Abstract
Background
Gastric electrical stimulation (GES) is known to improve vomiting with short pulses, normalize dysrhythmia with long pulses, and accelerate gastric emptying with two-channels. The aim of this study was to assess the effects of a new method GES – two-channel GES with dual pulses on gastric emptying of solids as well as gastric dysrhythmia and emetic responses.
Methods
Seven beagle dogs implanted with 4 pairs of electrodes were studied. A novel method of GES was proposed: two-channel dual-pulse GES in which each stimulus was composed of a short pulse followed with a long pulse, and stimulation was delivered at two different locations. The study was performed to test the effects of this new method of GES on vasopressin-induced delayed gastric emptying of solids, gastric dysrhythmia, and emetic responses.
Results
1) Vasopressin induced gastric dysrhythmia and emetic responses, as well as delayed gastric emptying of solids (p<0.01). 2). Two-channel, but not one-channel, dual-pulse GES was able to accelerate vasopressin-induced delayed gastric emptying of solids. 3) Both one- and two-channel dual-pulse GES was capable of improving dysrhythmia and emetic responses (p<0.01).
Conclusions
The novel method of two-channel dual-pulse GES is capable of accelerating gastric emptying of solids and improving dysrhythmia and emetic responses induced by vasopressin. This new method of GES may have a potential for gastroparesis.
Keywords: Gastric electrical stimulation, Gastrointestinal motility, Gastric emptying of solids, Gastric dysrhythmia, Nausea and vomiting, Gastroparesis
Introduction
Gastroparesis is defined as delayed gastric emptying of solids in the absence of mechanical obstruction (1, 2) and has been identified to some extent in 30% to 50% of individuals with diabetes (3–5). Patients with this condition usually present with nausea, vomiting, abdominal discomfort, and early satiety that affect quality of life adversely. In addition, gastric dysrhythmia is observed frequently in individuals with gastroparesis. Treatment options for diabetic gastroparesis are limited in the USA and include medical therapy, operative therapy, and nutritional support. The main therapy for gastroparesis is the use of prokinetic agents to enhance gastrointestinal motility (6–11) but often ineffective for gastroparesis. To date, none of the available therapies, including operative treatment, is completely satisfactory, and there is a need to develop safe and effective methods to treat patients with severe gastroparesis.
Recently, the therapeutic potential of gastric electrical stimulation (GES) for gastroparesis has been explored. A number of studies have investigated the effect of GES on gastric myoelectric activity, gastric motility, and gastrointestinal symptoms in dogs and humans (12–24). According to the type of stimuli applied, GES can be classified into a) long pulse stimulation (pulse width in the order of ms, stimulation frequency slightly higher than the physiologic frequency of gastric electrical activity) and b) short pulse stimulation (pulse width in the order of μs, stimulation frequency 4 times the physiologic frequency). According to its stimulation sites, GES includes one-channel stimulation and multi-channel stimulation (via one or multiple pairs of electrodes implanted on the serosal surface of the stomach, respectively). Long-pulse stimulation is able to normalize dysrhythmias and entrain slow waves (18), short-pulse stimulation is capable of preventing symptoms of nausea and vomiting (21–23), and two-channel GES is known to accelerate gastric emptying (24). None of the previously reported methods of GES possesses all of the following three capabilities: acceleration of gastric emptying, amelioration of emetic responses, and normalization of gastric dysrhythmia. These three capabilities are important for GES because patients with gastroparesis often have all these three abnormalities: delayed gastric emptying of solids, gastric dysrhythmia and emesis.
In this study, we proposed a novel method of GES: two-channel dual-pulse GES. This technique involved the use of a stimulus composed of both short and long pulses and two pairs of stimulation electrodes along the greater curvature of the stomach. We hypothesized that this new method GES would possess all of the above mentioned three capacities and would therefore be able to improve simultaneously gastric emptying of solids (via two-channel stimulation), symptoms of gastroparesis (via short pulses), and gastric dysrhythmia (via long pulses).
