Abstract
Behavioral threshold for a tone burst presented in a long-duration noise masker decreases as the onset of the tone burst is delayed relative to masker onset. The threshold difference between detection of early- and late-onset tone bursts is called overshoot. Although the underlying mechanisms are unclear, one hypothesis is that overshoot occurs due to efferent suppression of cochlear nonlinearity [von Klitzing, R., and Kohlrausch, A. (1994). J. Acoust. Soc. Am. 95, 2192–2201]. This hypothesis was tested by using overshoot conditions to elicit stimulus-frequency otoacoustic emissions (SFOAEs), which provide a physiological measure of cochlear nonlinearity. SFOAE and behavioral thresholds were estimated using a modified maximum-likelihood yes-no procedure. The masker was a 400-ms “frozen” notched noise. The signal was a 20-ms, 4-kHz tone burst presented at 1 or 200 ms after the noise onset. Behavioral overshoot results replicated previous studies, but no overshoot was observed in SFOAE thresholds. This suggests that either efferent suppression of cochlear nonlinearity is not involved in overshoot, or a SFOAE threshold estimation procedure based on stimuli similar to those used to study behavioral overshoot is not sensitive enough to measure the effect.
INTRODUCTION
The behavioral threshold of a brief tone burst or signal in the presence of a gated broadband or notched noise is higher when the burst is presented at the beginning of the noise than when the burst is delayed by approximately 200 ms within the duration of the gated noise (Zwicker, 1965; Elliott, 1965). The difference in thresholds between early and late bursts is called overshoot. In listeners with normal hearing, overshoot is around 10 dB in conditions with moderate narrow- or broadband noise levels, but this varies widely across individual listeners and stimulus conditions (e.g.,Bacon, 1990; Bacon and Liu, 2000; Carlyon and White, 1992; Formby et al., 2000; McFadden, 1989; Zwicker, 1965). The purpose of the current study is to examine a potential mechanism of the overshoot phenomenon. von Klitzing and Kohlrausch (1994) proposed that overshoot is the result of a shift in the basilar membrane (BM) input-output (I∕O) function, and that the shift may be a result of efferent adaptation as suggested by Schmidt and Zwicker (1991). If this is the case, then the effects should be observable in stimulus-frequency otoacoustic emissions (SFOAEs), which are generated by the same mechanism as the cochlear nonlinearity. The hypothesis is that if overshoot is the result of efferent adaptation of cochlear nonlinearity, then comparable amounts of overshoot should be observed in behavioral and SFOAE thresholds.
In the past, overshoot has been explained as resulting from short-term adaptation in afferent auditory-nerve fibers tuned close to the signal frequency (Smith and Zwislocki, 1975). A difficulty with that model is that off-frequency tones (Bacon and Viemeister, 1985) and notched-noise maskers (Bacon and Smith, 1991; Strickland, 2004) can produce an overshoot effect. That is, overshoot occurs in the absence of excitation of fibers (by the masker) that are near the signal frequency. Overshoot effects are reduced in ears with impaired cochlear functioning. For example, overshoot is reduced in ears following intense sound exposure (Champlin and McFadden, 1989), following aspirin administration (McFadden and Champlin, 1990), and in ears with sensorineural hearing loss (Bacon and Takahashi, 1992; Strickland and Krishnan, 2005). These results are consistent with an explanation of overshoot based on efferent adaptations of cochlear mechanics: a reduction in cochlear nonlinearity would leave a smaller possible range of adaptation of cochlear function, thereby reducing or even eliminating overshoot.
Evidence for involvement of the efferent system is derived from a wide range of studies. Efferent adaptation is mediated by the medial olivary complex (MOC) in the auditory brainstem. A “fast” MOC adaptation occurs on time scales on the order of 100 ms in humans (Guinan, 2006). This is similar to the time course of behavioral overshoot results in which the magnitude of overshoot increases as the delay in signal onset relative to the masker onset increases toward 100 ms, and begins to asymptote around 200 ms (e.g., Elliot, 1965 and Zwicker, 1965). This suggests a possible relation of MOC functioning to overshoot. Such a relationship has been examined in studies performed on patients who had undergone vestibular neurectomy, a procedure that severs vestibular and efferent nerves to alleviate severe vertigo. One study found differences within 1.1 dB in the overshoot measured in the surgery and non-surgery ears, thereby concluding that overshoot does not involve MOC efferent activity (Scharf et al., 1997). Another study using different procedures found reduced overshoot in ears with vestibular neurectomy compared to non-surgery ears, thus supporting the role of MOC efferents as a contributor to overshoot (Zeng et al., 2000). Interpretation of these data is complicated by the unknown effects of surgery on auditory function. Thus, there is no consensus of interpretation regarding available data from subjects who received surgery. Furthermore, due to their restriction to this class of patients, such studies cannot examine the role of the peripheral auditory system in overshoot in normal-hearing subjects.
Bacon and Liu (2000) tested listeners with normal hearing. Their results using both ipsilateral and contralateral precursors suggest either a possible role for peripheral adaptation in ipsilateral overshoot or a stronger overshoot effect in the ipsilateral ear due to the larger number of MOC efferent neurons responding best to ipsilateral stimulation, as was found by Liberman (1988) in cat ears. These results in normal-hearing listeners contradict theories of overshoot based solely on peripheral adaptation, but such adaptation may contribute to ipsilateral overshoot. Contralateral stimulation prior to noise onset reduced the overshoot effect in normal-hearing subjects but had no effect in hearing-impaired subjects (Turner and Doherty, 1997). These results support a role for the MOC system in overshoot, based on the assumption that MOC effects would be absent in hearing-impaired subjects.
Through experiments using cat in which the MOC fibers were cut, it is known that the MOC reflex enhances the compound action potential response to transient signals presented in a continuous background noise; this enhancement effect is termed antimasking (Kawase and Liberman, 1993). The MOC reflex also enhances the responses of single auditory-nerve fibers in cat for transients within a continuous background noise (Kawase et al., 1993). Kawase and Liberman (1993) explained antimasking on the basis of MOC activation of outer hair cells (OHCs). MOC fibers terminate on the base of OHCs and modulate OHC function, and by extension, the cochlear nonlinearity. As described in reviews of efferent effects (Pujol, 1994; Guinan, 2006), the MOC modulates the OHC activity responsible for the amplification of low-level sounds. BM mechanics adapt in the presence of the noise over time due to MOC activation. This produces a shift to higher stimulus levels and a larger slope of the BM I∕O function, i.e., the smaller, compressive slope is modified in the direction of a more nearly linear slope. The result is that the cochlear representation of noise is reduced and the internal signal-to-noise ratio (SNR) is increased for the late burst relative to the early burst. The detection threshold for the late burst is thereby lower. These effects are consistent with effects observed in animal studies in which the olivocochlear bundle was stimulated (Murugasu and Russell, 1996). Behavioral overshoot data support the antimasking mechanism (Strickland, 2001; Strickland, 2008), although it is not clear whether the improved representation of the tone burst in noise is due to adaptation of cochlear mechanics, neural processing, or both systems.
