Abstract
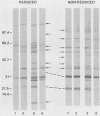
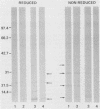
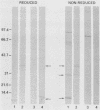
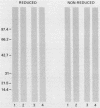
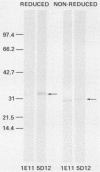
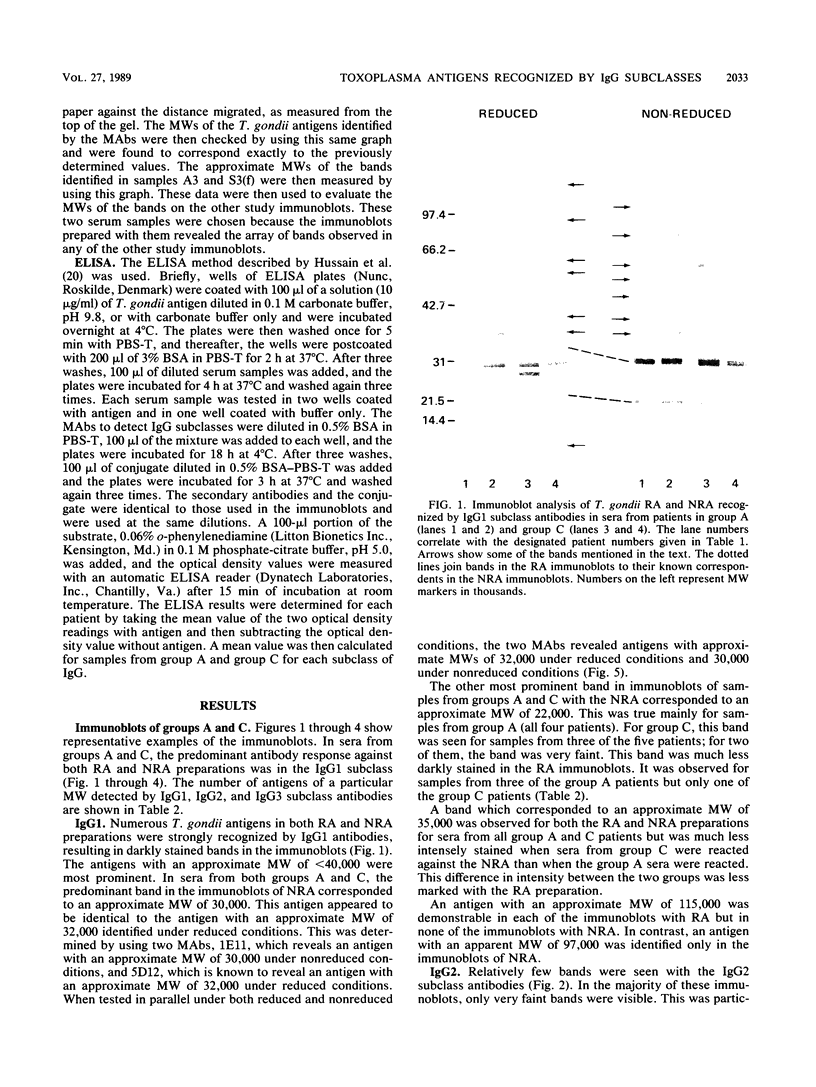
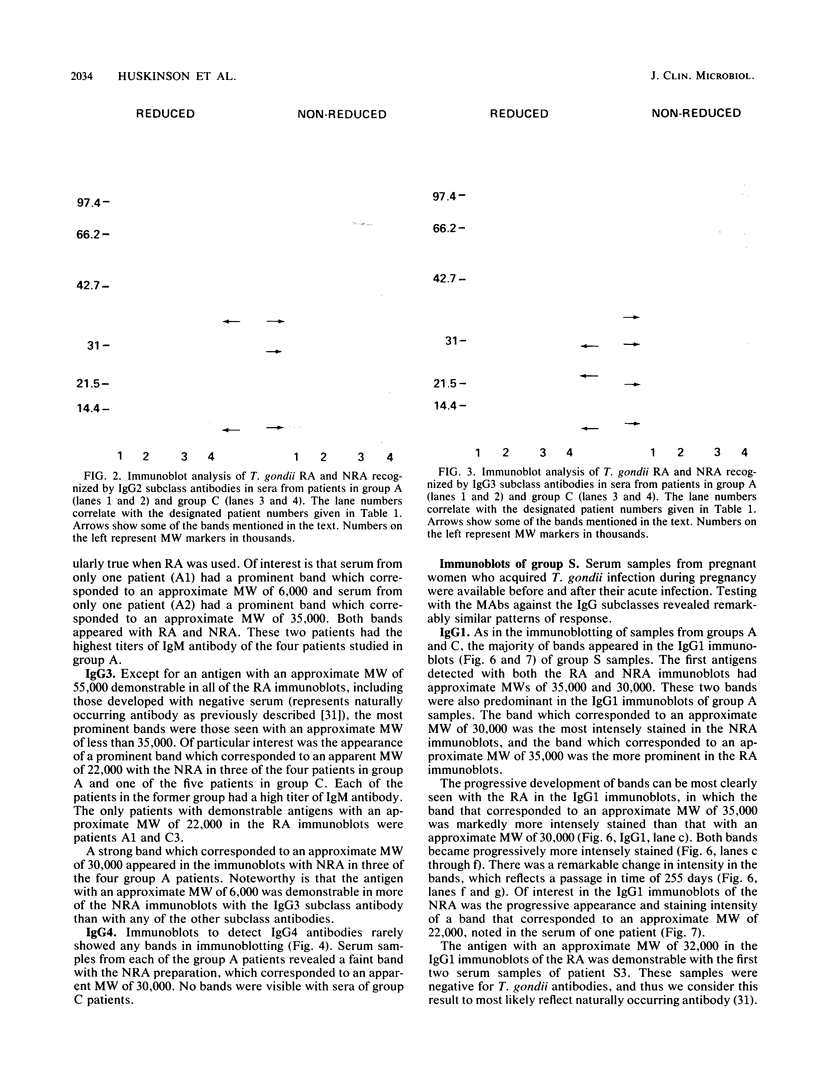
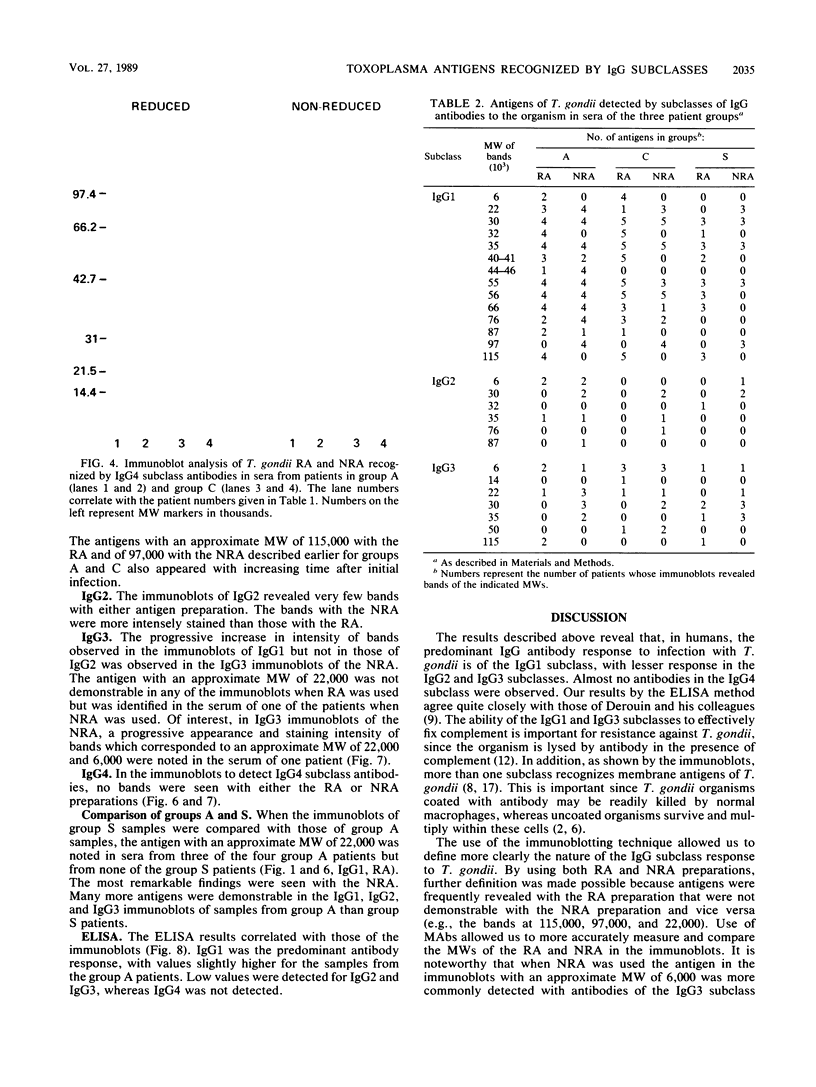
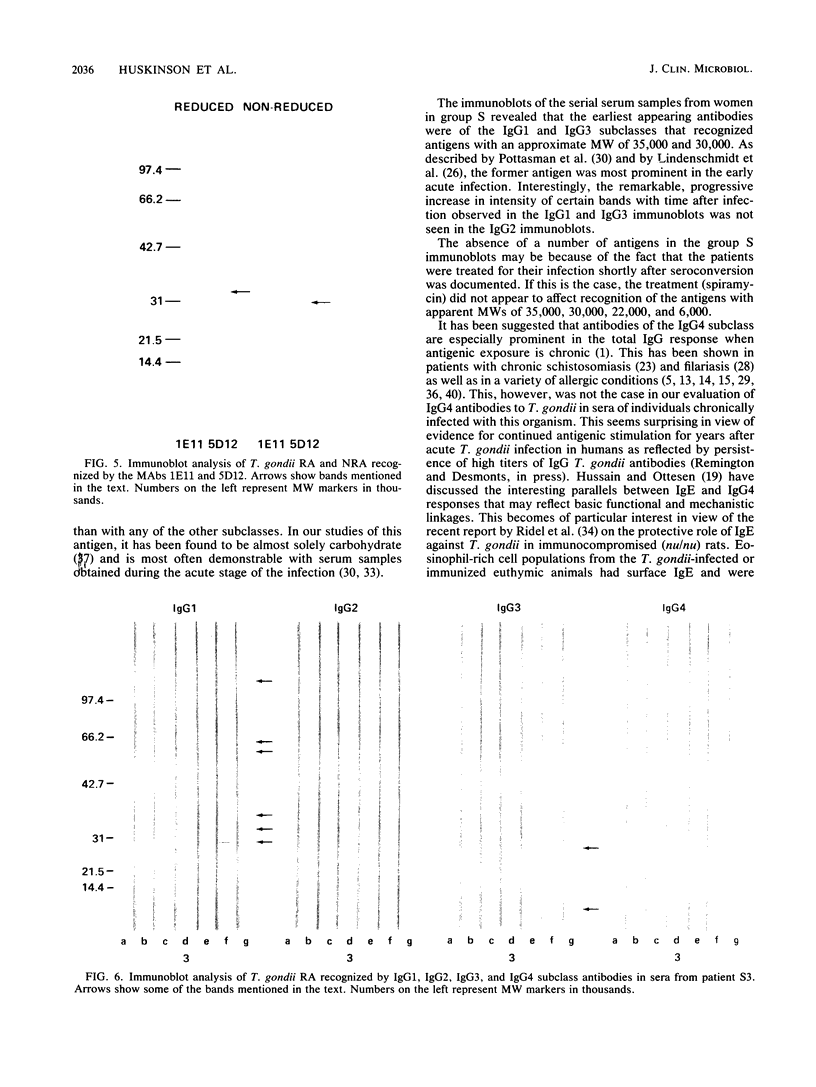
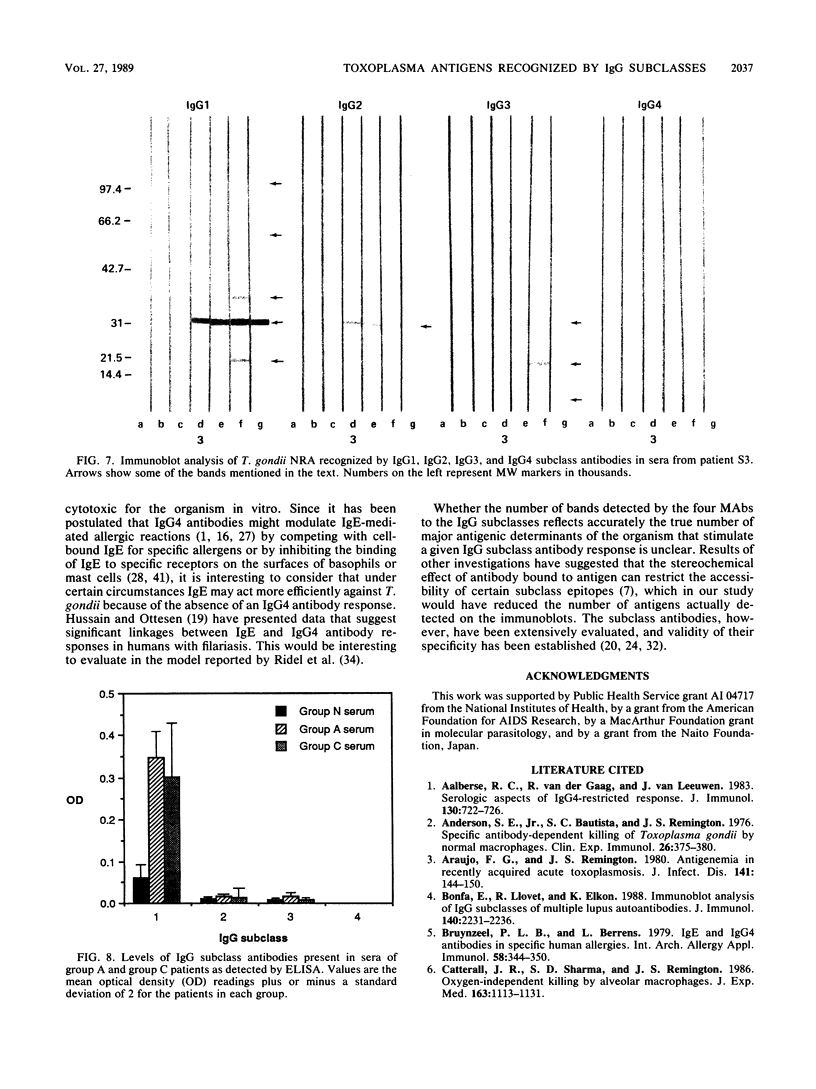
The immunoglobulin G (IgG) subclass response to Toxoplasma gondii antigens during the acute and chronic stages of T. gondii infection were studied by using immunoblots with reduced antigen (RA) and nonreduced antigen (NRA) preparations. Serum samples were from individuals with acute or chronic T. gondii infection, and sequential samples were from women who seroconverted during gestation and were treated with spiramycin. IgG1 antibodies were predominant in sera from each of the groups and recognized a large number of RA and NRA. In the latter group of patients, IgG1 and IgG3 were the first antibodies to appear in response to the infection. In all groups, an antigen with a molecular weight (MW) of 30,000 was the most intensely stained and frequently recognized by IgG1 antibodies in NRA preparations. In RA preparations, antigens of MW 35,000 and 30,000 were the most intensely stained and frequently recognized by IgG1 antibodies. An antigen with an MW of 22,000 was intensely stained in the IgG1 immunoblots of the NRA preparation and to a lesser extent in the RA preparation. In contrast to immunoblots with IgG1 antibodies, very few antigens in the RA and NRA preparations were detected by IgG2 and IgG3 antibodies; IgG4 antibodies rarely detected any antigens. Of interest was that IgG2 antibodies detected antigens distributed over the entire MW range, whereas those detected by IgG3 antibodies were located mostly below the 35,000 MW marker. Enzyme-linked immunosorbent assay results paralleled those of the immunoblots in that IgG1 antibodies were predominant.