The aim of this study was to investigate the efficacy of the proposed novel method of GES on vasopressin-induced delayed gastric emptying of solids, emetic responses, and gastric dysrhythmia in dogs.
Materials and Methods
Animal Preparation
Seven healthy female beagle dogs (14–21 kg) were used for the study. After an overnight fast, anesthesia was induced and maintained on pentothal (2% sodium thiopental 0.6 ml/kg, intravenous). During the operation, the dogs were monitored by assessment of tongue color, pulse rate, and breathing rate. Four pairs of 28-gauge cardiac pacing wires were implanted on the serosal surface of the stomach at an interval of 4 cm. The distance between two electrodes in each pair was 0.5 cm. The pacing wires were arranged in an arching line along the greater curvature from the corpus to the pylorus. The most distal pair of electrodes was 2 cm proximal to the pylorus. Using nonabsorbable sutures, the electrodes were sutured to the seromuscular layer without penetrating the gastric lumen. The electrode wires were tunneled subcutaneously through the anterior abdominal wall along the right side of the trunk and placed outside the skin around the hypochodrium for the attachment to the recorder or the stimulator. At the completion of the operation, the dogs were given bupromorphine for postoperative pain control and intravenous balance electrolyte solutions for blood loss, and then transferred to a recovery cage. The study was initiated after the dogs had recovered completely from the operation, usually 2 weeks later. The Animal Care and Use Committee of Union Hospital of Tongji Medical College approved the surgical and experimental protocols.
Experimental Protocol
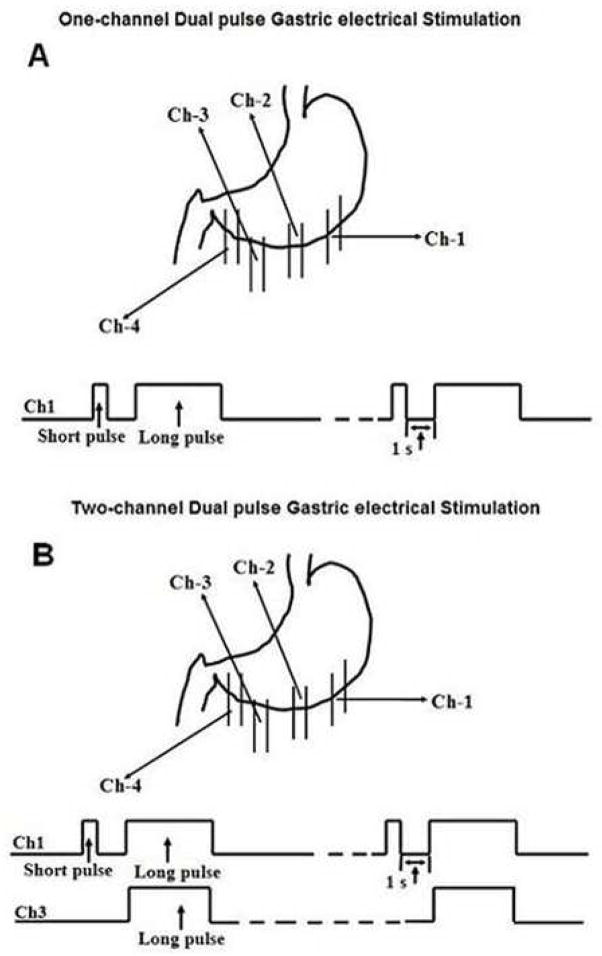
The study was performed after an overnight fast in four sessions (Saline; Vasopressin; Single-channel dual-pulse stimulation (DPS); Two-channel DPS) on four separate days with an interval of three days or more. The order of the four sessions was randomized. Each session consisted of 280-min: a 40-min period in the fasting state and a 240-min postprandial period immediately after a solid test meal labeled with isotopes. In the saline session, there was a 20-min baseline recording of gastric myoelectric activity and a 20-min recording with sham GES in the fasting state. Then the solid test meal was ingested and 154mM NaCl was infused intravenously during the first 20-min postprandial period; gastric emptying was monitored for a period of 4 h (Fig. 1). The protocol of the vasopressin session was the same as the saline session except the replacement of saline with vasopressin. Vasopressin (0.5u/kg, in 20ml of 154mM NaCl) was infused at a rate of 1ml/min with an infusion pump (KD, Scientific, INC. Boston, MA) via a 20-gauge intravenous catheter placed in the antecubital vein of the body during the second 20-min period. The protocol of one-channel or two-channel DPS session was the same as the vasopressin session except that one of the two kinds of GES was applied continuously after the 20-min baseline recording. In the one-channel or two-channel DPS session, GES was performed continuously via the 1st pair (14cm proximal to the pylorus) for one-channel DPS or both 1st pair and 3rd pair of electrodes (6cm proximal to the pylorus). The behaviors of the dogs during the 20-min vasopressin infusion period in each session were recorded.