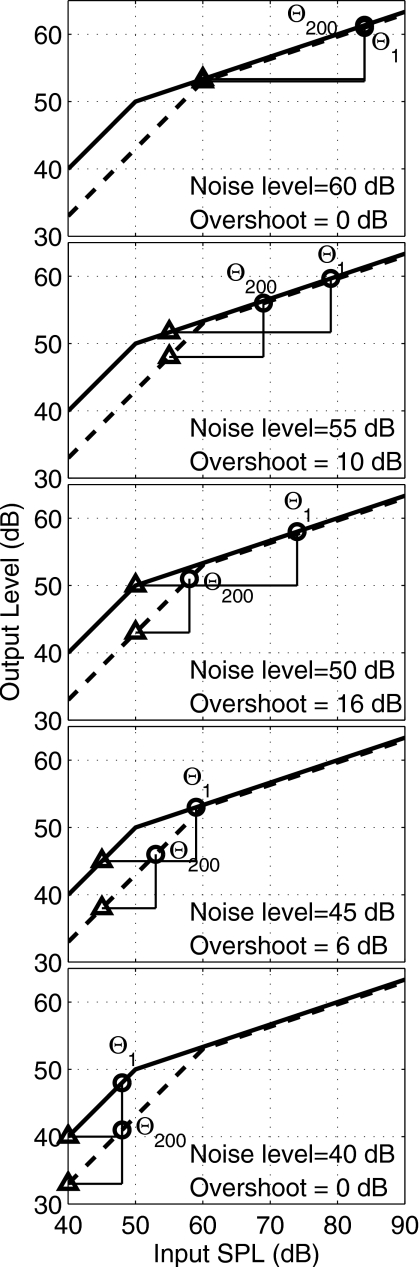
A shift in the BM I∕O function due to efferent adaptation is the overshoot mechanism proposed by von Klitzing and Kohlrausch (1994), and is illustrated in Fig. 1. Each panel shows schematic BM I∕O functions for the 200-ms delay condition (late burst), in which an efferent shift is present, versus the 1-ms delay condition (early burst), in which no efferent shift is present. Each I∕O function is assumed to have linear growth at low input levels and compressive growth at higher input levels. The pair of triangles on each panel shows the input noise sound pressure level (SPL) in each condition. In this example, a signal is assumed present at a threshold based on an output level that is 8 dB higher than the noise level. The resulting signal levels at threshold are shown by circle symbols on each of the early-burst and late-burst I∕O functions. The amount of overshoot reported in each panel is the input SPL difference of the threshold of the signal with 1-ms delay compared to the threshold of the signal with 200-ms delay.
Figure 1.
This figure shows the threshold shifts that are predicted from the von Klitzing and Kohlrausch (1994) model. The modeled BM I∕O functions (with arbitrary normalization) are plotted as a function of stimulus level at 1 ms after the noise onset (solid lines) and 200 ms after the noise onset (dashed line). The simulated noise sound pressure spectrum level is listed in each panel (as noise level) and represented on each plot by a triangle at this input level. The noise level decreases in 5-dB steps from 60 dB (top panel) to 40 dB (bottom panel). Each threshold is marked on each plot using a circle symbol and labeled with the appropriate threshold of the early (Θ1) or late (Θ200) burst. In this model, the SNR for threshold for the burst is assumed to be 8 dB above the noise level on the respective BM I∕O function (i.e., 8 dB difference between triangles and circles on the Y axis, or output). For example, in the middle panel, the noise level is 50 dB, so that the output level at threshold is 8 dB higher, or 58 dB, for the late burst (Θ200). The early-burst threshold Θ1 attains this output level at an input SPL of 74 dB. The overshoot is the difference in input SPL of the early burst to that of the late burst, or 16 dB in this example.
SFOAEs elicited using sine tones in noise are a non-invasive correlate to behavioral tone-in-noise tests. If the above mechanism for overshoot is correct, the MOC effects on OHC functioning should be reflected in SFOAE thresholds in comparable stimulus conditions. Shifts in SFOAE level in the presence of contralateral (Souter, 1995) and ipsilateral noise (Guinan et al., 2003; Goodman and Keefe, 2006; Guinan, 2006) have been used previously to study adaptation by the MOC efferent system.
In the present study, behavioral and SFOAE thresholds were measured in each subject using similar stimulus conditions delivered by the same insert earphones. OAE responses are often measured as I∕O functions, which allow the estimation of an OAE threshold as that input level resulting in an OAE detected according to a chosen criterion level. A commonly used approach to defining the threshold is a minimum SNR. This definition has the disadvantage that noise fluctuations occurring near threshold can produce a nonmonotonic growth of response at stimulus levels close to threshold. One conclusion of a preliminary study (Keefe et al., 2003) was that a better definition of SFOAE threshold was needed in order to accurately measure changes in threshold that might be associated with any overshoot effect. A novel aspect of the present study was the measurement of a SFOAE threshold using a sequential adaptive technique with properties that are well understood from psychophysical research. Both behavioral and SFOAE thresholds were measured at 4 kHz, which is a signal frequency often used in past overshoot studies, e.g., Turner and Doherty (1997), Bacon and Liu (2000), Smith et al. (2000), and Strickland (2001, 2004, 2008).
METHODS
Subjects
Subjects (N=14) were recruited from a young adult, normal-hearing population. The inclusion criteria for the experiments were that subjects have normal tympanograms and normal air-conduction thresholds, i.e., less than or equal to 15-dB hearing level (HL) at all octave frequencies from 0.5 to 8 kHz. A 0.226-kHz tympanogram was classified within normal limits according to criteria described in Ellison and Keefe (2005). All testing was performed in one ear of each subject. Thresholds of a 4-kHz tone burst were measured in quiet and in noise presented over a range of levels. All behavioral thresholds for each subject were measured on the same day, but a different day from that on which SFOAE thresholds were measured. The more extensive SFOAE data collection was completed in seven 2-h test sessions, one test session for measuring the threshold of a tone-in-quiet condition and six sessions for the thresholds of tone-in-noise conditions. All 14 subjects completed the SFOAE testing whereas only 12 of these subjects participated in the behavioral testing. The other two subjects did not return for behavioral testing.
Research suggests the possibility of an attentional control of MOC functioning (Guinan, 2006), which might vary with subject state. The theory under evaluation was that MOC adaption of cochlear nonlinearity produced changes in SFOAEs, so that subject state (e.g., whether awake or asleep) might influence any adaptation of SFOAEs. To control for possible effects of subject state, subjects were kept awake during the SFOAE test sessions with the assistance of a DVD movie with sub-titles and no audible sound. This likely resulted in lower average levels of physiological noise in the SFOAE recordings, which might otherwise be elevated in a sleeping subject who begins to snore or in a bored subject who begins to fidget.
General methods
Subject responses were measured using custom software on a laboratory Windows computer with a high-quality sound card (24-bit resolution, 22.05-kHz sample rate, CardDeluxe). SFOAEs were measured for tone-in-quiet and tone-in-noise conditions using a commercial OAE ear-probe assembly comprised of a microphone and a pair of receivers (Etymotic ER-10C). The ear-probe was inserted into the ear canal using a soft foam tip provided by the manufacturer. The ear-probe system was modified by the manufacturer to provide 20 dB of additional gain in the output receivers. Behavioral thresholds were measured in response to stimuli presented through one receiver of the same ear-probe.
