Abstract
Utilization of adult stem cells in regenerative therapies may require a thorough understanding of the mechanisms that establish, recruit and renew the stem cell, promote the differentiation of its daughters, or how the stem cell is repressed by its target tissue. Regeneration of melanocytes in the regenerating zebrafish caudal fin, or following larval melanocyte-specific ablation, or recruitment of new melanocytes during pigment pattern metamorphosis each provide evidence for melanocyte stem cells (MSCs) that support the melanocyte pigment pattern. We discuss the mechanisms of MSC regulation provided from analysis of normal or mutant regeneration in each of these systems, including the implications drawn from evidence that regeneration does not simply recapitulate ontogenetic development. These results suggest that analysis of melanocyte regeneration in zebrafish will provide a fine scale dissection of mechanisms establishing or regulating adult stem cells.
Keywords: Regeneration, Melanocyte Stem Cell (MSC), zebrafish, kit
Introduction
In developmental biology, we tend to use the term stem cell to describe cells with one or both of two features; the ability to produce multiple types of daughter cells (pluripotentiality), and the property of self renewal. For instance, the inner cell mass (ICM) of the blastula are a stem cell population for generating the diverse cell types of the embryo. These cells likely do not self renew during embryogenesis, but instead are consumed by the act of development. ICM cells can be coaxed to self renew in the petri-dish, an achievement that results in the laboratory embryonic stem cell (1). In contrast, cells of the skin, gut epithelium, and blood, tissues that are constantly turned over (physiological regeneration), are maintained by stem cells with limited potential to produce other cell types, but sufficient powers of self-renewal to maintain their target tissues through many decades of turnover. The different stem cells that maintain specific cells or tissues are collectively called adult stem cells. One such adult stem cell is the melanocyte stem cell (MSC). The existence of the MSC can be inferred from physiological regeneration of the mammalian hair follicle. At the end of each hair follicle cycle, matrix keratinocytes and the melanocytes that donate pigment to keratinocytes of the growing hair are shed, suggesting adult stem cell are responsible for generating both new keratinocytes and new melanocytes for the next hair follicle cycle (2). Use of the dct:lacZ transgenic mouse (3) together with BrdU incorporation studies provided direct evidence of the MSC in the bulge region of the mammalian hair follicle (4). A variety of human depigmenting syndromes, including vitiligo and age-associated graying, are the result of loss of MSCs from the hair follicle (5).
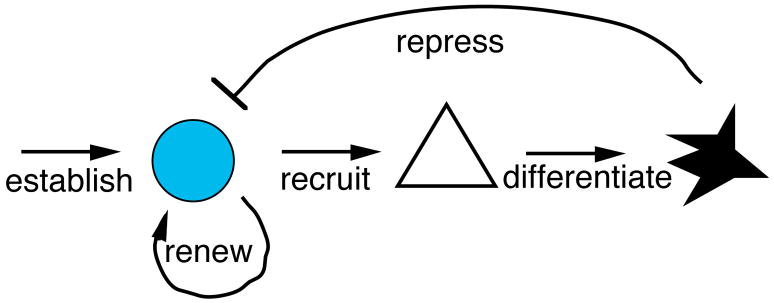
The goal of studying melanocyte regeneration in zebrafish is to understand the mechanisms underlying regulation of adult stem cells in general, or melanocyte stem cells in particular. More and more evidence is accumulating for the existence of a variety of different adult stem cells in mammals, but the nature of the mechanisms that regulate them, and can then be exploited to regenerate tissues for therapeutic purposes lags behind. The hope is that simple paradigms for ablating melanocytes and then challenging their ability to regenerate in zebrafish will lead to the identification of mutations or of drugs that affect the regulation of the melanocyte stem cell, that can then translate into applications for manipulating a variety of stem cells in therapeutic applications. We outline in Figure 1 some of the mechanisms that can be expected to be involved in adult stem cell regulation, including the embryonic mechanisms that establish the stem cells, that recruit the stem cell to produce new cells in response to growth or injury, and then differentiate them. It also seems likely that the stem cells are repressed by intact target cells or tissues. Finally, stem cells renew themselves when they generate new cells for development. However, unlike in mouse (4), the zebrafish melanocyte stem cell (MSC) has not been directly identified, and the inferences that we draw about the zebrafish melanocyte stem cell come indirectly from the analysis of their differentiated daughters, the melanocytes, following regeneration or pigment pattern metamorphosis. In this review, we discuss several examples of regenerating melanocytes that shed light on one or more of the mechanisms involved in adult stem cell regulation.
Figure 1.
Mechanisms utilized in Melanocyte Stem Cell regulation. This schematic shows different types of mechanisms that are likely involved regulating MSCs during regeneration experiments. Blue circle represents the MSC, triangle represents melanoblast intermediate, and the black cell represents a mature melanocyte.
In addition to describing the inferences that are drawn from these experiments, we also describe some of the outstanding questions that arise from these studies. It will then be interesting to see in the next few years whether the tools will be developed to allow these questions to be answered.
(1) Two populations of melanocytes in the regenerating fin
Fin regeneration in zebrafish was first described by Goodrich and his colleagues (6) in his studies to use re-establishment of pigment patterns in the regenerating fin to provide a different perspective on the mechanism responsible for the patterning of the melanocyte stripes. Those experiments are summarized in Figure 2. Briefly, within 4 days after amputation (converted to standard regeneration stages at 25 degrees) melanocytes are observed scattered throughout the regenerate. By 7–8 days, most of the melanocytes in the proximal, or developmentally oldest part of the regenerate, are now arranged in distinct stripes, and scattered melanocytes outside the presumptive stripe are only found in the most distal, or developmentally youngest region of the regenerate. This pattern of melanocytes in the regenerating fin persists until several weeks after amputation, at which time regeneration is largely completed and no melanocytes are found in the distal, presumptive interstripe regions of the regenerate. These studies suggested that the distal, scattered melanocytes are also developmentally younger, and that maturation of the regenerate then leads melanocytes in interstripe regions to either die, or migrate into stripe regions. These studies of Goodrich, together with more modern tools and the availability of mutants that affect pigment pattern in zebrafish later led to the studies of Rawls and Johnson (7,8) to explore the origins and genetic control of development of melanocytes in the regenerating fin.
