary movements from tropical to temperate breeding areas, and further that these changes have occurred in both the northern and southern hemispheres.
The relationship between losses of female song and increases in latitude is particularly striking in the orioles, in which both parsimony and maximum-likelihood reconstructions clearly indicate that ancestral taxa were tropical with similar song rates in males and females (figures 1 and 2). Losses of female song have occurred at least three separate times in this clade, and each case was associated with a change from tropical to temperate breeding. Previous reconstructions of migratory behaviours in orioles (Kondo & Omland 2007) show that migration evolved rapidly and relatively recently in these same species (Icterus spurius, Icterus cucullatus, I. galbula and Icterus parisorum). Kondo & Omland (2007) sampled more oriole taxa in their study than we include here (45 taxa from 25 species, rather than the 14 species here), and if losses of female song accompanied the evolution of migration to temperate areas, as these studies together suggest, such losses have occurred four separate times in the orioles and over relatively short time periods. These same lineages have lost elaborate female coloration (Hofmann et al. 2008) that may have also happened in parallel with the evolution of temperate breeding and migration.
Further evidence for rapid evolutionary changes in female song is provided by comparing A. phoeniceus, the red-winged blackbird of North America and its closest relative A. assimilis, the red-shouldered blackbird of Cuba. These sister taxa are very close genetically and in fact have been considered conspecific in the past (Barker et al. 2008), yet they differ in a variety of morphological and behavioural characteristics (Whittingham et al. 1992). Females of A. assimilis are nearly indistinguishable from conspecific males in song structure and song rate and are also similar in plumage and body size (Whittingham et al. 1992, 1996, 1997; Jaramillo & Burke 1999), whereas females of A. phoeniceus differ considerably from conspecific males in these traits (Beletsky 1983; Searcy & Yasukawa 1995; Yasukawa & Searcy 1995). Whether these male-like female characteristics were lost in A. phoeniceus or gained in A. assimilis was not resolved in our reconstructions of song (figures 1a and 2a) or in similar reconstructions of other traits in these birds (Barker et al. 2008). However, regardless of whether these differences resulted from losses or gains, it is clear that the changes in female song and plumage must have occurred quite rapidly.
Moreover, it is also clear from our study that the majority of recent evolutionary changes in these taxa and in many other icterids have occurred in females rather than males. In the Agelaius genus, for instance, females differ substantially between species in both their vocal behaviours and plumage colours, some being nearly indistinguishable from males (A. assimilis, Agelaius xanthomus and Agelaius humeralis) and others being strikingly different (A. phoeniceus and Agelaius tricolor), while all males produce similar buzzy songs and have black plumage with brightly coloured epaulets (Whittingham et al. 1992, 1996; Beedy & Hamilton 1999; Jaramillo & Burke 1999; Barker et al. 2008). A comparable pattern is seen in the orioles, in which differences between the sexes in song and plumage have also resulted from historical changes in females rather than males (figures 1 and 2; Hofmann et al. 2008; also see Irwin 1994). Our findings should therefore caution future studies of sexual selection against focusing too much on male traits. For example, the red-winged blackbird has long served as a model for investigating the function and evolution of male songs and colours (Searcy & Yasukawa 1995; Catchpole & Slater 2008). Yet it is likely that both traits have changed relatively little in males during the evolution of this species and that most differences between the sexes have resulted from selection on females.
Our evidence that singing by both sexes was the ancestral state for many temperate New World blackbirds is consistent with a recent phylogenetic study by Garamszegi et al. (2007), showing that female song was present in the ancestors of many European songbirds that now lack female song. That study did not, however, investigate ancestral breeding ranges (Garamszegi et al. 2007). Some authors have further suggested that song in both sexes was the ancestral state for all oscine passerines (Riebel et al. 2005), based on evidence for an Australasian origin of songbirds (Barker et al. 2002) combined with the prevalence of female song among Australian species today. These possibilities present interesting challenges for future researchers to identify the factors favouring loss of female song, and they further emphasize the importance of understanding, whether male-specific song in a species is primarily due to selection for complex song in males or selection against such songs in females.