Behavioral and SFOAE measurements were performed using the same procedures and stimuli to the maximum extent possible. The signal was a 4-kHz, 20-ms (5-ms ramps) tone burst. The rationale for choosing this duration is described at the end of Sec. 2. A 400-ms, fixed-length sample of noise was generated and used in all measurements as a frozen noise masker. The noise had a notch in the spectrum centered at 4 kHz, which coincided with the peak frequency in the tone-burst spectrum, and a notch width of 0.4 kHz, which corresponds to 0.14 octave. The lower and upper passbands each had a bandwidth of 1.6 kHz. The notch width was sufficiently broad that the main spectral lobe of the signal tone burst was contained completely within the notch. The early-burst condition presented the onset of the signal 1 ms after the onset of the noise, and the late-burst condition presented the onset of the signal 200 ms after the onset of the noise. The temporal fine structure of the frozen noise was identical for early and late bursts. Thresholds were measured at each of six noise masker levels, which varied over a 60-dB range in 12-dB increments. The noise sound pressure spectrum levels (reference bandwidth of 1 Hz) averaged across the upper and lower passbands were measured in an artificial ear simulator (IEC 711) to be −14, −2, 10, 22, 34, and 46 dB.
Adaptive behavioral threshold procedure using yes∕no task
An adaptive, maximum-likelihood (ML) procedure was used to estimate thresholds in a yes-no task in the behavioral experiments. Green (1993) introduced a ML method for estimating thresholds in a yes-no task using a modified logistic function to represent the underlying psychometric function. Gu and Green (1994) improved the ability of the ML method to estimate false-alarm rates. Such ML procedures are accurate in behavioral threshold testing (Formby et al., 1996; Leek et al., 2000). We used a ML procedure similar to that of Gu and Green (1994) to measure thresholds with the following modification: to overcome any sensitivity to the results of the initial trials, the stimulus levels of the first four trials were set independent of the subject’s responses to the trials. The stimulus dynamic range was split into four equal sub-ranges. The stimulus level in each of the first four trials was selected by randomly choosing (without replacement) one of these subranges for the trial, and then randomly choosing a stimulus level within the sub-range. A threshold was then calculated using the ML estimate based on these initial four yes-no responses. The basic idea was that the stimulus levels of the fifth and subsequent trials would be chosen as in Green (1993) based on the current threshold estimate. This initialization procedure substantially reduced the sensitivity of the ML procedure to errors when testing subjects. Preliminary behavioral data were acquired with N as large as 30 trials and demonstrated that fewer trials gave results of sufficient accuracy. Therefore, N=16 trials were used in the main experiment. Of these 16 trials, 4 catch trials, i.e., 4 signal-absent trials, were randomly included after the first stimulus presentation. These catch trials identified whether the listener was inattentive, as would be evidenced by responding that a signal was present when no stimulus was presented, and were used to assess the false-alarm rate (Gu and Green, 1994). The presence of these catch trials on any of trials 2–16 interacted with the initialization procedure, which was based on the first four signal-present trials irrespective of the number of catch trials that might have been interpolated during the initialization. Thus, the stimulus levels of the fifth and subsequent signal-present trials were chosen using the ML procedure.
During the task, the subjects were instructed to ignore the masker and listen for the tone bursts. The observation interval was marked by a row of asterisks on the text panel of a custom-built response box. For each observation interval, they were instructed to push one button for “yes” and another button for “no” to indicate whether the tone burst was heard. Feedback included a message informing the subject of the beginning of the first trial of a run, the yes or no response entered on each trial, an alert to the next upcoming trial, and a message announcing the end of the threshold test. After each stimulus presentation, the automated procedure waited until the subject depressed the yes or no button. To measure a single behavioral threshold, the stimulus buffer was 1 s, with an approximate 1.3-s response time (that varied across subjects), with 16 trials per run and two runs per estimate for a total measurement time on the order of 1.2 min per condition. There was an additional brief waiting period for the subject between runs and an initial alert displayed at the beginning of each run. The final threshold was calculated as the mean of two successive runs as long as the standard deviation (SD) between runs was within 3 dB. If the SD was larger, then another run was performed.
Behavioral thresholds at 4 kHz were measured in 12 subjects, which included six left and six right ears, six males and six females, and a mean age of 21.2 years (SD of 1.5 years) in the −14, −2, and 22 dB noise level conditions. Behavioral thresholds were measured in 11 of these 12 subjects in the 10, 34, and 46 dB noise spectrum level conditions (the 12th subject was not tested in these conditions due to the subject’s time constraints).
Adaptive SFOAE threshold procedure using yes∕no task
Basic SFOAE measurement procedures
For the tone-in-quiet condition, the signal s1 was the same 20-ms tone burst at 4 kHz as used in the behavioral task, and the suppressor stimulus s2 was a 30-ms tone burst at a frequency 4% higher than that of the signal. The centers of each tone burst were time aligned. The suppressor level was fixed at 15 dB above the signal level. Both signal and suppressor levels were varied by equal increments within an adaptive threshold procedure. The pressure waveform p1 was measured in response to stimulus s1 delivered through receiver 1 of the probe, response p2 was measured in response to stimulus s2 delivered through receiver 2 of the probe, and response p12 was measured in response to the simultaneous presentation of s1 through receiver 1 and s2 through receiver 2. The SFOAE distortion response pd was calculated as the nonlinear residual of p1+p2−p12 (Keefe, 1998), as has been used in previous SFOAE studies (Schairer et al., 2003; Schairer and Keefe, 2005; Schairer et al., 2006). The distortion response is zero for a purely linear system and otherwise contains a residual that originates from the compressively nonlinear mechanics associated with OHC functioning. Recordings using the ear-probe inserted into an artificial ear (IEC 711, B&K model 4157) confirmed minimal distortion in the measurement system.
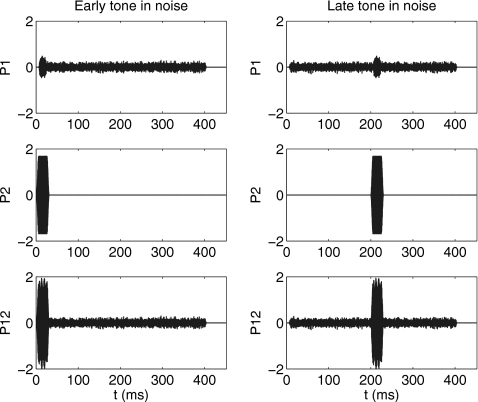
For the tone-in-noise conditions, the same tone-burst stimuli as described above were used, but the notched noise was added to the signal s1 with no change in s2. The early-burst condition presented the onset of the signal 1 ms after the onset of the noise (see left panels, Fig. 2), and the late-burst condition presented the onset of the signal 200 ms after the onset of the noise (see right panels, Fig. 2). The duration of the gated noise was 400 ms, which was followed by a silent interval of 600 ms (total buffer duration of 1 s). The presentation of early and late tone bursts in a noise background (top row, Fig. 2) is analogous to the behavioral overshoot stimuli. The s2 stimulus was identical to that used in quiet (middle row panels, Fig. 2). The third stimulus set consisted of the simultaneous presentation of s1 and s2 (bottom row panels, Fig. 2). The SFOAE distortion waveform pd was calculated using the p1, p2, and p12 responses labeled in Fig. 2.