Figure 2.
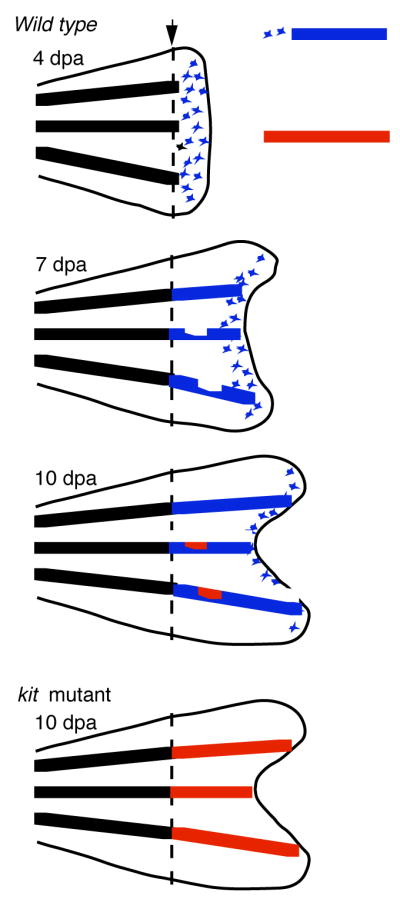
Two types of melanocytes contribute to the regenerating stripe in the regenerating zebrafish caudal fin. Schematic shows that primary regeneration melanocytes (in blue) first develop in scattered locations in distal or developmentally youngest part of fin. Secondary, or regulatory melanocytes (in red), develop in more proximal or developmentally older parts of the regenerate, in response to deficits in the primary regeneration melanocyte stripe. In kit mutants, that lack primary regeneration melanocytes, secondary regulatory melanocytes account for the entire population of regeneration melanocytes.
In principle, without evoking exotic models such as transdifferentiation or re-establishment of stem cells, regenerating melanocytes can come from one of two sources: cell division from existing, pigmented melanocytes, or recruitment of melanocyte precursors or stem cells to re-enter developmental pathways, reenter the cell cycle, and produce new melanocytes de novo. For instance, the first model, whereby differentiated cells re-enter the cell cycle and simply divide to grow new cells seems to be the case for regeneration of the bone or lepidotrichia in regenerating zebrafish fin, as revealed by BrdU incorporation or by lineage analysis of mosaic clones of transposons expressing GFP under strong ubiquitous promoters (H. Huang and S. Johnson, unpublished). In contrast, the second model seems to hold for hair cell regeneration in the lateral line, whereby supporting glial cells, that seem to serve as the stem cell for the neuromast, divide to produce new hair cells following neomycin induced hair cell ablation (9). Which of these models hold for melanocyte regeneration in the regenerating fin was solved by using the pre-existing melanin in differentiated melanocytes as a lineage marker, and preventing new melanin synthesis in any newly differentiated melanocytes by incubating fish in a melanin synthesis inhibitor, phenylthiourea (PTU). The finding that preexisting melanin is not partitioned to the vast majority of melanocytes that are found in the regenerating fin suggested that regeneration melanocytes develop de novo from undifferentiated precursors, rather than by division of differentiated, pigmented cells (7).
The existence of two distinct populations of regeneration melanocytes in the regenerating fin is suggested by the kinetics of melanocyte appearance in the kit mutant regenerates. In contrast to regeneration in the wild type fin, that produces de novo melanocytes throughout the regenerate beginning at 4 days, and then restricted to distal, developmentally younger tissue at 7 or 10 days following amputation, kit mutants have no new melanocytes in the regenerate at 4 or 7 days (8). However, by 8–10 days kit mutants begin to develop new melanocytes. In contrast to wild type fish, that develop melanocytes throughout the distal, or youngest, area of the regenerate, melanocytes in the kit mutant first appear restricted to the presumptive stripe, in the developmentally oldest regions of the regenerate (8). This finding reveals that there are two distinct populations of melanocytes that contribute to regeneration. First, an early, primary population develops throughout the newest, or distal, portion of the regenerate. The development of this population is promoted by the kit receptor tyrosine kinase. Lacking kit function, a second, regulatory population of melanocytes develops. This population appears after a delay of approximately 4 days, in the proximal or developmentally older part of the regenerate, and only in the presumptive stripe. This population plays little or no role in normal stripe regeneration, except perhaps to fill in gaps or holes left by the primary population. However, in the absence of the kit-dependent primary population, such as in kit mutants, the secondary or regulatory population generates as many melanocytes as does the wild-type regenerate (8).
In situ expression analysis revealed that kit transcript is upregulated in cells near the amputation plane as early as 1.5 days post amputation (dpa), suggesting an early role for kit in recruiting stem cells back into regeneration pathways (8). However, it remained possible that kit function was also required to establish these stem cells during embryonic development. To address this question, Rawls and Johnson (2001) isolated a temperature-sensitive allele of kit (8). Reciprocal shifts from embryonic stages through stages after amputation revealed that kit was not required for establishing the melanocyte stem cells during embryonic development, but rather was required beginning two days after amputation. Thus, the role for kit in fin melanocyte regeneration is not in establishing the stem cell population, but instead to recruit the stem cells back into development, or in some early stage of melanocyte differentiation (8).
