Abstract
Echolocating big brown bats (Eptesicus fuscus) frequently catch insects during aerial pursuits in open spaces, but they also capture prey swarming on vegetation, and from substrates. To evaluate perception of targets on cluttered surfaces, big brown bats were trained in a two-alternative forced-choice task to locate a target, varying in height, that was embedded partway in holes (clutter) cut in a foam surface. The holes were colocalized with the possible positions of the target at distances ranging from 25 to 35 cm. For successful perception of the target, the bat had to detect the echoes contributed by the target in the same time window that contained echoes from the clutter. Performance was assessed in terms of target reflective strength relative to clutter strength in the same time window. The bats detected the target whenever the target strength was greater than 1–2 dB above the clutter.
INTRODUCTION
Big brown bats (Eptesicus fuscus) emit frequency-modulated (FM) sonar sounds in the 20–100 kHz band (Saillant et al., 2007; Surlykke and Moss, 2000) and locate prey from echoes that return to their ears (Neuweiler, 2000). They are often observed flying in the evening in pursuit of insects, capturing prey in aerial interception maneuvers guided by echolocation (Fenton, 1995; Griffin, 1958). Video recordings made with thermal-imaging cameras document that these bats are able to successfully catch insects in flight (Simmons, 2005). However, the same infrared video recordings reveal that big brown bats can also capture beetles from vegetation and sometimes even land on the ground to seize prey (Simmons, 2005; Simmons et al., 2001). Some kinds of insects taken as prey by big brown bats, such as crickets or katydids, are not commonly observed to fly at night and presumably must be taken from substrates such as the ground or vegetation (Fullard et al., 2005; Kurta and Baker, 1990).
Beetles swarming in vegetation make buzzing sounds that are audible to bats (Hamr and Bailey, 1985), and crickets and katydids communicate with each other acoustically, in both cases providing bats with potential cues for passive hearing to detect and localize prey. However, when gleaning prey from clutter or substrates, the bat’s actual approach and certain details of capture must be guided by some contribution from echolocation, if only to avoid collisions with vegetation or the substrate itself, and under these conditions the bats continue to emit echolocation sounds (Fullard et al., 2005; Ratcliffe et al., 2005; Schmidt, et al., 2000). The presence of targets on surfaces or in vegetation creates a complex acoustic scene with echoes from the target and from the clutter competing to be perceived (Moss and Surlykke, 2001; Ratcliffe and Dawson, 2003). Bats that make use of sounds produced by their prey face a more tractable task than if the prey were silent, but they still have to maneuver in the clutter or approach the ground using echoes to guide their flight. For example, echoes from the ground arrive at nearly the same time as echoes from insects resting on the ground, which makes detecting the target difficult, although a grazing approach to the ground could minimize these cluttering reflections.
Insects in vegetation often are smaller than the leaves themselves so the clutter echoes are often more intense than the target, while also being located within the same time window. Among species of bats that pursue insects in aerial interception maneuvers, most species emit sounds with only one or two harmonics (Fenton, 1995). In contrast, bats that frequently hunt for prey or search for fruit in vegetation typically emit wideband FM sounds that contain three to five harmonic sweeps (Fenton, 1995; Schnitzler et al., 2003; Simmons et al., 1979). Big brown bats are unusual among aerial-feeding insectivorous bats in emitting sonar sounds that contain multiple harmonics, even when closing in to complete an aerial interception (Saillant et al., 2007). The prevalence of harmonics in the sounds combined with the evidence that these bats take prey from surfaces raises the possibility that big brown bats capture insects in clutter more than is realized.
It is important to distinguish between the completions of aerial interceptions of flying insects close to background objects from the capture of prey that rest or swarm on leaves or the ground (Schnitzler et al., 2003; see video clips in Simmons et al., 2001 and Simmons, 2005). In laboratory tests, free-flying insectivorous bats (five species of Myotis) were able to capture insects presented at varying distances (5–50 cm) from a clutter screen that was designed to mimic the edge of vegetation (Siemers and Schnitzler, 2004). At distances of 25–50 cm, the capture success rate was almost 100%. The minimum distance from the insect to the clutter that still allowed for successful captures varied from 5 to 10 cm across species. In a similar experiment, Eptesicus fuscus could capture tethered insects close to a background of clutter (artificial hanging plant) that simulated vegetation (Moss et al., 2006). The bats were able to catch insects within 20–40 cm (80%–90%, respectively), but not within 10 cm (40%) of the clutter. Yet other experiments of this type with Eptesicus nilssonii found that, while bats cannot capture moths located below the surface of grass, their capture success increases (40%–50%) when the moths fly 50 cm or more above the grass (Rydell, 1998). Although these bats did not capture any moths at distances below 50 cm from the grass surface, they did make attempts to capture, indicating that the bats detected the presence of the insect. When echoes of the moths were measured against the grass surface, they proved to be distinguishable from their spectrograms when the insect was located as close as 10 cm below or above the surface of the grass (Jensen et al., 2001). This minimum distance for acoustic detection is well below the 50 cm distance above the grass necessary for successful capture.
The aim of this study was to determine how effectively big brown bats can detect a target embedded in clutter under controlled laboratory conditions simulating an insect in vegetation. The expectation was that the bat’s detection threshold would be related to the target’s reflection strength in relation to the clutter, so the results were analyzed in terms of ratios of echo energy between the target and clutter.
METHODS
Subjects
Subjects were four adult male wild-caught big brown bats (Eptesicus fuscus) from Rhode Island. They were housed in a colony room on a reverse 12:12 light∕dark cycle with a controlled temperature of 22–25 °C and 60% relative humidity. The bats were fed mealworms (Tenebrio larvae) and provided with vitamin-enhanced (Poly-Vi-Sol) waterad libitum. All subjects weighed between 14 and 15 g. Animal procedures were consistent with guidelines established by the National Institutes of Health and were approved by the Brown University Animal Care and Use Committee.