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalberse R. C., van der Gaag R., van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983 Feb;130(2):722–726. [PubMed] [Google Scholar]
- Anderson S. E., Jr, Bautista S. C., Remington J. S. Specific antibody-dependent killing of Toxoplasma gondii by normal macrophages. Clin Exp Immunol. 1976 Dec;26(3):375–380. [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Remington J. S. Antigenemia in recently acquired acute toxoplasmosis. J Infect Dis. 1980 Feb;141(2):144–150. doi: 10.1093/infdis/141.2.144. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Llovet R., Elkon K. Immunoblot analysis of IgG subclasses of multiple lupus autoantibodies. J Immunol. 1988 Apr 1;140(7):2231–2236. [PubMed] [Google Scholar]
- Bruynzeel P. L., Berrens L. IgE and IgG4 antibodies in specific human allergies. Int Arch Allergy Appl Immunol. 1979;58(3):344–350. doi: 10.1159/000232211. [DOI] [PubMed] [Google Scholar]
- Catterall J. R., Sharma S. D., Remington J. S. Oxygen-independent killing by alveolar macrophages. J Exp Med. 1986 May 1;163(5):1113–1131. doi: 10.1084/jem.163.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. M., Nahmias A. J., Williams S. C., Phillips D. J., Black C. M., Reimer C. B. IgG subclass antibodies to herpes simplex virus. J Infect Dis. 1985 May;151(5):929–936. doi: 10.1093/infdis/151.5.929. [DOI] [PubMed] [Google Scholar]
- Couvreur G., Sadak A., Fortier B., Dubremetz J. F. Surface antigens of Toxoplasma gondii. Parasitology. 1988 Aug;97(Pt 1):1–10. doi: 10.1017/s0031182000066695. [DOI] [PubMed] [Google Scholar]