The disagreement between parsimony and maximum-likelihood reconstructions in our study underscores the importance of not relying on any single method in ancestral reconstructions (Cunningham et al. 1998). Both methods supported similar patterns of character change in breeding latitude across the family and in female song among the orioles, but they largely disagreed about ancestral changes in the oropendolas and caciques and in the grackles and allies. Maximum-likelihood uses information about phylogenetic branch lengths while parsimony does not, and this difference presumably explains much of the disagreement in these results, since both clades include relatively short internodes in their early histories (figure 2). Using parsimony alone therefore could have provided potentially misleading conclusions about many of these ancestral states, especially because parsimony reconstructions imply no uncertainty about the states of these nodes. But maximum-likelihood reconstructions alone might also be misleading, since they suggest both low female song rates and tropical breeding ranges in many deep ancestral nodes, which contradict the strong overall pattern of high rates of female song in tropical breeders today. Both the concentrated changes test and Pagel's (1994) test for correlated trait evolution supported a clear relationship between female song rates and breeding latitude, showing that such comparative methods can work well even when ancestral reconstruction methods fail to agree. Indeed, large numbers of evolutionary changes on a tree, such as those characterizing female song rates and breeding latitudes in the Icteridae, can provide more power for statistical comparative methods while at the same time reducing our confidence in ancestral states (Oakley & Cunningham 2000).
Although we found a strong general relationship between female song rates and breeding latitude across the Icteridae, the factors favouring each loss or gain in female song were not always clear and indeed may have differed among lineages. For example, unlike the many taxa that appear to have lost female song with the evolution of temperate migration, both the oropendola and cacique clade and the cowbird (Molothrus) clade present cases in which female song was apparently lost in tropical resident taxa (figure 1). In the oropendolas and caciques, species that lack female song are also known to be polygynous and nest colonially (Feekes 1981, 1982; Trainer 1989; Webster 1994; Price & Lanyon 2004; Fraga & Kreft 2007), while taxa with frequent female song appear to form year-round territorial pairs (Robinson 1986; Skutch 1996; Jaramillo & Burke 1999). The cowbirds, on the other hand, are interspecific brood parasites, a trait that presumably evolved in the ancestor of the genus (Jaramillo & Burke 1999; Searcy et al. 1999). Thus, in both the oropendolas and caciques and the cowbirds, female song appears to have been lost with changes in breeding behaviours, possibly from monogamous ancestors in which both sexes shared territorial duties and parental investment. These exceptions to the relationship between female song rates and breeding latitude suggest that it is not latitude per se influencing changes in female song, but rather other aspects of life history that tend to be associated with temperate or tropical habitats (Slater & Mann 2004). Future, more detailed work on this family should include additional characters such as mating system, territorial behaviours and migration to investigate the strength of any associations with female song. Studies of individual species will also be needed to better understand the selective mechanisms behind the evolutionary changes revealed here.
Acknowledgments
We thank Elizabeth Price, Dustin Reichard, three anonymous reviewers and especially Emily Cramer for their helpful comments on the paper. The University of Michigan Biological Station provided facilities for J.J.P. while writing the manuscript. K.E.O. was supported by a National Science Foundation grant DEB-0347083 and J.J.P. was supported by a Research Opportunity Award supplement to that grant.