Figure 1.
Gastric Electrical Stimulation
The stimulus, generated by a universal stimulator (WPI, Sarasota, Florida), was delivered via the 1st pair of electrodes (14cm proximal to the pylorus) for single-channel DPS and composed of a short pulse of 0.3ms followed with a long pulse with a pulse width of 500ms and amplitude of 4mA, repeated at 6 cycles/min. For two channel DPS, electrical stimulation was applied to both the 1st pair (dual pulses of 0.3ms and 200ms, amplitude of 1mA and frequency of 6cpm) and the 3rd pair of electrodes (6cm proximal to the pylorus, long pulses with a pulse width of 200ms, 0.6mA, 6 pulses/min) (Fig. 2). These parameters were selected based on a previous study (24).
Figure 2.
Gastric Emptying
Scintigraphic scanning was used to measure gastric emptying of solids. The test meal was composed of a scrambled egg and 375 g solid food (370 calories). One mCi of 99Tc labeled sulfur colloid was mixed with the egg, and the mixture was cooked in a microwave oven for 2 min and stirred once during cooking. This isotope-labeled solid meal was consumed within 10 min after the preparation. Anterior images were acquired at 0, 15, 30, 45, 60, 90, 120, 180 and 240 min after the meal. The region of interest was drawn around the stomach on the anterior and posterior images for each frame; a semi-auto-analysis system was used to calculate the emptying of the stomach by computer methodology (25).
Recording and Analysis of Gastric Myoelectric Activity
A multi-channel recorder (AcqknowledgeIII, EOG 100A, Biopac System, Inc. Santa Barbara, CA) was used to record gastric myoelectric activity via all available electrodes not used for stimulation during the entire study. The signals (4 channels at baseline, and 2 or 3 channels during GES) were displayed on a computer monitor and saved on the hard disk by an IBM-compatible 486PC. The low and high cutoff frequencies of the amplifier were 0.05Hz and 35 Hz, respectively. For the analysis of gastric slow waves, the signals were further lowpass filtered with a cutoff frequency of 1 Hz and down-sampled at 2 Hz. Previously validated computerized spectral analyses were performed to derive the percentage of normal gastric slow waves from the recordings (18). The myoelectric recordings obtained from the most distal pair of electrodes were used for the computation of the following parameter.
The percentage of normal gastric slow waves was defined as the percentage of time during which regular 4–6 cpm slow waves were presented over a specific analyzed period. It was computed using the adaptive spectral analysis method (18). In this method, each recording was divided into blocks of 1 min without overlapping. The power spectrum of each 1-min recording was calculated and examined to see if the peak power was within the range of 4–6 cpm. The 1-min recording was called normal if the peak power was within the 4–6 cpm range. Otherwise it was defined as dysrhythmia. The definition of normal slow wave frequency range (4–6 cpm) was based on a previous study (19, 20).