Figure 2.
The modeled stimulus waveform responses in the ear canal (in arbitrary units) are shown for the conditions used to estimate SFOAE thresholds. Modeled responses for SFOAEs elicited by tone bursts masked by notched noise in the early condition are shown in the left column, and responses in the late condition are shown in the right column. The top panels show the modeled signal+gated noise stimulus waveform responses p1, the middle panels show the tonal suppressor responses p2, and the bottom panels show the sum p12 of these responses.
Using a technique similar to that described in Goodman and Keefe (2006), it was verified in preliminary experiments that there was no middle-ear muscle reflex shift evoked at even the highest-level noise condition. This technique compared the responses to a moderate-level low-frequency tone (0.25 kHz) that was presented in quiet and in the notched-noise masker. The lower passband in the notched-noise spectrum had negligible energy below 1.5 kHz, so that there was no spectral overlap between the noise and the low-frequency tone.
Measuring SFOAE thresholds adaptively using a yes∕no task
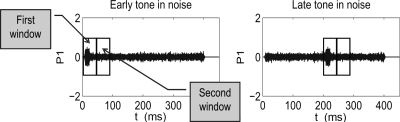
The use of a yes-no task requires a decision that the signal, in this case the SFOAE, be classified as present (i.e., yes) or absent (i.e., no). To minimize total test time, the tone burst eliciting the SFOAE was presented on every trial. A 1024-sample Hanning window (with duration of 46 ms) was applied to a first time interval of the response that included the 20-ms tone burst followed by the interval in which the SFOAE would be present (including a few milliseconds of cochlear round-trip travel time). The mean SFOAE latency is in the range of 3–4.5 ms at 4 kHz (Shera and Guinan, 2003; Schairer et al., 2006). As shown in simulated responses in Fig. 3, this first interval included all of the signal and suppressor tone energies for either the early or late tone burst. A second 1024-sample Hanning window was applied to the 1024 samples immediately following the first time interval. No signal or suppressor tone was present during this second interval, although the notched-noise signal was continuously present during both intervals. A discrete Fourier transform (DFT) of the windowed waveform was calculated for each of the intervals. A signal was classified as present if the energy level at 4 kHz in the first interval was at least 3 dB larger than the energy level at 4 kHz in the second interval. Classifier criteria of 0, 6, and 9 dB were also analyzed in preliminary experiments. The results appeared less consistent using the 0-dB criterion than with any other criterion. The 3-dB criterion was selected because results were reliable and thresholds were lower than with the 6- or 9-dB criteria.
Figure 3.
The simulated signal response p1 is plotted in arbitrary units as a function of time (in milliseconds) for early and late bursts in noise in the left and right panels, respectively. Each panel also shows the first and second windows, each 1024 samples (or 46 ms) in length, which were used for the signal-present and signal-absent detection conditions, respectively. Each window is drawn as a box.
The adaptive procedure to estimate SFOAE thresholds was generally similar to the behavioral procedure, except that no catch trials were included in the SFOAE procedure because it used an energy detection decision that was not subject to false alarms. On each trial of the adaptive procedure (i.e., a trial results in a yes or no decision), the stimulus set shown in Fig. 2 was presented multiple times to enable time averaging with real-time artifact rejection to exclude intermittent noise generated by the subject or other environmental source. In preliminary studies using two subjects, data were acquired using a number of repeated responses equal to 4, 8, 16, and 32. The response variability was analyzed in terms of the prevalence of far outliers in the sense defined by Tukey (1977). Such far outliers, when detected, were excluded from the time average. The artifact rejection method functioned better at excluding outliers with 16 or 32 responses than with 8 or fewer responses. The use of 32 responses did not reveal a clear benefit over the use of 16 responses. Thus, data in the main experiment were acquired using 16 responses per trial. After calculating the signal as the average of these 16 responses, the SFOAE threshold at each noise level or in quiet was the lowest signal level that produced a detectable SFOAE residual.
The final threshold was based on the median of three runs. The median was preferable to the mean because some runs were contaminated by excessively high SFOAE thresholds. A factor that possibly contributed to variability in thresholds is that some SFOAE I∕O functions are nonmonotonic with increasing stimulus level (Schairer et al., 2003; Schairer and Keefe, 2005). Notches in the SFOAE output level may appear at a relatively high stimulus level, whereas an adaptive procedure to estimate threshold assumes a monotonic relationship. The role of this factor was not systematically studied because the entire I∕O function was not recorded. Nevertheless, if present in any subject’s run, this factor would play a role if the adaptive yes-no procedure of Green (1993) were used because of its sensitivity to the results of the first few trials. When the initial trial detected no SFOAE, the Green procedure set the stimulus to its maximum allowed level. This often resulted in preliminary experiments in SFOAE thresholds lying near the maximum of the range. As in the behavioral procedure, SFOAE thresholds were acquired in a sequential adaptive procedure in which the stimulus levels of the first four trials were set independently of the yes-no responses in the trials. Preliminary results showed that this initialization reduced the variability of SFOAE threshold estimates. This typically resulted in an initial threshold estimate within the low-to-moderate range of input levels on the SFOAE I∕O function, so that there was reduced likelihood of an effect of a nonmonotonic notch in the I∕O function.
The 20-ms duration of the 4-kHz tone burst in the SFOAE (and behavioral) measurements was selected based on a preliminary pilot study. Increasing the tone-burst duration would be expected to increase the SNR and thus decrease the SFOAE threshold. The tone-burst durations were varied between 10 ms, which is typical in behavioral overshoot studies, and 100 ms, which is on the order of MOC latency, and thus was an upper limit in the experimental design. If the stimulus levels were high enough, then it might produce system distortion that would contaminate the SFOAE recording in an ear. Therefore, it was potentially advantageous to use a longer-duration stimulus to evoke the SFOAE so as to measure its threshold over a lower range of stimulus levels. As expected, SFOAE thresholds were lower with increasing stimulus duration and increasing signal averaging. The signal averaging to extract the SFOAE was performed prior to any classification of the SFOAE as present or absent. Such signal averaging is not used in a behavioral experiment. The task in each of the behavioral and SFOAE experiments is to detect a signal in the presence of internal and external noise. The external noise in each experiment includes the noise masker and any environmental noise transmitted with the stimulus. The internal noise sources differ in the behavioral and SFOAE experiments: the behavioral internal noise source is attributed to the listener’s internal state, while the SFOAE internal noise includes physiologic sources of noise and the noise at the output of the microphone preamplifier. The SFOAE internal noise is measured using the DFT at the frequency (4 kHz) of the signal tone burst. As described above (see Fig. 2), a signal duration of 20 ms with a suppressor duration of 30 ms was used in the main experiment. These durations were somewhat longer than tone bursts used in behavioral overshoot studies (e.g., Formby et al., 2000; McFadden, 1989; Zwicker, 1965). However, the longer durations were deemed to be appropriate because they improved the SNR of the SFOAE measurements, yet were shorter than the onset latencies associated with fast MOC effects. Their use did not detract from the behavioral measurements.