Appendix A
| species | female song | latitudea | references |
|---|---|---|---|
| Agelaioides badius | infrequent | S temperateb | Hauber et al. (1999) and Jaramillo & Burke (1999) |
| Agelaius assimilis | duet with male | tropical | Whittingham et al. (1992, 1997) and Jaramillo & Burke (1999) |
| Agelaius humeralis | duet with male | tropical | Whittingham et al. (1996) and Jaramillo & Burke (1999) |
| Agelaius phoeniceus | infrequent | N temperate | Yasukawa & Searcy (1995) and Jaramillo & Burke (1999) |
| Agelaius tricolor | infrequent | N temperate | Beedy & Hamilton (1999) and Jaramillo & Burke (1999) |
| Agelaius xanthomus | similar to male | tropical | Jaramillo & Burke (1999) |
| Agelasticus thilius | rare or absent | S temperate | Jaramillo & Burke (1999) |
| Agelasticus xanthophthalmus | similar to male | tropical | Orians & Orians (2000) |
| Amblycercus holocericeus | duet with male | tropical | Ridgely & Tudor (1989), Howell & Webb (1995) and Jaramillo & Burke (1999) |
| Amblyramphus holosericeus | infrequent | S temperateb | Jaramillo & Burke (1999) |
| Cacicus cela | infrequent | tropical | Feekes (1981, 1982), Trainer (1989) and Jaramillo & Burke (1999) |
| Cacicus chrysopterus | duet with male | S temperateb | Stiles & Skutch (1989), Skutch (1996) and Jaramillo & Burke (1999) |
| Cacicus haemorrhous | rare or absent | tropical | Feekes (1981) and Jaramillo & Burke (1999) |
| Cacicus solitarius | duet with male | tropical | Ridgely & Tudor (1989) and Jaramillo & Burke (1999) |
| Cacicus uropygialis | duet with male | tropical | Stiles & Skutch (1989), Skutch (1996) and Jaramillo & Burke (1999) |
| Chrysomus icterocephalus | rare or absent | tropical | Wiley & Wiley (1980) and Jaramillo & Burke (1999) |
| Chrysomus ruficapillus | rare or absent | S temperateb | Jaramillo & Burke (1999) |
| Curaeus forbesi | similar to male | tropical | Skutch (1996) and Jaramillo & Burke (1999) |
| Dives dives | duet with male | tropical | Orians (1983), Stiles & Skutch (1989) and Jaramillo & Burke (1999) |
| Dives warszewiczi | duet with male | tropical | Orians (1983) and Jaramillo & Burke (1999) |
| Dolichonyx oryzivorus | rare or absent | N temperate | Martin & Gavin (1995) and Jaramillo & Burke (1999) |
| Euphagus carolinus | infrequent | N temperate | Avery (1995) and Jaramillo & Burke (1999) |
| Euphagus cyanocephalus | infrequent | N temperate | Jaramillo & Burke (1999) and Martin (2002) |
| Gnorimopsar chopi | similar to male | tropicalb | Jaramillo & Burke (1999) |
| Gymnomystax mexicanus | similar to male | tropical | Skutch (1996) and Jaramillo & Burke (1999) |
| Gymnostinops bifasciatus | rare or absent | tropical | Jaramillo & Burke (1999) and Fraga & Kreft (2007) |
| Gymnostinops guatimozinus | rare or absent | tropical | Jaramillo & Burke (1999) |
| Gymnostinops montezuma | rare or absent | tropical | Jaramillo & Burke (1999) and Price et al. (2006) |
| Icterus bullockii | similar to male | N temperate | Miller (1931) and Rising & Williams (1999) |
| Icterus chrysater | similar to male | tropical | Howell (1972) and Jaramillo & Burke (1999) |
| Icterus chrysocephalus | duet with male | tropical | Jaramillo & Burke (1999) |
| Icterus croconotus | duet with male | tropical | Jaramillo & Burke (1999) |
| Icterus cucullatus | infrequent | N temperate | Pleasants & Albano (2001) |
| Icterus galbula | infrequent | N temperate | Beletsky (1982), Rising & Flood (1998) and Jaramillo & Burke (1999) |