Assessment of motion sickness-like signs
Animal behaviors/signs were observed and noted during the period of vasopressin infusion in the fasting state with or without GES, and included tongue licking, closing eyes, yawning, belching, murmuring, movement, and rapid breathing. These signs were assessed based on their severity and/or frequency (0: never; 1: seldom; 2: often; 3: continues or intolerable). Vomiting was scored differently from other signs and was scored 3, 4 or 5 if the dog vomited 1, 2 or 3 times. The total symptom score was computed from each animal (18, 24, 27). To make the evaluation more objective, the person who evaluated the signs was blinded from the study design and objectives.
Statistical Analysis
All values are expressed as mean±SEM. The analysis of variance (ANOVA) was applied to investigate the effects of GES or DPS on gastric slow waves, animal behaviors, and gastric emptying. Paired comparison was performed using the Student’s t-test only if the ANOVA showed a significant difference. The episode of vomiting during vasopressin infusion with and without stimulation was compared using the Chi-square test. P<0.05 was considered significant.
Results
Effects of two-channel DPS on vasopressin-induced delayed gastric emptying of solids
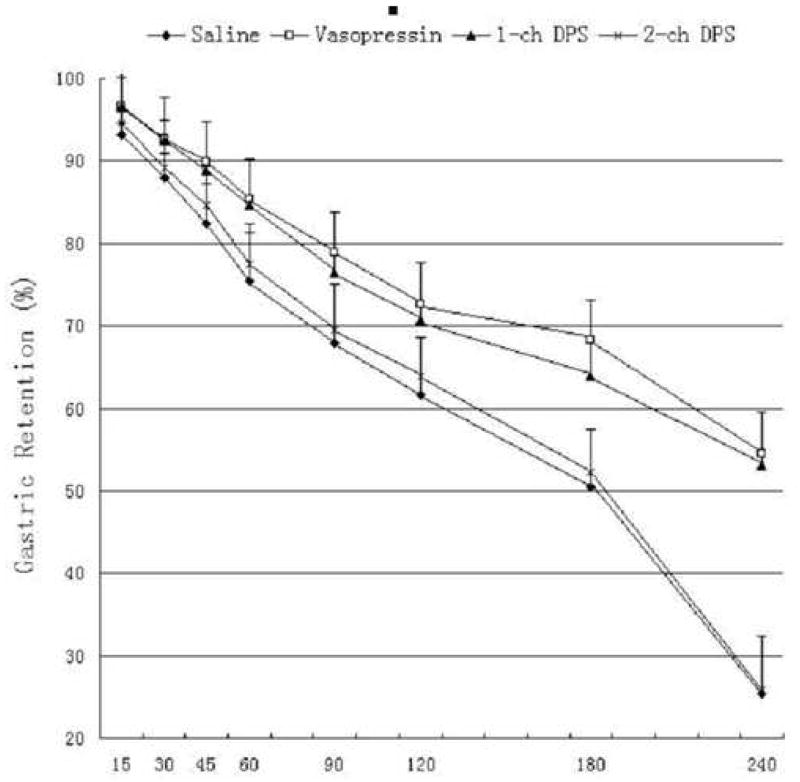
Vasopressin delayed gastric emptying. Two-channel, but not one-channel, DPS improved vasopressin-induced delay in gastric emptying (P<0.05, ANOVA). As shown in Fig. 3, the mean gastric retention at 60 min was increased from 75±4 % with saline to 85±3 % with vasopressin, and decreased to 78±6 % with two-channel DPS. Similar findings were also noted at 240 min (25±11 % with saline, 55±5 % with vasopressin and 26 ± 18 % with two-channel DPS). The mean half-time (T50) of gastric emptying was 180 ± 26 min with saline, increased to 269±44 min with vasopressin (P=0.0015 vs. the saline session) and decreased to 183 ± 46 min with two-channel DPS (P=0.011 vs. the vasopressin session without DPS), but was not altered with one-channel DPS (261±31 min, P>0.05 vs. the vasopressin session without DPS). It can also be seen from Fig. 3, there was no obvious lag time in gastric emptying, and the emptying of the stomach was close to linear in all four sessions. The rate (or slope of the emptying curve) of gastric emptying was about 0.6 per minute in the vasopressin session and the one-channel DPS session, and about 0.9 per minute in the control session and the two-channel DPS session.