To measure a single SFOAE threshold at a fixed noise level or in quiet, three buffers (p1, p2, and p12) comprising the OAE stimulus had a total duration of 3 s, with 16 averages per trial, 16 trials per run, and three runs per estimate for a total measurement time of 38.4 min. Note that only two runs per estimate were obtained in the behavioral paradigm because behavioral thresholds were less variable and the results more predictable than for the SFOAE paradigm. SFOAE thresholds at 4 kHz were measured in all 14 subjects, including six left and eight right ears, eight males and six females, and a mean age of 21.1 years (SD of 1.4 years).
RESULTS
Behavioral thresholds
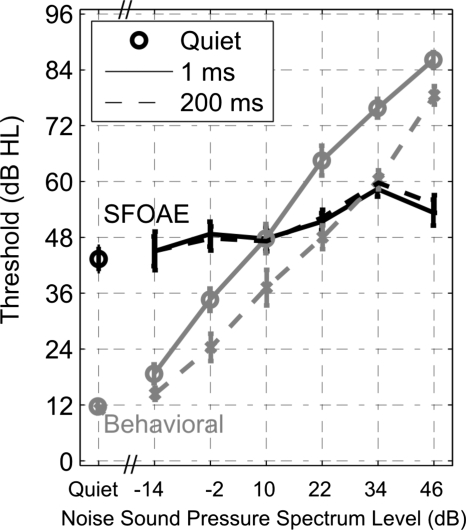
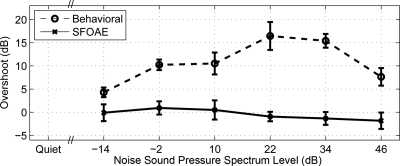
The mean ±1 standard error of the mean (SE) of the behavioral thresholds for the tone burst in quiet and in notched noise at each noise spectrum level is plotted in Fig. 4 for the early-burst (labeled 1 ms, gray solid line) and late-burst (labeled 200 ms, gray dashed line) conditions. The two thresholds in quiet for the early and late bursts provided replications of the threshold-in-quiet measurement (12-dB HL). This threshold in quiet was based on SPL measured in a 2 cm3 coupler in relation to its reference equivalent threshold sound pressure level (RETSPL) based on continuous tones. It lies above the nominal threshold of 0 dB HL due to the brief duration (20 ms) of the tone burst. The thresholds in noise were elevated above the threshold in quiet and the late-burst thresholds increased linearly with increasing noise spectrum level (except for a slightly steeper rate of increase for the threshold at the highest noise spectrum level). The slope of the early-burst threshold function was slightly larger than that of the late-burst function at lower noise spectrum levels and slightly smaller at higher noise spectrum levels, resulting in an overall concave-downwards shape. The difference in the early-burst and late-burst thresholds was calculated for each subject as a function of noise spectrum level. In Fig. 5 (dashed line), the mean ±1 SE of this difference across subjects is plotted as the group-averaged overshoot. The mean overshoot had a maximum of 16 dB at an intermediate noise spectrum level of 22 dB. Mean overshoot decreased to 4 dB at the lowest noise sound pressure spectrum level (−14 dB) and to 7 dB at the highest noise level (46 dB).
Figure 4.
Mean behavioral (gray lines) and SFOAE (black lines) thresholds in decibel HL as a function of the masker level, i.e., the noise sound pressure spectrum level (reference bandwidth of 1 Hz) for the tone burst presented in noise at 1 ms after the masker onset (solid lines) and 200 ms after the masker onset (dashed lines). The thresholds in quiet for each measure are plotted as detached symbols on the left side. Each error bar extends ±1 SE from its mean.
Figure 5.
The group mean ±1 SE of the behavioral overshoot (dashed line) and SFOAE overshoot (solid line) calculated from the group responses in Fig. 4 are plotted as a function of the masker level, i.e., the noise sound pressure spectrum level (reference bandwidth 1 Hz).
SFOAE thresholds
The mean ±1 SE of the SFOAE thresholds evoked by the tone burst in quiet and in notched noise is plotted in Fig. 4 for the early-burst (labeled 1 ms, black solid line) and late-burst (labeled 200 ms, black dashed line) conditions. The mean threshold in quiet was 43-dB HL with a SE of approximately 3 dB. The SFOAE thresholds for tone-in-noise conditions were similar to the threshold for the tone in quiet at the lower noise spectrum levels (up to 10-dB SPL) and were more elevated at high noise spectrum levels (22 dB SPL and above). The thresholds in noise increased with noise spectrum level with a slope of 0.26 dB∕dB for noise sound pressure spectrum levels in the range −14 to 34 dB. The threshold at the highest noise level (46 dB) was lower than that at the next highest level (34 dB). This SFOAE threshold slope of 0.26 dB∕dB was much less than the behavioral-threshold slope of approximately 1 dB∕dB.
There was little difference in the mean SFOAE thresholds of the early and late bursts in Fig. 4. This is quantified by the mean SFOAE overshoot in Fig. 5 (solid line), which represents the mean ±1 SE of the difference across subjects in the early-burst and late-burst thresholds. This mean was equal to 0 dB to within the measurement variability, i.e., the early and late SFOAE thresholds were equal.
As explained in Sec. 2, there was concern in the adaptive SFOAE threshold procedure with the threshold variability between the three adaptive runs from which the threshold was estimated as the median. This variability was analyzed in terms of the SD across the three runs. This SD was calculated for the quiet and each of the noise conditions, and then averaged across subjects. This average SD was generally similar for the early- and late-bursts in noise conditions. The average SD for the tone-in-noise conditions did not appear to increase with increasing noise spectrum level. The grand average across all conditions and subjects of the SD across the three runs was 4.5 dB.
DISCUSSION
The ability to accurately measure thresholds has been an important experimental test in psychoacoustics, with adaptive techniques to measure thresholds in widespread use. An adaptive procedure to measure the threshold of a SFOAE response was developed using an energy detection step embedded in a yes-no task. Each run in the adaptive procedure used maximum likelihood to set the stimulus levels on the 5th through the 16th trials and to calculate the threshold. The resulting SFOAE thresholds had sufficiently small standard errors to examine the predictions of a theory of behavioral overshoot.