The finding that fin melanocytes develop by two different pathways, one dependent on kit function, and a second regulatory pathway that produces melanocytes independent of kit, raises several important issues in adult stem cell regulation. One question is: how do stem cells know when they have generated sufficient cells to repair the missing tissue? The MSCs of the regenerating fin seems to have solved this problem by using two distinct mechanisms. First, dependent on kit function, the stem cells generate excess melanocytes that are distributed throughout the distal, or developmentally youngest, part of the regenerate. Rather than responding to loss of repression from pre-existing melanocytes, the primary melanocytes seem to respond positively to a fin-wide regeneration signal, that then generates excess melanocytes, which are then pruned down to the numbers needed for the appropriate stripe. A compelling question is: what is the nature of this positive signal that recruits stem cells into the primary regeneration pathway, and is this the same signal used to evoke regeneration of other tissues in the regenerating fin?
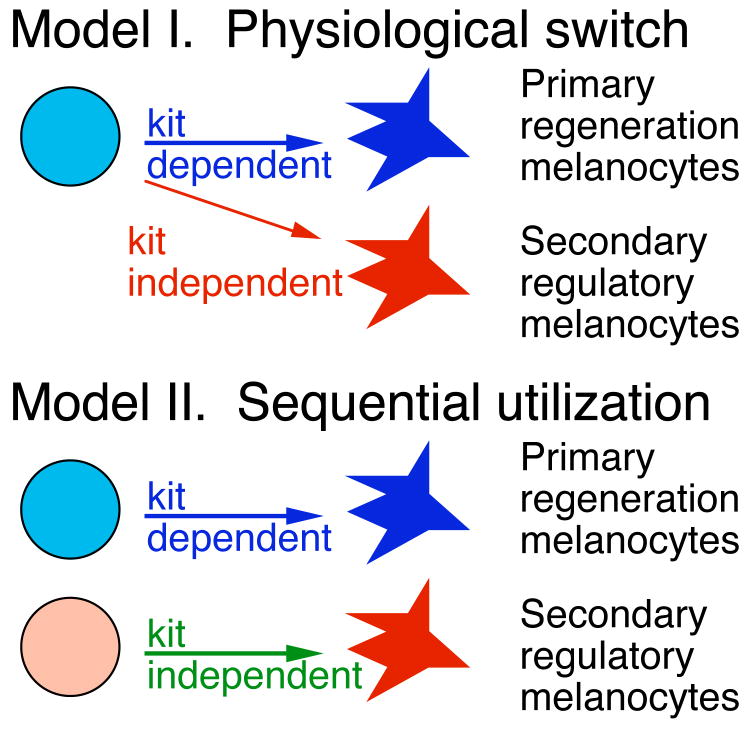
In contrast to the primary, kit-dependent melanocytes, the second, kit-independent population of melanocytes seems exquisitely sensitive to the presence of melanocytes, and appears to generate new melanocytes only to fill in holes or gaps in the stripe pattern after the primary population has done its best. A question raised by these two different types of melanocytes is whether they arise from the same stem cells, that change physiology of their response as they come to reside in developmentally older tissue, or instead identify two distinct populations of stem cells that fulfill the different roles of first producing an excess of melanocytes, and then filling in the holes and gaps left by the first mechanism. These different models are outlined in Figure 3.
Figure 3.
Models proposing roles for one or two types of MSCs to account for different melanocyte populations. In the physiological switch model, the MSC first generates primary regeneration melanocytes by kit-dependent means (in blue), then switches to making secondary regulatory melanocytes by kit-independent means (in red). In the second model, two distinct stem cell populations contribute sequentially to development of the melanocyte stripe.
(2) Specific ablation of larval melanocytes reveals MSCs are repressed by their targets
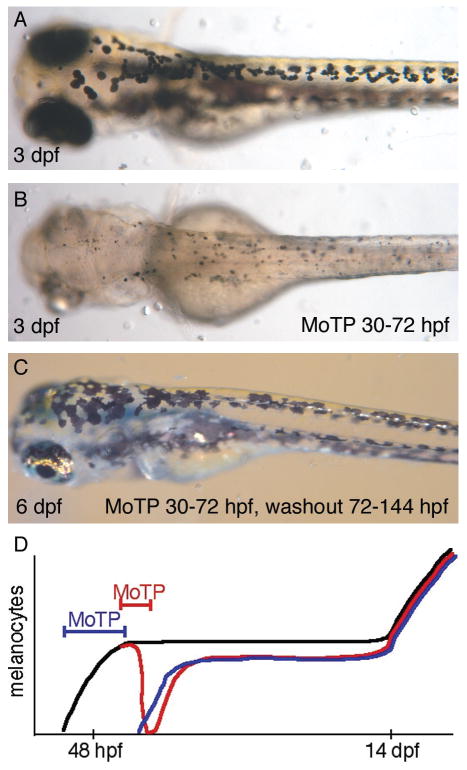
A thorough dissection of the mechanisms regulating MSCs outlined in Figure 1 requires the development of a simpler system than regeneration of the complex tissues of the adult fin, described above. Ideally, this could be achieved by melanocyte specific ablation, employed in stages of the life history with little or no other role for melanocyte development. The development of methods to use clinical tattoo-removal lasers on one hand (10) or melanotoxic drugs to kill late stage melanoblasts or newly born melanocytes on the other hand (11), meets the first of these goals. The relative lack of new melanocytes during the larval stage from 60 hours post fertilization (hpf) through 14 days post fertilization (dpf) (see Figure 4) meets the second, and sets the stage for a sensitive dissection of the regulation of the MSC.
Figure 4.