| Icterus graduacauda | similar to male | tropicalb | Flood (1990), Jaramillo & Burke (1999) and Flood et al. (2002) |
| Icterus icterus | similar to male | tropical | Skutch (1996) and Jaramillo & Burke (1999) |
| Icterus leucopteryx | similar to male | tropical | Scott (1893) and Jaramillo & Burke (1999) |
| Icterus mesomelas | duet with male | tropical | Stiles & Skutch (1989) and Jaramillo & Burke (1999) |
| Icterus parisorum | infrequent | N temperate | Scott (1885), Bent (1958) and Jaramillo & Burke (1999) |
| Icterus pectoralis | similar to male | tropical | Stiles & Skutch (1989), Skutch (1996) and Jaramillo & Burke (1999) |
| Icterus pustulatus | similar to male | tropicalb | Jaramillo & Burke (1999) and Price et al. (2008) |
| Icterus spurius | infrequent | N temperate | Bent (1958), Scharf & Kren (1996) and Jaramillo & Burke (1999) |
| Macroagelaius imthurni | similar to male | tropical | Jaramillo & Burke (1999) |
| Molothrus aeneus | rare or absent | N temperateb | Lowther (1995), Skutch (1996), Jaramillo & Burke (1999) and Warren (2002) |
| Molothrus ater | rare or absent | N temperate | Lowther (1993) and Jaramillo & Burke (1999) |
| Molothrus bonariensis | rare or absent | S temperateb | Skutch (1996), Hauber et al. (1999), Jaramillo & Burke (1999) and Lowther & Post (1999) |
| Molothrus oryzivorus | rare or absent | tropical | Stiles & Skutch (1989), Skutch (1996) and Jaramillo & Burke (1999) |
| Molothrus rufoaxillaris | rare or absent | S temperate | Ridgely & Tudor (1989), Skutch (1996), Hauber et al. (1999) and Jaramillo & Burke (1999) |
| Nesopsar nigerrimus | similar to male | tropical | Wiley & Cruz (1980), Skutch (1996) and Jaramillo & Burke (1999) |
| Psarocolius angustifrons | rare or absent | tropical | Jaramillo & Burke (1999) and Fraga & Kreft (2007) |
| Psarocolius atrovirens | rare or absent | tropical | Jaramillo & Burke (1999) |
| Psarocolius decumanus | rare or absent | tropical | Jaramillo & Burke (1999) |
| Psarocolius oseryi | rare or absent | tropical | Ridgely & Tudor (1989) and Jaramillo & Burke (1999) |
| Psarocolius viridis | rare or absent | tropical | Jaramillo & Burke (1999) |
| Psarocolius wagleri | rare or absent | tropical | Jaramillo & Burke (1999) |
| Quiscalus lugubris | rare or absent | tropical | Ridgely & Tudor (1989) |
| Quiscalus major | infrequent | N temperate | Post et al. (1996) |
| Quiscalus mexicanus | rare or absent | N temperateb | Skutch (1996), Jaramillo & Burke (1999) and Johnson & Peer (2001) |
| Quiscalus nicaraguensis | infrequent | tropical | Jaramillo & Burke (1999) |
| Quiscalus niger | infrequent | tropical | Jaramillo & Burke (1999) |
| Quiscalus quiscula | infrequent | N temperate | Wiley (1976) and Jaramillo & Burke (1999) |
| Sturnella magna | infrequent | N temperateb | Lanyon (1995) and Jaramillo & Burke (1999) |
| Sturnella neglecta | rare or absent | N temperate | Lanyon (1994) and Jaramillo & Burke (1999) |
| Xanthocephalus xanthocephalus | infrequent | N temperate | Twedt & Crawford (1995) and Jaramillo & Burke (1999) |
| Xanthopsar flavus | infrequent | S temperate | Jaramillo & Burke (1999) and Fraga (2005) |
Scored based on whether more or less than 80 per cent of breeding range is above or below 23.5° latitude north (N) or south (S), based on range maps in Jaramillo & Burke (1999).
Species in which less than 80 per cent of breeding range is included in either the tropical or a temperate zone; breeding ranges in these taxa were classified based on the locations of published observations of singing behaviour.