- Derouin F., Sulcebe G., Ballet J. J. Sequential determination of IgG subclasses and IgA specific antibodies in primary and reactivating toxoplasmosis. Biomed Pharmacother. 1987;41(8):429–433. [PubMed] [Google Scholar]
- Desmonts G., Naot Y., Remington J. S. Immunoglobulin M-immunosorbent agglutination assay for diagnosis of infectious diseases: diagnosis of acute congenital and acquired Toxoplasma infections. J Clin Microbiol. 1981 Nov;14(5):486–491. doi: 10.1128/jcm.14.5.486-491.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman R. F., Remington J. S. Value of lymph-node biopsy in the diagnosis of acute acquired toxoplasmosis. N Engl J Med. 1973 Oct 25;289(17):878–881. doi: 10.1056/NEJM197310252891702. [DOI] [PubMed] [Google Scholar]
- Feldman H. A. Maxwell Finland lecture. J Infect Dis. 1980 Apr;141(4):525–529. doi: 10.1093/infdis/141.4.525. [DOI] [PubMed] [Google Scholar]
- Gwynn C. M., Almosawi T., Stanworth D. R. Clinical associations with serum allergen-specific IgG4 antibodies. Clin Allergy. 1982 Sep;12(5):459–464. doi: 10.1111/j.1365-2222.1982.tb01644.x. [DOI] [PubMed] [Google Scholar]
- Gwynn C. M., Smith J. M., Leon G. L., Stanworth D. R. IgE and IgG4 subclass in atopic families. Clin Allergy. 1979 Mar;9(2):119–123. doi: 10.1111/j.1365-2222.1979.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Gwynn C. M., Smith J. M., Leon G. L., Stanworth D. R. Role of IgG4 subclass in childhood allergy. Lancet. 1978 Apr 29;1(8070):910–911. doi: 10.1016/s0140-6736(78)90685-2. [DOI] [PubMed] [Google Scholar]
- Handman E., Goding J. W., Remington J. S. Detection and characterization of membrane antigens of Toxoplasma gondii. J Immunol. 1980 Jun;124(6):2578–2583. [PubMed] [Google Scholar]