Figure 3.
Effect of two-channel DPS on vasopressin-induced dysrhythmia
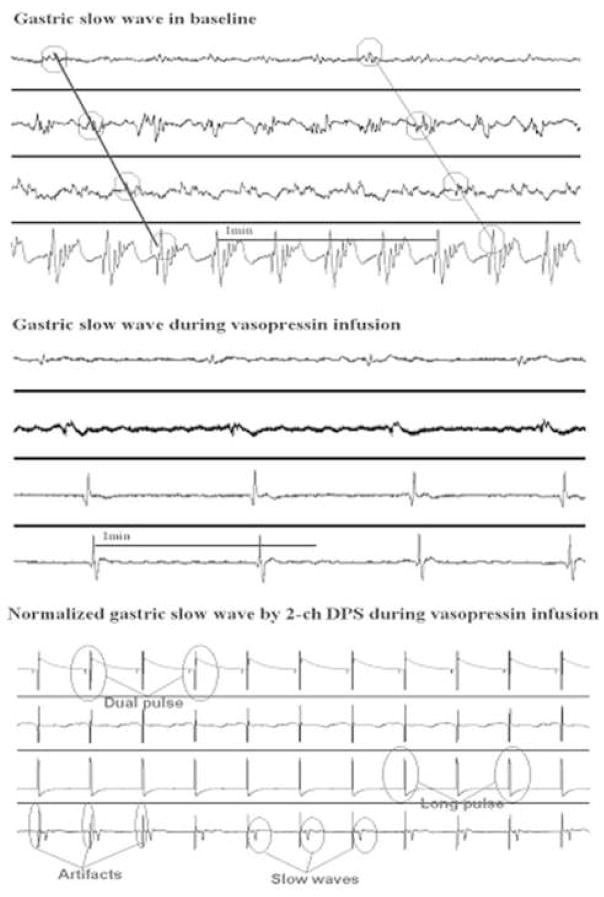
Vasopressin induced gastric dysrhythmia (P<0.01) (Fig. 4). In the saline session, the percentage of regular 4- to 6-cpm slow waves in the antrum (distal channel) was 98±1% at baseline (20-min recording before infusion of saline) and 96±2% during 20-min saline infusion. In the vasopressin session, the percentage of regular slow waves (4–6cpm) in the antrum was 97±2% at baseline and decreased to 41±8% during the 20-min infusion of vasopressin (P<0.01). Figure 4 represented typical tracings of regular gastric slow waves at baseline and irregular slow waves during the infusion of vasopressin.
Figure 4.
Both one- and two- channel DPS normalized gastric dysrhythmia (P<0.01, ANOVA). In the one- and two-channel DPS session, the percentage of regular 4- to 6-cpm slow waves was 98±1% and 99±1% at baseline and 75±6% and 79±5% during vasopressin infusion with GES (p<0.01 vs. the corresponding period in the vasopressin session without GES). The normalization of gastric dysrhythmia was attributed to complete or partial entrainment of gastric slow waves with GES. As shown in Figure 4, the gastric slow waves were phase-locked with the stimuli.
Effects of two-channel DPS on vasopressin-induced motion sickness-like signs
Two-channel DPS improved emetic responses induced by vasopressin (P<0.01, ANOVA). In the vasopressin session, all dogs had signs of discomfort or emesis, and the average total symptom score was 6.3±2.1, which was greater than that during saline infusion (1.0±0.3, p<0.001), but suppressed by one-channel DPS (3.9±1.8, P=0.043) as well two-channel DPS (3.3±1.4, P=0.01). The average episode of vomiting was 6/7 with during vasopressin infusion and reduced to 0 with two-channel DPS (P<0.001).