The behavioral thresholds of the late tone burst increased linearly with increases in noise sound pressure spectrum level with the exception of the change in threshold from the 34- to 46-dB level conditions (Fig. 4). This linear relationship is consistent with the simultaneous masking of the tone burst by the notched noise, in which the notch width was relatively narrow (0.14 octave). The rate of change in behavioral thresholds of the early tone burst varied with noise spectrum level, that is, it was not linear as in the late tone-burst condition. This is expected because overshoot is larger in moderate masker level conditions than in lower and higher masker level conditions, and is attributed to nonlinear changes in the early tone-burst threshold relative to the linear changes in the late-burst threshold. The SFOAE thresholds increased more slowly with increasing noise spectrum level, with a slope of 0.26 dB∕dB except at the highest noise spectrum level. The SFOAE thresholds would not be expected to increase linearly with increasing noise spectrum level, because the SFOAE was measured at 4 kHz, which is the center frequency within the notch of the notched-noise masker. The SFOAE threshold slope of 0.26 dB∕dB appears consistent with a compressive growth of SFOAE suppression on the BM at higher notched-noise levels. This disparity in slopes in Fig. 4 resulted in more elevated SFOAE thresholds than behavioral thresholds in quiet and at low noise levels, and more elevated behavioral thresholds than SFOAE thresholds at high noise levels.
The absolute levels of the SFOAE thresholds were slightly elevated overall through the use of the 3-dB SNR criterion in the energy detection procedure used at the 4-kHz DFT bin (see Sec. 2). SFOAE thresholds would have been reduced in quiet and for each noise condition if a 0-dB SNR criterion had been used or if additional averaging of responses had been performed. The fact that the SFOAE energy detector used the same SNR criterion in quiet and in each noise condition means that differences in physiological noise would not be expected to account for the shallow slope of the SFOAE threshold function in Fig. 4.
The variability in mean thresholds across subjects was slightly larger for the SFOAE threshold data than the behavioral threshold data, as is evident in the size of the SEs in Figs. 45. This larger SFOAE variability must be interpreted in the context of the fact that these estimates were based on 16 averages per trial within the adaptive procedure. Thus, the adverse effects of noise were greater for SFOAE threshold detection than for behavioral threshold detection. SFOAE measurements included additional effects of noise and variability from reverse transmission, small biological signals, and any measurement equipment artifacts that are not a factor in behavioral thresholds.
The theory summarized in Sec. 1 explains the lower thresholds for the late burst in noise relative to the early burst in noise in terms of an adaptation mechanism associated with OHC and MOC efferent functioning, the effects of which are present for the late burst and absent for the early burst. Thus, noise excitation activates the MOC system, which affects OHC functioning to reduce the amount of compression associated with the cochlear-amplifier mechanism. As shown in Fig. 1, the predicted overshoot has a maximum at intermediate noise spectrum levels. This midlevel maximum in overshoot was reported in Bacon and Smith (1991) and in other studies cited in Sec. 1, and, in particular, was observed in behavioral overshoot in the present study using notched noise (see Fig. 5).
OAEs are produced through the activity of OHCs evoked by a sound stimulus. A 4 kHz tone burst at moderate levels in quiet produces a transient SFOAE at 4-kHz in normal ears (Konrad-Martin and Keefe, 2003) and in some ears with mild hearing loss (Konrad-Martin and Keefe, 2005). It was hypothesized that if OHC functioning differs in the early- and late-bursts in noise conditions, then differences in evoked SFOAEs would be observed. Such differences were not observed. There was no mean SFOAE overshoot despite the presence of a behavioral overshoot as large as 16 dB (Fig. 5).
There are three possible explanations that might account for the absence of SFOAE overshoot. First, efferent adaptation of cochlear nonlinearity may not be the dominant underlying mechanism of overshoot. Second, although normal cochlear function is needed for sufficient afferent stimulation to elicit the MOC, the resulting MOC effects may be neural. That is, the effect might reflect MOC adaptation of neural elements rather than cochlear mechanisms. The detection of the late burst presented 200 ms after the noise onset is influenced by this adaptation, but the detection of the early burst is not. The adaptation may be regarded as lowering the internal gain of the noise, i.e., its internal level is reduced at 200 ms after the noise onset in comparison to immediately following the noise onset. This adaptation does not necessarily occur at the OHC level. When the late burst is presented at a given spectral level, its internal SNR in some neural representation(s) may be larger than that of the early burst. Because the burst is presented at a higher spectrum level than the noise spectrum level, and because the auditory system has a compressively nonlinear response with increasing stimulus level, the adaptation effect on the noise in the neural representation may be greater than that on the brief burst. This is generally similar to the antimasking effects observed in cat (Kawase and Liberman, 1993; Kawase et al., 1993). However, antimasking effects in cat were explained as due to adaptation of OHC functioning, whereas the present results in humans could imply that the effects rely on factors other than OHC functioning. In Sec. 1, reduction in overshoot in listeners with noise and aspirin exposure was interpreted as evidence for cochlear involvement. However, noise exposure and sensorineural hearing loss can also be associated with changes in neural functioning, and aspirin administration has systemic effects on neural functioning in addition to its effects on OHC function; salicylate effects are reviewed in Cazals (2000). Thus, the reduction in overshoot by any of these effects does not necessarily demonstrate that overshoot is produced by changes in OHC functioning alone, although it is consistent with this involvement by reducing the afferent auditory innervation received by MOC neurons.
Neural sources of excitatory and inhibitory feedback may contribute to the mechanisms underlying overshoot (McFadden, 1989). Neural circuits involving the MOC system are of particular interest. One possible circuit in the ventral cochlear nucleus (VCN) relies on cholinergic modulation of stellate cells (Fujino and Oertel, 2001). MOC efferent fibers projecting to OHCs also project through collateral branches to the cochlear nuclei, including to T stellate cells and D stellate cells in the VCN. T stellate cells are part of an excitatory auditory pathway from the VCN through the ventral nucleus of the trapezoid body (VNTB) to the contralateral inferior colliculus. The activity of T stellate cells is modulated by cholinergic inputs via feedback from the VNTB including MOC neurons. D stellate cells inhibit the activity of T stellate cells and are not modulated by cholinergic inputs. Stellate cells are tonotopically organized, with T stellate cells having a narrower excitatory tuning and D stellate cells having a broader inhibitory tuning. As further described by Fujino and Oertel (2001), this combination produces a lateral inhibition effect that may enhance the encoding of spectral peaks in noise. More research is needed to evaluate this and other possible neural sources of overshoot. Experiments using non-human mammals are also relevant, e.g., MOC lesions in macaques produced changes in behavioral thresholds for tones in continuous contralateral noise (Smith et al., 2000).
A third explanation to account for the absence of SFOAE overshoot is that efferent adaptation of cochlear nonlinearity is an important underlying mechanism of behavioral overshoot, but the SFOAE threshold estimation procedure was not sensitive enough to detect SFOAE overshoot. It may be that a large behavioral overshoot effect is accompanied by a SFOAE overshoot effect that would require much more averaging to reveal. The variability in thresholds across subjects and the limits to threshold detection in the lower level masker conditions (Fig. 4) may obscure much smaller SFOAE effects. If this third explanation has merit, one implication is that the stimulus conditions that will be effective for studying the cochlear mechanisms underlying behavioral overshoot may not resemble the stimulus conditions used in the present experiment. The latter were designed to be similar to the corresponding behavioral stimuli. The present results may not completely rule out the possibility that MOC efferent adaptation of cochlear nonlinearity is an important contributor to behavioral overshoot, but they constrain the possible set of models to those that can also account for the absence of a detectable MOC adaptation in SFOAE responses of the type reported in the present study. A difficulty with this explanation is that the SFOAE thresholds were approximately equal or lower than the behavioral thresholds for the tone-in-noise conditions at noise levels of 34 and 46 dB (see Fig. 4). Although behavioral overshoot was observed at these higher noise levels, no SFOAE overshoot was observed (see Fig. 5). This finding supports the view that MOC adaptation of OHC function cannot explain overshoot at these higher noise masker levels.