- Huskinson J., Stepick-Biek P., Remington J. S. Detection of antigens in urine during acute toxoplasmosis. J Clin Microbiol. 1989 May;27(5):1099–1101. doi: 10.1128/jcm.27.5.1099-1101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain R., Grögl M., Ottesen E. A. IgG antibody subclasses in human filariasis. Differential subclass recognition of parasite antigens correlates with different clinical manifestations of infection. J Immunol. 1987 Oct 15;139(8):2794–2798. [PubMed] [Google Scholar]
- Hussain R., Ottesen E. A. IgE responses in human filariasis. IV. Parallel antigen recognition by IgE and IgG4 subclass antibodies. J Immunol. 1986 Mar 1;136(5):1859–1863. [PubMed] [Google Scholar]
- Hussain R., Poindexter R. W., Ottesen E. A., Reimer C. B. Use of monoclonal antibodies to quantify subclasses of human IgG. II. Enzyme immunoassay to define antigen specific (anti-filarial) IgG subclass antibodies. J Immunol Methods. 1986 Nov 20;94(1-2):73–80. doi: 10.1016/0022-1759(86)90217-6. [DOI] [PubMed] [Google Scholar]
- Hussain R., Poindexter R. W., Wistar R., Reimer C. B. Use of monoclonal antibodies to quantify subclasses of human IgG. I. Development of two-site immunoenzymometric assays for total IgG subclass determinations. J Immunol Methods. 1986 Oct 23;93(1):89–96. doi: 10.1016/0022-1759(86)90437-0. [DOI] [PubMed] [Google Scholar]
- Iskander R., Das P. K., Aalberse R. C. IgG4 antibodies in Egyptian patients with schistosomiasis. Int Arch Allergy Appl Immunol. 1981;66(2):200–207. doi: 10.1159/000232819. [DOI] [PubMed] [Google Scholar]