MoTP mediated larval melanocyte ablation and regeneration. (A) 3 dpf zebrafish larvae with normal or ontogenetically developed embryonic melanocytes. (B) Similar 3 dpf larvae exposed to melanotoxin MoTP from 30–72 hpf to kill melanocytes. Note faint pigmented detritus. (C) MoTP treated larvae after drug washout and melanocyte regeneration. (D) Schematic shows that wild-type zebrafish (black line) develop embryonic melanocytes prior to 60 hpf, and that between 60 hpf and 14 dpf, few new melanocytes are added to the larval pigment pattern. Treatment with MoTP following melanocyte differentiation (red line) results in rapid melanocyte death. Washout of MoTP results in regeneration of approximately 80% of embryonic melanocyte numbers. Treatment with MoTP before overt melanization (green line) kills developing embryonic melanocytes, but has no affect on melanocyte regeneration. The development of new melanocytes during metamorphosis (beginning at 14 days) is not affected by earlier MoTP treatments.
One of the most salient features of the embryonic melanocyte is that it is the only darkly pigmented cell in the embryo. One property of dyes such as melanin is that they are efficient at absorbing energy from light. Yang and colleagues hypothesized that if they could deliver sufficient visible light to the embryo, they could specifically heat and kill melanocytes without affecting surrounding tissue (10). To achieve this, these investigators tried clinical tattoo-removal lasers. These lasers provide intense visible wavelength light in pulses on the order of 5 nanoseconds. Moreover, the light is delivered in 1–3 mm diameter beams, allowing for exposure of much of the embryo in a single pulse. When 2–3 dpf larvae were irradiated, they showed the typical phenotype of melanocyte cell death, including contraction of dendritic processes in melanocytes in the beam path, followed by their extrusion from the larva. Moreover, when exposed embryos were then incubated with the melanin synthesis inhibitor PTU (see above), the region of the embryo in the beam path remained clear of pigmented melanocytes. Subsequent washout of the PTU resulted in the appearance of melanized melanocytes in the beam path. Following the logic used for demonstrating MSCs in the regenerating fin (above), this result indicated that MSCs support and regenerate missing larval melanocytes (10). In this case, and unlike the case for the regenerating fin, the regeneration of melanocytes following melanocyte-specific ablation demonstrates that the larval melanocyte is actively repressing the MSC, and that this repression is relieved when melanocytes are ablated. Whether this repression is direct between the melanocyte and its stem cell, or instead is mediated by other cells or a MSC niche, remains to be determined.
A more easily applied method of melanocyte-specific ablation is provided by the discovery of the small molecule 4-(4-morpholinobutylthio)phenol (MoTP) that effectively kills embryonic and larval melanocytes. MoTP was first identified in the small molecule screens of (12), that showed that embryos could develop in this drug, but lacked melanocytes. Yang and Johnson subsequently showed that MoTP is a prodrug that is converted by tyrosinase to a cytotoxin (11). Tyrosinase is an enzyme that converts tyrosine to melanin and is only expressed at high levels in the melanocyte, thus accounting for the melanocyte specificity of MoTP cell ablation. Following MoTP incubation of melanized larvae, melanocytes retract their dendritic processes and are extruded from the fish, similar to that described for melanocyte death in kit mutants (13) and following laser ablation. Unlike the laser ablation discussed above, all the melanocytes in the embryo are killed. As seen in Figure 4B, these cells leave behind pigmented detritus. When embryos are incubated in MoTP prior to melanization, (from 14 hpf, Figure 4D), the melanoblasts are killed without leaving behind a pigmented detritus, that facilitates subsequent quantitative analysis and enables screens for melanocyte regeneration mutants (below). That MoTP ablation is revealing activity of a MSC is demonstrated by the findings that regeneration melanocytes have undergone at least two rounds of cell division as determined by BrdU incorporation, and that the larvae can be induced to regenerate melanocytes again, with a new round of MoTP exposure (11). These results indicate that the melanocyte regeneration observed is not due to recruitment of a post-mitotic melanoblast, or poised precursor, and further suggest that the precursors recruited for regeneration are renewed.
Similar drugs, such as 4-hydroxyanisole, that are also converted by tyrosinase to cytotoxins, were developed in the 1970s in pursuit of chemotherapies for melanoma (14). Unfortunately, in mammals, these drugs were also converted to cytotoxins in the liver, rendering the drugs unsuitable for clinical use against melanoma. In zebrafish, at high concentrations or when applied for more extensive periods, MoTP is also lethal to embryos, perhaps due to less efficient conversion to cytotoxins by other enzymes. Another limitation of MoTP is that it is only effective at killing melanocytes prior to 5–6 dpf, or in newly regenerated melanocytes, that have sufficiently high expression of tyrosinase (11). This tends to limit chemical ablation of melanocytes to studies of larval regeneration. Identification of additional drugs that kill old or mature melanocytes in the adult will allow analysis of the regeneration potential of the adult melanocyte, and the properties of the stem cells that support them as well.
The finding that the larvae recruits from a MSC to repair damage to the embryonic melanocyte pattern raises new questions about the establishment and regulation of the MSCs that generalize to all adult stem cells. One question that comes to mind is whether the MSC is restricted in its lineage, generating only melanocytes. An alternative model is that the MSC activity that we infer from the properties of melanocyte regeneration is actually a multipotent precursor, which can generate all pigment cells, or all neural crest derivatives, such as the neural crest stem cell (15). Solving this question in vivo in zebrafish will require the direct identification of the MSC, or lineage and clonal analysis techniques that vitally and persistently mark all derivatives of the neural crest.