References
- Arcese P., Stoddard P.K., Hiebert S.M. The form and function of song in female song sparrows. Condor. 1988;90:44–50. doi:10.2307/1368431 [Google Scholar]
- Avery, M. L. 1995 Rusty blackbird (Euphagus carolinus). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Barker F.K., Barrowclough G.F., Groth J.G. A phylogenetic hypothesis for passerine birds: taxonomic and biogeographic implications of an analysis of nuclear DNA sequence data. Proc. R. Soc. B. 2002;269:295–308. doi: 10.1098/rspb.2001.1883. doi:10.1098/rspb.2001.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker F.K., Vandergon A.J., Lanyon S.M. Species status of the red-shouldered blackbird (Agelaius assimilis): implications for ecological, morphological, and behavioral evolution in Agelaius. Auk. 2008;125:87–94. doi:10.1525/auk.2008.125.1.87 [Google Scholar]
- Beedy, E. C. & Hamilton III, W. J. 1999 Tricolored blackbird (Agelaius tricolor) In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Beletsky L.D. Vocalizations of female northern orioles. Condor. 1982;84:445–447. doi:10.2307/1367454 [Google Scholar]
- Beletsky L.D. Aggressive and pair bond maintenance songs of female red-winged blackbirds (Agelaius phoeniceus) Z. Tierpsychol. 1983;62:47–54. [Google Scholar]
- Bent A.C. Dover; New York, NY: 1958. Life histories of North American blackbirds, orioles, tanagers, and allies. [Google Scholar]
- Catchpole C.K., Slater P.J.B. 2nd edn. Cambridge University Press; New York, NY: 2008. Bird song, biological themes and variations. [Google Scholar]
- Clements J.F. 6th edn. Cornell University Press; Ithaca, NY: 2007. The Clements checklist of birds of the world. [Google Scholar]
- Cunningham C.W., Omland K.E., Oakley T.H. Reconstructing ancestral character states: a critical reappraisal. Trends Ecol. Evol. 1998;13:361–366. doi: 10.1016/s0169-5347(98)01382-2. doi:10.1016/S0169-5347(98)01382-2 [DOI] [PubMed] [Google Scholar]
- Farabaugh, S. M. 1982 The ecological and social significance of duetting In Acoustic communication in birds. Song learning and its consequences (eds D. E. Kroodsma & E. H. Miller), pp. 85–124. New York, NY: Academic Press.
- Feekes F. Biology and colonial organization of two sympatric caciques, Cacicus c. cela and Cacicus h. hemorrhous (Icteridae, Aves) in Suriname. Ardea. 1981;69:83–107. [Google Scholar]
- Feekes F. Song mimesis within colonies of Cacicus c. cela (Icteridae, Aves): a colonial password? Z. Tierpsychol. 1982;58:119–152. [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. doi:10.1086/284325 [DOI] [PubMed] [Google Scholar]
- Flood N.J. Aspects of the breeding biology of Audubon's oriole. J. Field Ornithol. 1990;61:290–302. [Google Scholar]
- Flood, N. J., Rising, J. D. & Brush, T. 2002 Audubon's oriole (Icterus graduacauda). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Fraga R.M. Ecology, behavior and social organization of saffron-cowled blackbirds (Xanthopsar flavus) Ornitol. Neotrop. 2005;16:15–29. [Google Scholar]
- Fraga R.M. Phylogeny and behavioral evolution in the family Icteridae. Ornitol. Neotrop. 2008;19:61–71. [Google Scholar]