- Jefferis R., Reimer C. B., Skvaril F., de Lange G., Ling N. R., Lowe J., Walker M. R., Phillips D. J., Aloisio C. H., Wells T. W. Evaluation of monoclonal antibodies having specificity for human IgG sub-classes: results of an IUIS/WHO collaborative study. Immunol Lett. 1985;10(3-4):223–252. doi: 10.1016/0165-2478(85)90082-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindenschmidt E. G. Demonstration of immunoglobulin M class antibodies to Toxoplasma gondii antigenic component p35000 by enzyme-linked antigen immunosorbent assay. J Clin Microbiol. 1986 Dec;24(6):1045–1049. doi: 10.1128/jcm.24.6.1045-1049.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottesen E. A., Kumaraswami V., Paranjape R., Poindexter R. W., Tripathy S. P. Naturally occurring blocking antibodies modulate immediate hypersensitivity responses in human filariasis. J Immunol. 1981 Nov;127(5):2014–2020. [PubMed] [Google Scholar]
- Ottesen E. A., Skvaril F., Tripathy S. P., Poindexter R. W., Hussain R. Prominence of IgG4 in the IgG antibody response to human filariasis. J Immunol. 1985 Apr;134(4):2707–2712. [PubMed] [Google Scholar]
- Parish W. E. The clinical relevance of heat-stable, short-term sensitizing anaphylactic IgG antibodies (IgG S-TS) and of related activities of IgG4 and IgG2. Br J Dermatol. 1981 Aug;105(2):223–231. doi: 10.1111/j.1365-2133.1981.tb01210.x. [DOI] [PubMed] [Google Scholar]
- Potasman I., Araujo F. G., Desmonts G., Remington J. S. Analysis of Toxoplasma gondii antigens recognized by human sera obtained before and after acute infection. J Infect Dis. 1986 Oct;154(4):650–657. doi: 10.1093/infdis/154.4.650. [DOI] [PubMed] [Google Scholar]
- Potasman I., Araujo F. G., Remington J. S. Toxoplasma antigens recognized by naturally occurring human antibodies. J Clin Microbiol. 1986 Dec;24(6):1050–1054. doi: 10.1128/jcm.24.6.1050-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer C. B., Phillips D. J., Aloisio C. H., Moore D. D., Galland G. G., Wells T. W., Black C. M., McDougal J. S. Evaluation of thirty-one mouse monoclonal antibodies to human IgG epitopes. Hybridoma. 