CONCLUSIONS
A sequential adaptive method to measure an OAE threshold based on a ML procedure was developed and applied to the measurement of the threshold of a SFOAE elicited by a tone burst in quiet and in noise. Such an adaptive method is a promising tool for efficient measurements of OAE thresholds. SFOAE thresholds were lower for the burst in quiet than for the burst in moderate-to-high noise levels. SFOAE thresholds did not differ for detection of early and late tone bursts to within the measurement variability. Thus, there was no overshoot in SFOAE threshold data despite confirming the presence of overshoot in behavioral thresholds in the same subject group. The results provide no support for a theory of behavioral overshoot based on temporal adaptation of cochlear OHC function. This negative result does not exclude the possibility that behavioral overshoot has its origins in efferent adaptation of nonlinear cochlear mechanics at lower and moderate noise levels, but it remains to be explained how such a mechanism would not lead to detectable overshoot in SFOAE responses at the higher noise levels. It is concluded that there are important neural contributors of overshoot.
ACKNOWLEDGMENTS
The first author benefited from discussions with Dr. Donata Oertel, who suggested the possible relevance of the functioning of stellate cells in the VCN to theories of behavioral overshoot. Two anonymous reviewers provided helpful critiques of a previous version of the manuscript. This research was supported by the NIH Grant Nos. DC003784 and DC006342, with core support provided by NIH Grant No. DC004662.
Preliminary reports of this research were presented at meetings of the American Auditory Society (Keefe et al. 2006) and the Association for Research in Otolaryngology (Keefe et al. 2005, 2003).
References
- Bacon, S. P. (1990). “Effect of masker level on overshoot,” J. Acoust. Soc. Am. 10.1121/1.399773 88, 698–702. [DOI] [PubMed] [Google Scholar]
- Bacon, S. P., and Liu, L. (2000). “Effects of ipsilateral and contralateral precursors on overshoot,” J. Acoust. Soc. Am. 10.1121/1.1290246 108, 1811–1818. [DOI] [PubMed] [Google Scholar]
- Bacon, S. P., and Smith, M. A. (1991). “Spectral, intensive and temporal factors influencing overshoot,” Q. J. Exp. Psychol. 43A, 373–399. [DOI] [PubMed] [Google Scholar]
- Bacon, S. P., and Takahashi, G. A. (1992). “Overshoot in normal-hearing and hearing-impaired subjects,” J. Acoust. Soc. Am. 10.1121/1.402967 91, 2865–2871. [DOI] [PubMed] [Google Scholar]
- Bacon, S. P., and Viemeister, N. F. (1985). “The temporal course of simultaneous tone-on-tone masking,” J. Acoust. Soc. Am. 10.1121/1.392891 78, 1231–1235. [DOI] [PubMed] [Google Scholar]
- Carlyon, R. P., and White, L. J. (1992). “Effect of signal frequency and masker level on the frequency regions responsible for the overshoot effect,” J. Acoust. Soc. Am. 10.1121/1.402629 91, 1034–1041. [DOI] [PubMed] [Google Scholar]
- Cazals, Y. (2000). “Auditory sensori-neural alterations induced by salicylate,” Prog. Neurobiol. 62, 583–631. [DOI] [PubMed] [Google Scholar]
- Champlin, C. A., and McFadden, D. (1989). “Reductions in overshoot following intense sound exposures,” J. Acoust. Soc. Am. 10.1121/1.397853 85, 2005–2011. [DOI] [PubMed] [Google Scholar]
- Elliott, L. L. (1965). “Changes in the simultaneous masked threshold of brief tones,” J. Acoust. Soc. Am. 10.1121/1.1909798 38, 738–746. [DOI] [PubMed] [Google Scholar]
- Ellison, J. C., and Keefe, D. H. (2005). “Audiometric predictions using SFOAE and middle-ear measurements,” Ear Hear. 10.1097/01.aud.0000179692.81851.3b 26, 487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formby, C., Sherlock, L. P., and Green, D. M. (1996). “Evaluation of a maximum likelihood procedure for measuring pure-tone thresholds under computer control,” J. Am. Acad. Audiol. 7, 125–129. [PubMed] [Google Scholar]
- Formby, C., Sherlock, L. P., and Ferguson, S. H. (2000). “Enhancement of the edges of temporal masking functions by complex patterns of overshoot and undershoot,” J. Acoust. Soc. Am. 10.1121/1.428498 107, 2169–2187. [DOI] [PubMed] [Google Scholar]
- Fujino, K., and Oertel, D. (2001). “Cholinergic modulation of stellate cells in the mammalian ventral cochlear nucleus,” J. Neurosci. 21, 7372–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, S. S., and Keefe, D. H. (2006). “Simultaneous measurement of noise-activated middle-ear muscle reflex and stimulus frequency otoacoustic emissions,” J. Assoc. Res. Otolaryngol. 7, 125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, D. (1993). “A maximum-likelihood method for estimating thresholds in a yes-no task,” J. Acoust. Soc. Am. 10.1121/1.406696 93, 2096–2105. [DOI] [PubMed] [Google Scholar]
- Gu, X., and Green, D. M. (1994). “Further studies of a maximum-likelihood yes-no procedure,” J. Acoust. Soc. Am. 10.1121/1.410378 96, 93–101. [DOI] [PubMed] [Google Scholar]
- Guinan, J. J., Jr. (2006). “Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans,” Ear Hear. 10.1097/01.aud.0000240507.83072.e7 27, 589–607. [DOI] [PubMed] [Google Scholar]
- Guinan, J. J., Jr., Backus, B. C., Lilaonitkul, W., and Aharonson, V. (2003). “Medial olivocochlear efferent reflex in humans: Otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs,” J. Assoc. Res. Otolaryngol. 4, 521–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase, T., and Liberman, M. C. (1993). “Antimasking effects of the olivocochlear reflex. I. Enhancement of compound action potentials to masked tones,” J. Neurophysiol. 70, 2519–2532. [DOI] [PubMed] [Google Scholar]
- Kawase, T., Delgutte, B., and Liberman, M. C. (1993). “Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones,” J. Neurophysiol. 70, 2533–2549. [DOI] [PubMed] [Google Scholar]
- Keefe, D. H. (1998). “Double-evoked otoacoustic emissions. I. Measurement theory and nonlinear coherence,” J. Acoust. Soc. Am. 10.1121/1.423057 103, 3489–3498. [DOI] [PubMed] [Google Scholar]
- Keefe, D. H., Schairer, K. S., Ellison, J. C., Fitzpatrick, D. F., and Jesteadt, W. (2005). “Stimulus frequency otoacoustic emissions elicited by transient tones in noise,” Assoc. Res. Otolaryngol. Abstr. 28, 1269. [Google Scholar]
- Keefe, D. H., Schairer, K. S., Ellison, J. C., Fitzpatrick, D. F., and Jesteadt, W. (2006). “Thresholds of behavioral and OAE responses to tones in noise,” American Auditory Society Meeting, Scottsdale, AZ.