- Fraga R.M., Kreft S. Natural history and breeding behavior of the olive (Psarocolius yuracares) and yellow-billed (P. angustifrons alfredi) oropendolas in Chapare Province, Bolivia. Ornitol. Neotrop. 2007;18:251–261. [Google Scholar]
- Garamszegi L.Z., Pavlova D.Z., Eens M., Moller A.P. The evolution of song in female birds in Europe. Behav. Ecol. 2007;18:86–96. doi:10.1093/beheco/arl047 [Google Scholar]
- Hall M.L. A review of hypotheses for the functions of avian duetting. Behav. Ecol. Sociobiol. 2004;55:415–430. doi:10.1007/s00265-003-0741-x [Google Scholar]
- Harvey P.H., Pagel M.D. Oxford University Press; Oxford, UK: 1991. The comparative method in evolutionary biology. [Google Scholar]
- Hauber M.E., Clayton N.S., Kacelnik A., Reboreda J.C., DeVoogd T.J. Sexual dimorphism and species differences in HVC volumes of cowbirds. Behav. Neurosci. 1999;113:1095–1099. doi: 10.1037//0735-7044.113.5.1095. doi:10.1037/0735-7044.113.5.1095 [DOI] [PubMed] [Google Scholar]
- Hofmann C.M., Cronin T.W., Omland K.E. Evolution of sexual dichromatism. 1. Convergent losses of elaborate female coloration in New World orioles (Icterus spp.) Auk. 2008;125:778–789. doi:10.1525/auk.2008.07112 [Google Scholar]
- Howell T.R. Birds of the lowland pine savanna of northeastern Nicaragua. Condor. 1972;74:316–340. doi:10.2307/1366592 [Google Scholar]
- Howell S.N.G., Webb S. Oxford University Press; Oxford, UK: 1995. A guide to the birds of Mexico and northern Central America. [Google Scholar]
- Irwin R.E. The evolution of plumage dichromatism in the New World blackbirds: social selection on female brightness? Am. Nat. 1994;144:890–907. doi:10.1086/285717 [Google Scholar]
- Jaramillo A., Burke P. Princeton University Press; Princeton, NJ: 1999. New World blackbirds, the icterids. [Google Scholar]
- Johnson, K. & Peer, B. D. 2001 Great-tailed grackle (Quiscalus mexicanus). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Kondo B., Omland K.E. Ancestral state reconstruction of migration: multistate analysis reveals rapid changes in New World orioles (Icterus spp.) Auk. 2007;124:410–419. doi:10.1642/0004-8038(2007)124[410:ASROMM]2.0.CO;2 [Google Scholar]
- Kroodsma D.E., Viellard J.M.E., Stiles F.G. Study of bird song in the Neotropics: urgency and opportunity. In: Kroodsma D.E., Miller E.H., editors. Ecology and evolution of acoustic communication in birds. Cornell University Press; Ithaca, NY: 1996. pp. 269–281. [Google Scholar]
- Langmore N.E. Functions of duet and solo songs of female birds. Trends Ecol. Evol. 1998;13:136–140. doi: 10.1016/s0169-5347(97)01241-x. doi:10.1016/S0169-5347(97)01241-X [DOI] [PubMed] [Google Scholar]
- Langmore N.E. Why female birds sing. In: Epmark Y., Amundsen T., Rosenqvist G., editors. Animal signals, signaling and signal design in animal communication. Tapir Academic Press; Trondheim, Norway: 2000. pp. 317–327. [Google Scholar]
- Lanyon, W. E. 1994 Western meadowlark (Sturnella neglecta) In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Lanyon, W.E. 1995 Eastern meadowlark (Sturnella magna). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Lanyon, S. M. & Barker, F. K. 2007 Exploring patterns of morphological evolution in the New World blackbirds. In 125th Meeting of the American Ornithologists' Union Laramie, WY.