1984 Fall;3(3):263–275. doi: 10.1089/hyb.1984.3.263. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Araujo F. G., Desmonts G. Recognition of different Toxoplasma antigens by IgM and IgG antibodies in mothers and their congenitally infected newborns. J Infect Dis. 1985 Nov;152(5):1020–1024. doi: 10.1093/infdis/152.5.1020. [DOI] [PubMed] [Google Scholar]
- Ridel P. R., Auriault C., Darcy F., Pierce R. J., Leite P., Santoro F., Neyrinck J. L., Kusnierz J. P., Capron A. Protective role of IgE in immunocompromised rat toxoplasmosis. J Immunol. 1988 Aug 1;141(3):978–983. [PubMed] [Google Scholar]
- Sabin A. B., Feldman H. A. Dyes as Microchemical Indicators of a New Immunity Phenomenon Affecting a Protozoon Parasite (Toxoplasma). Science. 1948 Dec 10;108(2815):660–663. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- Shakib F., McLaughlan P., Stanworth D. R., Smith E., Fairburn E. Elevated serum IgE and IgG4 in patients with atopic dermatitis. Br J Dermatol. 1977 Jul;97(1):59–63. doi: 10.1111/j.1365-2133.1977.tb15428.x. [DOI] [PubMed] [Google Scholar]
- Sharma S. D., Mullenax J., Araujo F. G., Erlich H. A., Remington J. S. Western Blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol. 1983 Aug;131(2):977–983. [PubMed] [Google Scholar]
- Siegel J. P., Remington J. S. Comparison of methods for quantitating antigen-specific immunoglobulin M antibody with a reverse enzyme-linked immunosorbent assay. J Clin Microbiol. 1983 Jul;18(1):63–70. doi: 10.1128/jcm.18.1.63-70.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torfason E. G., Reimer C. B., Keyserling H. L. Subclass restriction of human enterovirus antibodies. J Clin Microbiol. 