- Keefe, D. H., Schairer, K. S., and Jesteadt, W. (2003). “Is there an OAE correlate to behavioral overshoot?” Assoc. Res. Otolaryngol. Abstr. 26, 397. [Google Scholar]
- Konrad-Martin, D., and Keefe, D. H. (2003). “Time-frequency analyses of transient-evoked stimulus-frequency and distortion-product otoacoustic emissions: Testing cochlear model predictions,” J. Acoust. Soc. Am. 10.1121/1.1596170 114, 2021–2043. [DOI] [PubMed] [Google Scholar]
- Konrad-Martin, D., and Keefe, D. H. (2005). “Transient-evoked stimulus-frequency and distortion-product otoacoustic emissions in normal and impaired ears,” J. Acoust. Soc. Am. 10.1121/1.1904403 117, 3799–3815. [DOI] [PubMed] [Google Scholar]
- Leek, M. R., Dubno, J. R., He, N., and Ahlstrom, J. B. (2000). “Experience with a yes-no single-interval maximum-likelihood procedure,” J. Acoust. Soc. Am. 10.1121/1.428653 107, 2674–2684. [DOI] [PubMed] [Google Scholar]
- Liberman, M. C. (1988). “Response properties of cochlear efferent neurons: Monaural vs binaural stimulation and the effects of noise,” J. Neurophysiol. 60, 1779–1798. [DOI] [PubMed] [Google Scholar]
- McFadden, D. (1989). “Spectral differences in the ability of temporal gaps to reset the mechanisms underlying overshoot,” J. Acoust. Soc. Am. 10.1121/1.397732 85, 254–261. [DOI] [PubMed] [Google Scholar]
- McFadden, D., and Champlin, C. (1990). “Reductions in overshoot during aspirin use,” J. Acoust. Soc. Am. 10.1121/1.399056 87, 2634–2642. [DOI] [PubMed] [Google Scholar]
- Murugasu, E., and Russell, I. J. (1996). “The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea,” J. Neurosci. 16, 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol, R. (1994). “Lateral and medial efferents: A double neurochemical mechanism to protect and regulate inner and outer hair cell function in the cochlea,” Br. J. Audiol. 28, 185–191. [DOI] [PubMed] [Google Scholar]
- Schairer, K. S., Ellison, J. C., Fitzpatrick, D., and Keefe, D. H. (2006). “Use of stimulus-frequency otoacoustic emission latency and level to investigate cochlear mechanics in human ears,” J. Acoust. Soc. Am. 10.1121/1.2214147 120, 901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schairer, K. S., Fitzpatrick, D., and Keefe, D. H. (2003). “Input-output functions for stimulus-frequency otoacoustic emissions in normal-hearing adult ears,” J. Acoust. Soc. Am. 10.1121/1.1592799 114, 944–966. [DOI] [PubMed] [Google Scholar]
- Schairer, K. S., and Keefe, D. H. (2005). “Simultaneous recording of stimulus-frequency and distortion-product otoacoustic emission input-output functions in adult ears,” J. Acoust. Soc. Am. 10.1121/1.1850341 117, 818–832. [DOI] [PubMed] [Google Scholar]
- Scharf, B., Magnan, J., and Chays, A. (1997). “On the role of the olivocochlear bundle in hearing: 16 case studies,” Hear. Res. 10.1016/S0378-5955(96)00168-2 103, 101–122. [DOI] [PubMed] [Google Scholar]
- Schmidt, S., and Zwicker, E. (1991). “The effect of masker spectral asymmetry on overshoot in simultaneous masking,” J. Acoust. Soc. Am. 10.1121/1.400656 89, 1324–1330. [DOI] [PubMed] [Google Scholar]
- Shera, C. A., and Guinan, J. J., Jr. (2003). “Stimulus-frequency-emission group delay: A test of coherent reflection filtering and a window on cochlear tuning,” J. Acoust. Soc. Am. 10.1121/1.1557211 113, 2762–72. [DOI] [PubMed] [Google Scholar]
- Smith, D. W., Turner, D. A., and Henson, M. M. (2000). “Psychophysical correlates of contralateral efferent suppression. I. The role of the medial olivocochlear system in ‘central masking’ in nonhuman primates,” J. Acoust. Soc. Am. 10.1121/1.428274 107, 933–941. [DOI] [PubMed] [Google Scholar]
- Smith, R. L., and Zwislocki, J. J. (1975). “Short-term adaptation and incremental responses of single auditory-nerve fibers,” Biol. Cybern. 10.1007/BF00364166 17, 169–182. [DOI] [PubMed] [Google Scholar]
- Souter, M. (1995). “Suppression of stimulus frequency otoacoustic emissions by contralateral noise,” Hear. Res. 91, 167–177. [DOI] [PubMed] [Google Scholar]
- Strickland, E. A. (2001). “The relationship between frequency selectivity and overshoot,” J. Acoust. Soc. Am. 10.1121/1.1357811 109, 2062–2073. [DOI] [PubMed] [Google Scholar]
- Strickland, E. A. (2004). “The temporal effect with notched-noise maskers: Analysis in terms of input-output functions,” J. Acoust. Soc. Am. 10.1121/1.1691036 115, 2234–2245. [DOI] [PubMed] [Google Scholar]
- Strickland, E. A. (2008). “The relationship between precursor level and the temporal effect,” J. Acoust. Soc. Am. 10.1121/1.2821977 123, 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland, E. A., and Krishnan, L. A. (2005). “The temporal effect in listeners with mild to moderate cochlear hearing impairment,” J. Acoust. Soc. Am. 10.1121/1.2074787 118, 3211–3217. [DOI] [PubMed] [Google Scholar]
- Tukey, J. W. (1977). Exploratory Data Analysis (Addison-Wesley, Reading, MA: ). [Google Scholar]
- Turner, C. W., and Doherty, K. A. (1997). “Temporal masking and the ‘active process’ in normal and hearing-impaired listeners,” in Modeling Sensorineural Hearing Loss, edited by Jesteadt W. (Erlbaum, Hillsdale, NJ: ), pp. 387–396. [Google Scholar]
- von Klitzing, R., and Kohlrausch, A. (1994). “Effect of masker level on overshoot in running- and frozen-noise maskers,” J. Acoust. Soc. Am. 10.1121/1.408679 95, 2192–2201. [DOI] [PubMed] [Google Scholar]
- Zeng, F. G., Martino, K. M., Linthicum, F. H., and Soli, S. D. (2000). “Auditory perception in vestibular neurectomy subjects,” Hear. Res. 10.1016/S0378-5955(00)00011-3 142, 102–112. [DOI] [PubMed] [Google Scholar]
- Zwicker, E. (1965). “Temporal effects in simultaneous masking by white-noise bursts,” J. Acoust. Soc. Am. 10.1121/1.1909389 37, 653–663. [DOI] [PubMed] [Google Scholar]