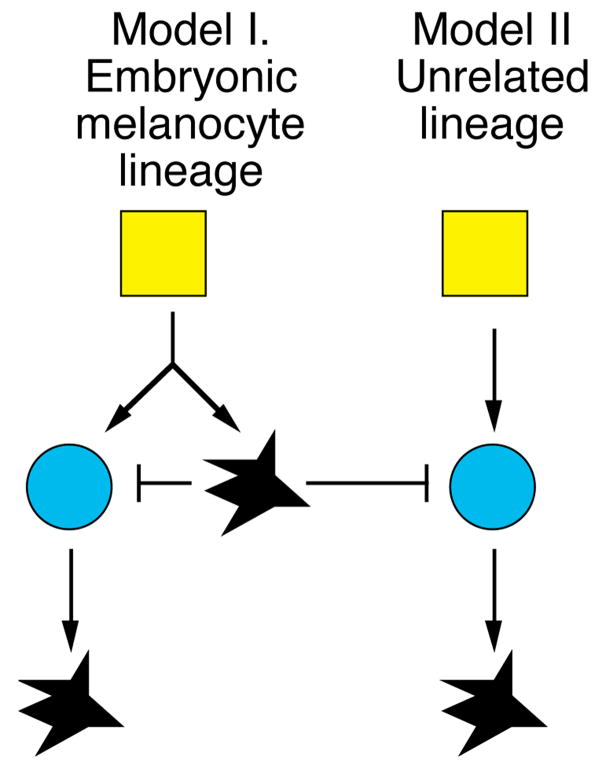
An additional problem suggested by these findings is: what is the origin of the MSC in the embryo? There is no reason to doubt that the MSC comes from the neural crest, but there is no particular reason to imagine that it comes from the same lineages as those that give rise to the embryonic melanocytes. This question is schematized in Figure 5. An alternative model is that the MSC arises from parallel, or unrelated lineages. Again, such distinctions await the development of new tools for the clonal analysis of lineages in zebrafish. The remarkable rates of transposition of the tol2 transposon (16) following injection in newly fertilized eggs may facilitate the development of these tools.
Figure 5.
Models proposing different embryonic origins of MSCs. Yellow squares represent early embryonic or neural crest precursors, blue circles represent MSCs, and black cells represent embryonic or larval regeneration melanocytes. In first model (on left) MSCs develop in same lineages as the embryonic melanocytes. In contrast, in the second model, MSCs develop in distinct lineages from the embryonic melanocytes. In both models, embryonic melanocytes repress the stem cells, and this repression is relieved by melanocyte ablation to allow larval melanocyte regeneration.
(3) Mutant screens for melanocyte regeneration
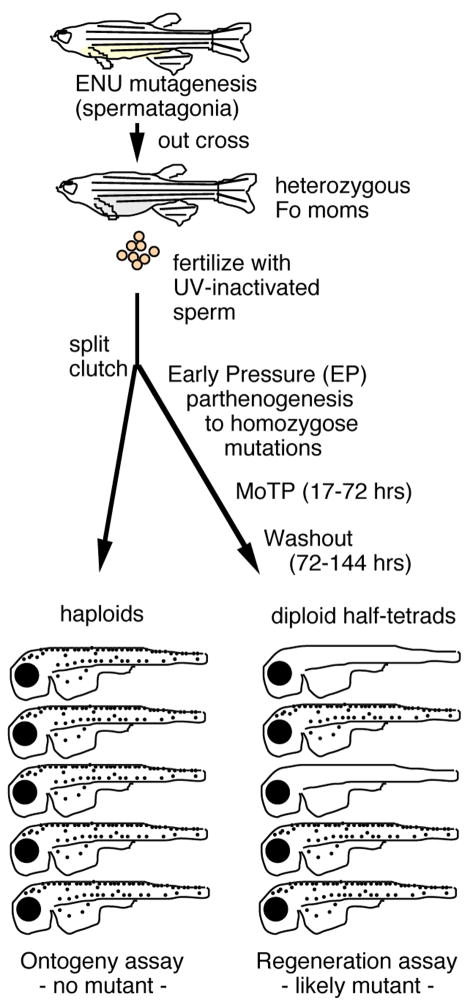
Although regeneration of the adult caudal fin is easily achieved and several mutant screens have been performed to find mutations that inhibit fin regeneration (17, 18, 19, 20), no mutants that have specific defects in fin melanocyte regeneration have been found from these screens. Mutants with profound defects in fin melanocyte regeneration, such as kit, were identified first as mutations that affect the embryonic pigment pattern (21). Moreover, it is likely that many of the genes that control the MSC are also required for viability of the embryo, and die before adult stages. A fine scale genetic dissection of melanocyte regeneration awaited the development of a practical regeneration assay in embryonic or larval stages. To accomplish this, Yang and Johnson developed a larval melanocyte regeneration screen (22) (see Figure 6). This screen combines the MoTP melanocyte ablation and regeneration assay with Early Pressure (EP) parthenogenesis (23) that allows for homozygosis of newly induced mutations. Unlike classic mutant screens in fruit flies or mice, that require that the investigator first generate sibships segregating the mutation, that are then interbred to generate mutant animals, EP screens allow the investigator to skip the sibship-building generation, thus saving both time and animal facility space.
Figure 6.
Mutagenesis screen for melanocyte regeneration mutants. Schematic shows Early Pressure Parthenogenesis coupled with MoTP melanocyte ablation to screen for melanocyte regeneration mutants.
The first important feature of mutant screens is the mutagenesis itself. Although several mutagenesis protocols have been developed, the most popular are those that target the spermatogonia with the chemical mutagen ethylnitrosourea (ENU) (24, 25). Thus, adult males are treated with the chemical mutagen, ethylnitrosourea (ENU) for several periods over approximately one month. It is also important to allow the mutagenized males another several weeks before breeding to generate the heterozygous females, to allow the mutagenized spermatagonia to replace the sperm. Because each male has at least several hundred proliferating spermatagonial stem cells, typically each heterozygous F0 female represents an independently mutagenized genome. Specific locus tests for mutagenesis rates range from one to three new mutations per thousand/loci tested, suggesting that saturation mutagenesis, or finding an average of one mutation per gene that could be revealed by mutation in the screen, can be approached by screening the progeny of approximately 1000 F0 females.