Discussion
GES has been under investigation for its therapeutic potential for gastroparesis. Patients with gastroparesis complain of symptoms of nausea and vomiting and the effective treatment of these symptoms is of great clinical significance. Delayed gastric emptying and gastric dysrhythmia, although often disassociated with symptoms, are two of major pathophysiologic factors involved in gastroparesis. Accordingly, an effective therapy for gastroparesis should not only ameliorate symptoms but also improve delayed gastric emptying of solids and gastric dysrhythmia.
Various methods of GES have been proposed and their effects on gastric emptying, gastric dysrhythmia and emesis have been investigated (13–27). GES with short pulses improves nausea and vomiting in both animals and humans. The most widely applied method of short pulse GES is called the Enterra® Therapy (Medtronic, Inc., Minneapolis, MN, USA). A number of clinical studies have demonstrated the anti-emetic effect of this method in patients with gastroparesis (21–23); however, short pulse GES does not have any direct effects on gastric emptying or gastric slow waves (18). Long pulse GES was studied intensively (16) and has been shown to pace the stomach, i.e., entrain gastric slow waves or normalize gastric dysrhythmia (15, 17, 26). Long pulse was also shown to improve gastric emptying in a canine model of gastroparesis and patients with gastroparesis (13, 17). None of previous studies have shown a prokinetic effect of long pulse GES in healthy dogs. Recently, a method of multi-channel GES has been proposed (19, 24). Four-channel GES accelerated gastric emptying of liquids in healthy dogs (19) and two-channel GES normalized vasopressin-induced delayed gastric emptying of liquids (24). Nevertheless, none of existing methods of GES are capable of simultaneously accelerating gastric emptying of solids, improving emesis, and normalizing gastric dysrhythmia.
The two-channel dual pulse GES proposed in this study is a combination of three methods: short pulse GES, long pulse GES, and two-channel GES. This novel method of GES was expected to accelerate gastric emptying (via two-channel stimulation), reduce emetic responses (via short pulses), and improve gastric dysrhythmia (via long pulses). Indeed, the results presented in this study demonstrated these capabilities. As shown in Fig. 3, vasopressin-induced delayed gastric emptying of solids was normalized with this new method of GES as the rate of gastric emptying and gastric retention at different time points were similar to those in the control session. This study is the first to show an improvement in pharmacologically delayed gastric emptying of solids with GES in healthy dogs. Mechanisms involved in the improvement of gastric emptying of solids with the proposed method of GES were not clear. One possible mechanism may involve the improvement in gastric slow waves with the stimulation. This explanation may play only a partial role as one-channel dual pulse GES also improved vasopressin-induced dysrhythmia but did not accelerate gastric emptying. The other possible mechanism might be the improved coordination of slow waves with the two-channel stimulation.
Improvement in emetic responses was also noted with the proposed method of two-channel dual pulse GES. The improvement was believed to be attributed to the component of short pulses in the proposed stimuli. None of previous methods of GES without short pulses showed any improvement in emetic responses in dogs (18, 20, 24). The finding and interpretation of the current study were also in agreement with the clinical studies with the Enterra® therapy (21–23). Mechanisms involved in the improvement of emetic responses with short pulse GES are not completely understood. Previous studies in dogs and rats have suggested the involvement of the vagal pathway as vagotomy or blockage of the vagal afferents was reported to abolish the anti-emetic effect of short pulse GES (18, 28).
The acceleration of gastric emptying, normalization of gastric dysrhythmia, and suppression of the emetic responses with the proposed method of two-channel dual pulse GES suggest its therapeutic potential for treating gastric motility disorders, such as gastroparesis. The risk involved in this novel method is low and the procedure (placement of electrodes and stimulator) can be performed relatively easily via a laparoscopic approach. The patient can be discharged on the same day without hospital stay. These suggest a high benefit-risk ratio. Though this study was focused on a pharmacologically induced gastric motility disorder, the same methodology may apply for the treatment of other motor disorders of the stomach such as idiopathic, diabetic, or postvagotomy gastroparesis but no data are available as yet in these disorders.