- Lanyon S.M., Omland K.E. A molecular phylogeny of the blackbirds (Icteridae): five lineages revealed by cytochrome-b sequence data. Auk. 1999;116:629–639. [Google Scholar]
- Lowther, P. E. 1993 Brown-headed cowbird (Molothrus ater). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Lowther, P. E. 1995 Bronzed cowbird (Molothrus aeneus). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Lowther, P. E. & Post, W. 1999 Shiny cowbird (Molothrus bonariensis). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Maddison W.P. A method for testing the correlated evolution of two binary characters: are gains or losses concentrated on certain branches of a phylogenetic tree? Evolution. 1990;44:539–557. doi: 10.1111/j.1558-5646.1990.tb05937.x. doi:10.2307/2409434 [DOI] [PubMed] [Google Scholar]
- Maddison D.R., Maddison W.P. Sinauer Associates, Inc; Sunderland, MA: 2003. MacClade 4. [Google Scholar]
- Maddison, D. R. & Maddison, W. P. 2008 Mesquite: a modular system for evolutionary analysis, v. 2.5: http://mesquiteproject.org
- Marler P., Slabbekoorn H. Elsevier Academic Press; San Diego, CA: 2004. Nature's music, the science of birdsong. [Google Scholar]
- Martin, S. G. 2002 Brewer's blackbird (Euphagus cyanocephalus). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Martin, S. G. & Gavin, T. A. 1995 Bobolink (Dolichonyx oryzivorus). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Miller A.H. Notes on the song and territorial habits of Bullock's oriole. Wilson Bull. 1931;43:102–108. [Google Scholar]
- Morton E.S. A comparison of vocal behavior among tropical and temperate passerine birds. In: Kroodsma D.E., Miller E.H., editors. Ecology and evolution of acoustic communication in birds. Cornell University Press; Ithaca, NY: 1996. pp. 258–268. [Google Scholar]
- Oakley T.H., Cunningham C.W. Independent contrasts succeed where ancestor reconstruction fails in a known bacteriophage phylogeny. Evolution. 2000;54:397–405. doi: 10.1111/j.0014-3820.2000.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Orians G.H. Notes on the behavior of the melodious blackbird (Dives dives) Condor. 1983;85:453–460. doi:10.2307/1367986 [Google Scholar]
- Orians G.H. University of Washington Press; Seattle, WA: 1985. Blackbirds of the Americas. [Google Scholar]
- Orians G.H., Orians E.N. Observations of the pale-eyed blackbird in southeastern Peru. Condor. 2000;102:956–958. doi:10.1650/0010-5422(2000)102[0956:OOTPEB]2.0.CO;2 [Google Scholar]
- Pagel M. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B. 1994;255:37–45. doi:10.1098/rspb.1994.0006 [Google Scholar]
- Pleasants, B. Y. & Albano, D. J. 2001 Hooded oriole (Icterus cucullatus). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Post, W., Poston, J. P. & Bancroft, G. T. 1996 Boat-tailed grackle (Quiscalus major). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Price J.J., Lanyon S.M. Patterns of song evolution and sexual selection in the oropendolas and caciques. Behav. Ecol. 2004;15:485–497. doi:10.1093/beheco/arh040 [Google Scholar]
- Price J.J., Earnshaw S.M., Webster M.S. Montezuma oropendolas modify a component of song constrained by body size during vocal contests. Anim. Behav. 2006;71:799–807. doi:10.1016/j.anbehav.2005.05.025 [Google Scholar]
- Price J.J., Yunes-Jimenez L., Osorio-Beristain M., Omland K.E., Murphy T.G. Sex-role reversal in song? Females sing more frequently than males in the streak-backed oriole. Condor. 2008;110:387–392. doi:10.1525/cond.2008.8430 [Google Scholar]
- Ridgely R.S., Tudor G. The oscine passerines. vol. 2. University of Texas Press; Austin, TX: 1989. The birds of South America. [Google Scholar]
- Riebel K. The “mute” sex revisited: vocal production and perception learning in female songbirds. In: Slater P.J.B., Rosenblatt J.S., Snowdon C.T., Roper T.J., Naguib M., editors. Advances in the study of behavior. vol. 33. Elsevier Academic Press; New York, NY: 2003. pp. 49–86. [Google Scholar]