1987 Aug;25(8):1376–1379. doi: 10.1128/jcm.25.8.1376-1379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay H. M., Perelmutter L. Inhibition of reagin-mediated PCA reactions in monkeys and histamine release from human leukocytes by human IgG4 subclass. Int Arch Allergy Appl Immunol. 1977;53(1):78–87. doi: 10.1159/000231734. [DOI] [PubMed] [Google Scholar]
- Wagner D. K., Nelson D. L., Walsh E. E., Reimer C. B., Henderson F. W., Murphy B. R. Differential immunoglobulin G subclass antibody titers to respiratory syncytial virus F and G glycoproteins in adults. J Clin Microbiol. 1987 Apr;25(4):748–750. doi: 10.1128/jcm.25.4.748-750.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M., Berzins K., Perlmann P., Persson M. Characterization of the humoral immune response in Plasmodium falciparum malaria. II. IgG subclass levels of anti-P. falciparum antibodies in different sera. Clin Exp Immunol. 1983 Oct;54(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Wedege E., Michaelsen T. E. Human immunoglobulin G subclass immune response to outer membrane antigens in meningococcal group B vaccine. J Clin Microbiol. 1987 Aug;25(8):1349–1353. doi: 10.1128/jcm.25.8.1349-1353.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch P. C., Masur H., Jones T. C., Remington J. S. Serologic diagnosis of acute lymphadenopathic toxoplasmosis. J Infect Dis. 1980 Aug;142(2):256–264. doi: 10.1093/infdis/142.2.256. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Toorenenbergen A. W., Aalberse R. C. Allergen-specific IgE and IgG4 antibody levels in sera and the capacity of these sera to sensitize basophil leucocytes in vitro. Clin Allergy. 1982 Sep;12(5):451–458. doi: 10.1111/j.1365-2222.1982.tb01643.x. [DOI] [PubMed] [Google Scholar]