In conclusion, two-channel dual pulse GES not only normalizes delayed gastric emptying of solids and improves gastric dysrhythmia but also improves emetic responses induced by vasopressin. This novel method of GES may have a good potential for gastroparesis.
Acknowledgments
This study was partially support by grants from NIH (DK066709 and DK063733).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The American Gastroenterological Association Clinical Practice Committee. American Gastroenterological Association Technical Review on the Diagnosis and Treatment of Gastroparesis. Gastroenterology. 2004;127:1592–1622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 2.Hornbuckle K, Barnett JL. The diagnosis and work-up of the patient with gastroparesis. J Clin Gastroenterol. 2000;30:117–24. doi: 10.1097/00004836-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Horowitz M, O’Donovan D, Jones KL, Feinle C, Rayner CK, Samsom M. Gastric emptying in diabetes: clinical significance and treatment. Diabetic Medicine. 2002;19(3):177–94. doi: 10.1046/j.1464-5491.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz M, Edelbroek M, Fraser R, Maddox A, Wishart J. Disordered gastric motor function in diabetes mellitus. Recent insights into prevalence, pathophysiology, clinical relevance, and treatment Scand J Gastroenterol. 1991;26:673–684. doi: 10.3109/00365529108998583. [DOI] [PubMed] [Google Scholar]
- 5.Zitomer BR, Gramm HF, Kozak GP. Gastric neuropathy in diabetes mellitus: Clinical and radiographic observations. Metabolism. 1968;17:199–211. doi: 10.1016/0026-0495(68)90123-6. [DOI] [PubMed] [Google Scholar]
- 6.McCallum RW. Cisapride: a new class of prokinetic agent. The ACG committee on FDA-related matters. American College of Gastroenterology Am J Gastroenterol. 1991;86(2):135–49. [PubMed] [Google Scholar]
- 7.McCallum RW, George SJ. Gastric Dysmotility and Gastroparesis. Curr Treat Options Gastroenterol. 2001;4(2):179–191. doi: 10.1007/s11938-001-0030-6. [DOI] [PubMed] [Google Scholar]
- 8.Koch KL, Stern RM, Stewart WP, Vasey MW. Gastric emptying and gastric myoelectric activity in patients with diabetic gastroparesis: effect of long-term domperidone treatment. Am J Gastroenterol. 1998;84:1069–75. [PubMed] [Google Scholar]
- 9.Riezzo G, Cucchiara S, Chiloiro M, Minella R, Guerra V, Giorgio I. Gastric emptying and myoelectric activity in children with non-ulcer dyspepsia: effect of cisapride. Dig Dis Sci. 1995;40:1428–34. doi: 10.1007/BF02285188. [DOI] [PubMed] [Google Scholar]
- 10.Rothstein RD, Alavi A, Reynolds JC. Electrogastrography in patients with gastroparesis and effect of long-time cisapride. Dig Dis Sci. 1993;38:1518–24. doi: 10.1007/BF01308614. [DOI] [PubMed] [Google Scholar]
- 11.Chen JDZ, Ke MY, Lin XM, Wang Z, Zhang M. Cisapride provides symptomatic relief in functional dyspepsia associated with gastric myoelectric abnormalities. Aliment Pharmacol Ther. 2000;14:1041–7. doi: 10.1046/j.1365-2036.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Lin ZY, Yi XB, McCallum RW. Abnormal electrogastrogram predicts delayed gastric emptying. Am J Gastroenterol. 1993;88:1504. [Google Scholar]
- 13.Bellahsène B-E, Lind CD, Schirmer BD, Updike OL, McCallum RW. Acceleration of gastric emptying with electrical stimulation in a canine model of gastroparesis. Am J Physiol. 1992;262:G826–G834. doi: 10.1152/ajpgi.1992.262.5.G826. [DOI] [PubMed] [Google Scholar]