The second important feature of the mutant screen is the EP Parthenogenesis, which renders portions of the mutagenized genomes of the heterozygous females homozygous in a single generation (23). Thus, eggs from heterozygous F0 females are first fertilized with UV-inactivated sperm. If nothing else were done to these eggs, they would develop as haploid embryos, which typically live from 1–3 days, and can reveal defects in the embryonic or ontogenetic melanocyte pattern (23). Thus, in the melanocyte regeneration screen (22), a few of the fertilized eggs are set aside and scored after several days to determine if the mutagenized genome carries any mutants that affect embryonic melanocyte development. The bulk of the eggs, however, are subjected to EP Parthenogenesis. Eggs are arrested at the onset of the second meiotic division. At this stage, the egg has two nuclei, each the diploid, half-tetrad products of the first meiotic division. Activation of the egg activates the spindles of the second division, to generate the female pronucleus and the 3 polar bodies. When the egg is placed under very high pressure immediately after fertilization (from 40 seconds to 6 minutes), the second meiotic spindles collapse, and one of the half-tetrads is now packaged as a diploid female pronucleus. Since these eggs are fertilized with UV-inactivated sperm, which makes no genetic contribution to the embryo, the eggs develop as half-tetrad diploids. Approximately 50% of embryos from unmutagenized stocks survive to adult stages, while survival to adult stages in mutagenized stocks is typically half of that, due to homozygosis of lethal mutations. EP screens have been successfully used to identify mutations in adult fin regeneration, adult pigment pattern, embryonic neural crest development as well as larval melanocyte regeneration (17, 22, 26, 27).
The final important feature of the larval melanocyte regeneration screen is that following ablation with the drug MoTP, melanocytes regenerate, as discussed above. In Figure 4B, we show the detritus of dying melanocytes that were allowed to partially pigment prior to MoTP ablation. In such ablations, the dying melanocytes and detritus are typically extruded from the fish. However, for screening purposes, such detritus may obscure mutants that fail to regenerate melanocytes. Our experience is that adding MoTP prior to overt differentiation of the melanocyte also kills developing melanocytes. Because these have not yet made melanin, the detritus of their death does not confound identification of mutants that fail to regenerate melanocytes. Such mutants might also be due to simple defects in ontogenetic development, which will be revealed if one-half the haploid embryos that were set aside from the clutch develop with ontogenetic defects. Unlike normal sexual crosses, where Mendelian ratios of 25% are expected for mutants, the ratios in EP half-tetrad clutches can range from 5%, for some telomeric loci, to 50% for loci near centromeres. A more thorough discussion of half-tetrad genetics in zebrafish is provided by Johnson et al. (28).
(4) Regeneration is not ontogeny
A reasonable expectation of the genetic requirements for regeneration is that many of same genes that promote the development of the cells or tissue will also have similar roles in its regeneration. Differences in genetic requirements between ontogenetic formation of a tissue and its regeneration might then reflect mechanisms that act specifically in one or the other. One example comes from the regeneration-specific defect of the temperature-sensitive fin regeneration mutation, reg6 (17, 29). This mutant has specific defects in branching morphogenesis used in growing the vascular plexus of the regenerating fin. Since ontogenetic growth of the fin proceeds without a vascular plexus and with little role for branching morphogenesis, the regeneration specificity of the mutation is explained by its role in a regeneration specific mechanism. Similarly, we can expect that identification of melanocyte regeneration mutations may identify mechanisms specific for regulating the stem cells that support the melanocyte pattern, or alternatively, identify differences in how melanocytes differentiate during ontogeny and regeneration.
One such mutation that differentiates between melanocyte ontogeny and regeneration is the temperature-sensitive allele of the kit receptor tyrosine kinase (kit-ts) (8). This allele was discussed above to help show that kit is not involved in establishing the stem cell population that regenerates melanocytes in the regenerating fin, but instead is involved in recruiting the stem cell or differentiating the regeneration melanocytes (8). In the adult fin, the requirement for kit in ontogeny and regeneration appear identical. In contrast, when embryonic melanocytes are ablated in the kit-ts mutant, either by laser or by MoTP, melanocytes largely fail to regenerate, even when embryos are held at the permissive temperature (10, 11). Note also that melanocytes indeed develop in mutants bearing either null or temperature-sensitive alleles of kit. Thus, the requirement on kit for melanocyte differentiation is specific to regeneration melanocytes, or to various adult melanocyte populations. It is interesting to speculate that these results reflect a role for kit in recruiting the MSC back into developmental pathways, and further speculate that embryonic melanocytes develop without an adult stem cell-like intermediate. This speculation is supported by findings discussed below involving the picasso metamorphosis mutant (30).
Two additional melanocyte regeneration-specific mutants were identified in a melanocyte regeneration screen as described above (22). Mutants for eartha and julie develop embryonic melanocytes, but fail to regenerate them after MoTP ablation. In situ expression analysis for dopachrome tautamerase (dct), a gene that promotes melanin formation and marks late melanoblasts, show that the eartha mutants accumulate dct-‘positive melanoblasts but fail to differentiate them into pigmented melanocytes. Closer examination of eartha mutants in ontogenetic development revealed that embryonic melanocytes begin to melanize normally, but fail to fully darken. Transplant experiments showed that the eartha gene acts cell autonomously in the melanocyte to promote this darkening. Positional cloning of the eartha gene showed that it is a defect in the enzyme glutamine:fructose-6-phosphate aminotransferase. This enzyme is the first step in the hexosamine biosynthesis pathway, that are used either in long chain sugars in the extracellular matrix, or monomeric modifications that affect activity of specific proteins. Not surprisingly, the eartha mutation is pleiotropic, with defects in synthesizing the cartilage of the jaw and late larval lethality. The finding that eartha acts in the melanocyte tends to rule out roles in the extracellular matrix as that responsible for the regeneration defect and raises the possibility that eartha-dependent glycosylation of a specific protein may function to promote early differentiation steps in regeneration, that acts at late stages in ontogenetic development of embryonic melanocytes (22).