- Riebel K., Hall M.L., Langmore N.E. Female songbirds still struggling to be heard. Trends Ecol. Evol. 2005;20:419–420. doi: 10.1016/j.tree.2005.04.024. doi:10.1016/j.tree.2005.04.024 [DOI] [PubMed] [Google Scholar]
- Rising, J. D. & Flood, N. J. 1998 Baltimore oriole (Icterus galbula). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Rising, J. D. & Williams, P. L. 1999 Bullock's oriole (Icterus bullockii). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Robinson S.K. The evolution of social behavior and mating systems in the blackbirds (Icterinae) In: Rubenstein P.I., Wrangham R.A., editors. Ecological aspects of social evolution. Princeton University Press; Princeton, NJ: 1986. pp. 175–200. [Google Scholar]
- Scharf, W. C. & Kren, J. 1996 Orchard oriole (Icterus spurius). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Scott W.E.D. On the breeding habits of some Arizona birds. Auk. 1885;11:1–7. [Google Scholar]
- Scott W.E.D. Observations on the birds of Jamaica, West Indies. Auk. 1893;10:177–181. [Google Scholar]
- Searcy W.A., Yasukawa K. Princeton University Press; Princeton, NJ: 1995. Polygyny and sexual selection in red-winged blackbirds. [Google Scholar]
- Searcy W.A., Yasukawa K., Lanyon S. Evolution of polygyny in the ancestors of red-winged blackbirds. Auk. 1999;116:5–19. [Google Scholar]
- Skutch A.F. University of Arizona Press; Tucson, AZ: 1996. Orioles, blackbirds, and their kin. [Google Scholar]
- Slater P.J.B., Mann N.I. Why do the females of many bird species sing in the tropics? J. Avian Biol. 2004;35:289–294. doi:10.1111/j.0908-8857.2004.03392.x [Google Scholar]
- Stiles F.G., Skutch A.F. Cornell University Press; Ithaca, NY: 1989. A guide to the birds of Costa Rica. [Google Scholar]
- Trainer J.M. Cultural evolution in song dialects of yellow-rumped caciques in Panama. Ethology. 1989;80:190–204. [Google Scholar]
- Twedt, D. J. & Crawford, R. D. 1995 Yellow-headed blackbird (Xanthocephalus xanthocephalus). In The birds of North America online (ed. A. Poole). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna
- Warren P.S. Geographic variation and dialects in songs of the bronzed cowbird (Molothrus aeneus) Auk. 2002;119:349–361. doi:10.1642/0004-8038(2002)119[0349:GVADIS]2.0.CO;2 [Google Scholar]
- Webster M.S. Female-defense polygyny in a Neotropical bird, the Montezuma oropendola. Anim. Behav. 1994;48:779–794. doi:10.1006/anbe.1994.1302 [Google Scholar]
- Whittingham L.A., Kirkconnell A., Ratcliffe L.M. Differences in song and sexual dimorphism between Cuban and North-American red-winged blackbirds (Agelaius phoeniceus) Auk. 1992;109:928–933. [Google Scholar]
- Whittingham L.A., Kirkconnell A., Ratcliffe L.M. Breeding behavior, social organization and morphology of red-shouldered (Agelaius assimilis) and tawny-shouldered (A. humeralis) blackbirds. Condor. 1996;98:832–836. doi:10.2307/1369864 [Google Scholar]
- Whittingham L.A., Kirkconnell A., Ratcliffe L.M. The context and function of duet and solo songs in the red-shouldered blackbird. Wilson Bull. 1997;109:279–289. [Google Scholar]
- Wiley R.H. Affiliation between the sexes in common grackles II: spatial and vocal coordination. Z. Tierpsychol. 1976;40:244–264. doi: 10.1111/j.1439-0310.1976.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Wiley R.H., Cruz A. The Jamaican blackbird: a ‘natural experiment’ in socioecology. In: Hecht M.K., Steere W.C., Wallace B., editors. Evolutionary biology. vol. 13. Plenum Press; New York, NY: 1980. pp. 261–293. [Google Scholar]
- Wiley R.H., Wiley M.S. Spacing and timing in the nesting ecology of a tropical blackbird: comparison of populations in different environments. Ecol. Monogr. 1980;50:153–178. doi:10.2307/1942477 [Google Scholar]
- Yasukawa, K. & Searcy, W. A. 1995 Red-winged blackbird (Agelaius pheoniceus). In The birds of North America online (eds A. Poole & F. Gill). Ithaca, NY: Cornell Lab of Ornithology. See http://bna.birds.cornell.edu/bna