- 14.Eagon JC, Kelly KA. Gastric pacing reverses canine peristalsis, slows emptying, and strengthens contractions. Am J Surg. 1993;163:628. [Google Scholar]
- 15.Hocking MP, Vogel SB, Sninsky CA. Human gastric myoelectric activity and gastric emptying following gastric surgery and with pacing. Gastroenterology. 1992;103:1811–1816. doi: 10.1016/0016-5085(92)91439-b. [DOI] [PubMed] [Google Scholar]
- 16.Miedema BW, Sarr MG, Kelly KA. Pacing the human stomach. Surgery. 1992;111(2):143–50. [PubMed] [Google Scholar]
- 17.McCallum RW, Chen JDZ, Lin ZY, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterol. 1998;114:456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 18.Chen JDZ, Qian LW, Ouyang H, Yin JY. Gastric electrical stimulation with short pulses improves vomiting but not gastric dysrhythmia in dogs. Gastroenterology. 2003;124:401–409. doi: 10.1053/gast.2003.50048. [DOI] [PubMed] [Google Scholar]
- 19.Chen ZJD, Xu X, Zhang J, Abo M, Lin X, Mccallum RW, Ross B. Efficiency and efficacy of multi-channel gastric electrical stimulation. Neurogastroenterol Motil. 2005;17:1–5. doi: 10.1111/j.1365-2982.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Brining DL, Chen JD. Effects of vasopressin and long pulse-low frequency gastric electrical stimulation on gastric emptying, gastric and intestinal myoelectric activity and symptoms in dogs. Neurogastroenterol Motil. 2005;17(2):236–44. doi: 10.1111/j.1365-2982.2004.00616.x. [DOI] [PubMed] [Google Scholar]
- 21.Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–28. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 22.Abell T, Lou J, Tabbaa M, Batista O, Malinowski S, Al-Juburi A. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27(4):277–81. doi: 10.1177/0148607103027004277. [DOI] [PubMed] [Google Scholar]
- 23.Forster J, Sarosiek I, Delcore R, Lin Z, Raju GS, McCallum RW. Gastric pacing is a new surgical treatment for gastroparesis. Am J Surg. 2001;182:676–681. doi: 10.1016/s0002-9610(01)00802-9. [DOI] [PubMed] [Google Scholar]
- 24.Song GQ, Hou XH, Yang B, Liu JS, Qian W, Chen JDZ. Two-channel gastric electrical stimulation accelerates delayed gastric emptying induced by vasopressin. Dig Dis Sci. 2005;50(4):662–668. doi: 10.1007/s10620-005-2553-5. [DOI] [PubMed] [Google Scholar]
- 25.Iwanaga Y, Wen J, Thollander MS, Kost LJ, Thomforde GM, Allen RG, Phillips SF. Scintigraphic measurement of regional gastrointestinal transit in the dog. Am J Physiol. 1998;275(5 Pt 1):G904–10. doi: 10.1152/ajpgi.1998.275.5.G904. [DOI] [PubMed] [Google Scholar]
- 26.Lin ZY, McCallum RW, Schirmer BD, Chen JDZ. Effects of pacing parameters in the entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol (Gastrointest Liver Physiol) 1998;37:G186–191. doi: 10.1152/ajpgi.1998.274.1.G186. [DOI] [PubMed] [Google Scholar]
- 27.Song GQ, Hou XH, Yang B, Sun Y, Liu JS, Qian W, Chen JDZ. Efficacy and Efficiency of Gastric Electrical Stimulation with Short Pulses in the Treatment of Vasopressin-induced Emetic Responses in Dogs. Neurogastroenterol Motil. 2006;18:385–391. doi: 10.1111/j.1365-2982.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Qiao X, Chen JDZ. Vagal Afferent Is Involved in Short-Pulse Gastric Electrical Stimulation in Rats. Dig Dis Sci. 2004;49(5):729–737. doi: 10.1023/b:ddas.0000030081.91006.86. [DOI] [PubMed] [Google Scholar]