Similarly, mutants for julie have defects specific to melanocyte regeneration, that are not shared by embryonic melanocytes. Unlike eartha mutants, julie mutants fail to accumulate dct-positive melanoblasts, indicating an earlier role, perhaps in recruitment from the MSC. Positional cloning of the julie mutation showed that it is a defect in skiv2l2 (22), the vertebrate ortholog of yeast MTR4 gene, an ATP-dependent RNA helicase thought to be involved in RNA processing or degradation through the exosome (31). Like eartha mutants, julie is pleiotropic, with general defects in cellular proliferation during larval stages. In situ expression analysis of skiv2l2 in larval zebrafish shows it is expressed similarly to the cell proliferation marker pcna, especially in the brain and eyes, regions that largely fail to grow in julie larvae after 3.5 dpf. Consistent with defects in general cellular proliferation, julie mutant larvae fail to regenerate their tails following amputation (22). Thus, here the regeneration-specific requirement for julie tempts us to speculate that the difference between ontogeny and regeneration results from whether there is an adult stem cell-like intermediate in the affected lineage. One possibility that explains the apparently normal appearance of julie mutants through embryonic development (through 3.5 days) is that most or all of the embryonic cells develop without an adult stem cell-like intermediate, and that cell division following an adult stem cell requires the function of the skiv2l2 RNA helicase.
(5) MSC development may be revealed by pigment pattern metamorphosis mutants
Developmental biologists have long noted that the adult pigment pattern of poikilotherms such as fish or amphibians differs strikingly from that of embryonic or larval patterns (32). This has led to the notion that the embryo sets aside undifferentiated precursors, that are then recruited during metamorphosis, to generate the adult pattern. In zebrafish, this pigment pattern metamorphosis begins at approximately 14 dpf, continuing through 4 weeks, at which time the transition to adult pattern is complete and the fish look like small adults. Because these fish continue to grow and add melanocytes to their pattern throughout their lives, it is likely that a self-renewing stem cell is responsible both for the new melanocytes that arise during metamorphosis and the new melanocytes that arise during growth. This has led Parichy and his colleagues (26, 30, 33, 34) to explore how stem cell regulation is responsible for generating new cells in zebrafish metamorphosis, or responsible for the different deployment of new melanocytes observed in closely related species. Of particular interest are the mutants puma and picasso (26, 30). Each of these mutants appears identical to wild-type individuals during embryonic and larval stages, but show deficits in recruiting new melanocytes upon reaching the onset of pigment pattern metamorphosis (2 weeks). Interestingly, puma was isolated in screens for temperature-sensitive metamorphosis (26). In this mutant, the melanocyte deficit is partially suppressed when fish are allowed to mature more slowly at the permissive temperature, or when growth was slowed by limiting nutrition, leading to the model that the mutant defect was slow expansion of the MSC population during larval growth.
A second pigment pattern metamorphosis mutant, picasso, shows temperature and growth rate independent defects in generation of new melanocytes at metamorphosis. Positional cloning revealed a defect in the EGF receptor-like tyrosine kinase gene erbb3b (30). Mutants in this gene have also been described for their defects in glial cell development (35). In each study, analysis of the mutant phenotype was aided by the availability of drugs that specifically block the function of erbb receptor tyrosine kinases. Because of the role of EGF receptor and its erbb homologs in a variety of cancers, several highly specific inhibitors of erbb receptor functions have been developed by the drug industry. Interestingly, only when these drugs are applied to zebrafish embryos between 14 and 22 hours (about the time when neural crest cells are migrating into the periphery) is the metamorphosis melanocyte deficit observed (30). Thus, the early time of drug action suggests that it blocks the establishment of the MSC population. An interesting question now is whether the MSCs that support metamorphosis are the same cells that are recruited during larval stages following MoTP ablation.
(6) Conclusions
Methods to ablate and regenerate melanocytes, or analyze melanocyte development across discrete stages of development have provided several insights into the regulation of undifferentiated, quiescent precursors that have several properties expected of adult stem cells. These include how adult stem cells are established during embryonic development, repressed by their target cells, recruited to enter developmental pathways, or differentiate. Each of the examples we discuss reveal profound differences between the developmental mechanisms used make the embryonic melanocyte pattern, that may develop independently of adult stem cell like intermediates, and those used to regenerate it from MSCs. Our variety of different means to assess MSCs in post-embryonic development and regeneration, together with ability to perform screens for mutations or drugs that specifically affect the regulation of the MSC suggests that we can achieve a rich understanding of the mechanisms regulating MSC in particular, and adult stem cells in general.
Acknowledgments
We thank Keith Hultmann and Rob Tryon for help with figures and for reading the manuscript. This work was supported by NIH grant GM56988 to SLJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin GR. Isolation of a pluripotent ell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–45. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 3.Mackenzie MA, Jordan SA, Budd PS, Jackson IJ. Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Dev Biol. 1997;192:99–107. doi: 10.1006/dbio.1997.8738. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–60. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete stem cell maintenance in the niche. Science. 2005;307:720–4. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 6.Goodrich HB, Nichols R. The development and the regeneration of the color pattern in brachydanio rerio. J Morph. 1931;52:513–523. [Google Scholar]
- 7.Rawls JF, Johnson SL. Zebrafish kit mutation reveals primary and secondary regulation of melanocyte development during fin stripe regeneration. Development. 2000;127:3715–24. doi: 10.1242/dev.127.17.3715. [DOI] [PubMed] [Google Scholar]
- 8.Rawls JF, Johnson SL. Requirements for the kit receptor tyrosine kinase during regeneration of zebrafish fin melanocytes. Development. 2001;128:1943–9. doi: 10.1242/dev.128.11.1943. [DOI] [PubMed] [Google Scholar]
- 9.Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28:2261–73. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang CT, Sengelmann RD, Johnson SL. Larval melanocyte regeneration following laser ablation in zebrafish. J Invest Dermatol. 2004;123:924–9. doi: 10.1111/j.0022-202X.2004.23475.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang CT, Johnson SL. Small molecule-induced ablation and subsequent regeneration of larval zebrafish melanocytes. Development. 2006;133:3563–73. doi: 10.1242/dev.02533. [DOI] [PubMed] [Google Scholar]
- 12.Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci U S A. 2000;97(24):12965–9. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parichy DM, Rawls JF, Pratt SJ, Whitfield TT, Johnson SL. Zebrafish sparse corresponds to an orthologue of c-kit and is required for the morphogenesis of a subpopulation of melanocytes, but is not essential for hematopoiesis or primordial germ cell development. Development. 1999;126:3425–36. doi: 10.1242/dev.126.15.3425. [DOI] [PubMed] [Google Scholar]
- 14.Naish S, Cooksey CJ, Riley PA. Initial mushroom tyrosinase-catalysed oxidation product of 4-hydroxyanisole is 4-methoxy-ortho-benzoquinone. Pigment Cell Res. 1988;1:379–381. doi: 10.1111/j.1600-0749.1988.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 15.Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell. 2004;7:133–44. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Johnson SL, Weston JA. Temperature-sensitive mutations that cause stage-specific defects in Zebrafish fin regeneration. Genetics. 1995;141:1583–95. doi: 10.1093/genetics/141.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellgren EM, Johnson SL. pyewacket, a new zebrafish fin pigment pattern mutant. Pigment Cell Res. 2006;19:232–8. doi: 10.1111/j.1600-0749.2006.00311.x. [DOI] [PubMed] [Google Scholar]
- 19.Nechiporuk A, Poss KD, Johnson SL, Keating MT. Positional cloning of a temperature-sensitive mutant emmental reveals a role for sly1 during cell proliferation in zebrafish fin regeneration. Dev Biol. 2003;258:291–306. doi: 10.1016/s0012-1606(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 20.Poss KD, Nechiporuk A, Hillam AM, Johnson SL, Keating MT. Mps1 defines a proximal blastemal proliferative compartment essential for zebrafish fin regeneration. Development. 2002;129:5141–9. doi: 10.1242/dev.129.22.5141. [DOI] [PubMed] [Google Scholar]
- 21.Johnson SL, Africa D, Walker C, Weston JA. Genetic control of adult pigment stripe development in zebrafish. Dev Biol. 1995;167:27–33. doi: 10.1006/dbio.1995.1004. [DOI] [PubMed] [Google Scholar]
- 22.Yang CT, Hindes AE, Hultman KA, Johnson SL. Mutations in gfpt1 and skiv2l2 cause distinct stage-specific defects in larval melanocyte regeneration in zebrafish. PLoS Genet. 2007;3:e88. doi: 10.1371/journal.pgen.0030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio) Nature. 1981;291:293–6. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- 24.Solnica-Krezel L, Schier AF, Driever W. Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics. 1994;136:1401–20. doi: 10.1093/genetics/136.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullins MC, Nüsslein-Volhard C. Mutational approaches to studying embryonic pattern formation in the zebrafish. Curr Opin Genet Dev. 1993;3:648–54. doi: 10.1016/0959-437x(93)90102-u. [DOI] [PubMed] [Google Scholar]
- 26.Parichy DM, Turner JM, Parker NB. Essential role for puma in development of postembryonic neural crest-derived cell lineages in zebrafish. Dev Biol. 2003;256:221–41. doi: 10.1016/s0012-1606(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 27.Henion PD, Raible DW, Beattie CE, Stoesser KL, Weston JA, Eisen JS. Screen for mutations affecting development of Zebrafish neural crest. Dev Genet. 1996;18:11–7. doi: 10.1002/(SICI)1520-6408(1996)18:1<11::AID-DVG2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Johnson SL, Africa D, Horne S, Postlethwait JH. Half-tetrad analysis in zebrafish: mapping the ros mutation and the centromere of linkage group I. Genetics. 1995;139:1727–35. doi: 10.1093/genetics/139.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang CC, Lawson ND, Weinstein BM, Johnson SL. reg6 is required for branching morphogenesis during blood vessel regeneration in zebrafish caudal fins. Dev Biol. 2003;264:263–74. doi: 10.1016/j.ydbio.2003.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Budi EH, Patterson LB, Parichy DM. Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation. Development. 2008 doi: 10.1242/dev.019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernstein J, Patterson DN, Wilson GM, Toth EA. Characterization of the essential activities of Saccharomyces cerevisiae Mtr4p, a 3′->5′ helicase partner of the nuclear exosome. J Biol Chem. 2008;283:4930–42. doi: 10.1074/jbc.M706677200. [DOI] [PubMed] [Google Scholar]
- 32.Niu MC, Twitty VC. The origins of epidermal melanophores during metamorphosis in trituris torusis. J. Expt. Zool. 1950;113:633–648. [Google Scholar]
- 33.Parichy DM, Ransom DG, Paw B, Zon LI, Johnson SL. An orthologue of the kit-related gene fms is required for development of neural crest-derived xanthophores and a subpopulation of adult melanocytes in the zebrafish, Danio rerio. Development. 2000;127:3031–44. doi: 10.1242/dev.127.14.3031. [DOI] [PubMed] [Google Scholar]
- 34.Quigley IK, Turner JM, Nuckels RJ, Manuel JL, Budi EH, MacDonald EL, et al. Pigment pattern evolution by differential deployment of neural crest and post-embryonic melanophore lineages in Danio fishes. Development. 2004;131:6053–69. doi: 10.1242/dev.01526. [DOI] [PubMed] [Google Scholar]
- 35.Lyons DA, Pogoda HM, Voas MG, Woods IG, Diamond B, Nix R, et al. erbb3 and erbb2 are essential for schwann cell migration and myelination in zebrafish. Curr Biol. 2005;15:513–24. doi: 10.1016/j.cub.2005.02.030. [DOI] [PubMed] [Google Scholar]