Psychophysical procedure
The experimental setup is shown in Fig. 1. Each bat was placed on an elevated Y-shaped platform (12 cm wide and 20 cm front to back) and trained in a two-alternative forced-choice procedure to locate, via echolocation, the presence of the target presented on either the bat’s left or right. Each trial lasted 2–3 s. All trials were run double blind and in complete darkness so that one experimenter (trainer) was unaware of the target location until after the conclusion of the trial and potential visual cues were controlled. For each trial, the target was positioned and the bat’s response was recorded by a second experimenter (recorder). The location of the target varied from left to right on a schedule using pseudorandom sequences (Gellermann, 1933) grouped together to make sets of 50 consecutive trials. These sets imposed the constraint that the target would not be present on the same side for more than three successive trials and that there were an equal number of left-side and right-side presentations. The bat was rewarded with a piece of a mealworm offered in plastic forceps by the trainer for each correct trial. An incorrect response was followed by a high frequency broadband sound indicating that an error had been made.
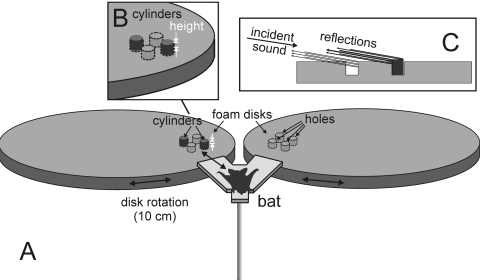
Figure 1.
(A) Diagram of the two-alternative forced-choice detection task. The bat was placed on the Y-shaped platform and trained to detect a target consisting of two small cylinders located on the right or left side. The cylinders were 5 cm apart and were embedded in holes cut into the surface of the 2.5 cm thick foam disks 90 cm in diameter. They varied in height by 12, 8, 4, or 2 mm above the surface of the foam (see inset in B). To receive food reward, the bat walked down (arrow) the side of the platform that corresponded to the target. The target’s cylinders occupied two holes on the left (as indicated in B) or the right (alternated pseudorandomly), and they reflected echoes whose strength depended on the cylinders’ protruding height. The other holes were empty from one trial to the next, but they generated their own reflections (inset in C) to compete with echoes from the cylinders and serve as clutter. For successful detection, the bat had to discriminate the reflections of the cylinders from these added clutter reflections. The experiment measured the bat’s detection performance while the strength of the target’s reflections was decreased in several steps by reducing the protruding height of the cylinders until target strength was similar to the clutter.
Trials were videotaped using a black-and-white charge coupled device (CCD) video camera (Supercircuits, Inc., Type 15-CB22-1) mounted on the ceiling to look down on the bat and the apparatus. To keep the room dark for experiments, illumination for the video recordings was provided by two infrared light emitting diode (LED) panels (Supercircuits, Inc.) mounted beside the video camera. A bat detector (Mini-3 model from Ultra Sound Advice, Ltd.) tuned to 28 kHz was located in front of the bat at a distance of 1.4 m to record its sonar broadcasts. The video signal and the audio output from the bat detector were recorded on 8 mm digital video tape using a Sony Video Walkman. During the experiments, an audible display of the bat’s echolocation emissions was provided by the bat detector. For some sessions, the echolocation emissions were recorded with two ultrasonic microphones (Titley Electronics, Australia, Ltd.) at a sampling rate of 384 kHz on a digital instrumentation recorder (Sony SIR-1000W) that also recorded the video signal.
Target and clutter
The target to be detected consisted of two black plastic cylinders, each with a diameter of 1.6 cm, separated by 5 cm to form a dipole target. A dipole target was used because the size of the individual cylinders (1.6 cm diameter, 0.2–1.2 cm in height) approximates those of June beetles and crickets resting on a surface. Moreover, baseline information about detection and discrimination of dipoles by bats was available from the results of new behavioral experiments (DeLong et al., 2008) The dipole configuration caused echoes returning to the bat to vary slightly in amplitude and spectrum as the aspect angle was varied from one trial to the next, so that no single amplitude increment or spectral profile could be used by the bat to find the target. This variation provides more naturalistic stimuli for the detection task.
The target was presented on one of the two circular foam covered Plexiglas surfaces (90 cm in diameter and 2.5 cm thick) on the bat’s left and right [Fig. 1A]. The target was placed into holes cut partway into the foam surface. In each (right or left) foam disk, there were four holes 1.7 cm deep and 1.7 cm in diameter, arranged in a diamond-shaped pattern with a 5 cm diagonal spacing [Fig. 1B]. The holes marking the four corners of the diamond were positioned as mirror images between sides. Thus, the square pattern of the holes on the left was about 90° different from the pattern on the right.
The target was inserted into two of the holes in the square pattern—always on the opposite corners of the square, so that the target had an aspect angle of about 40°–50° as a consequence of the slight trial-to-trial rotation of the disks (counterclockwise when on the left foam disk, and clockwise when on the right foam disk; see below). On any given trial, the cylinders occupied two of the holes either on the left or on the right foam disk; the remaining holes (two in the “target” side, and four on the “nontarget” side) were left to reflect echoes of the bat’s sounds from their inside surfaces [see Fig. 1C].
The two circular foam pads were held in place on Plexiglas disks that also had a diameter of 90 cm. The Plexiglas disks under the foam could be rotated to change the location of the target relative to the bat on the Y-shaped platform. Both disks were always rotated the same distance [up to a 10 cm movement at the circumference of each disk; see arrows in Fig. 1A], so that the positions of the holes on both sides were at approximately the same distance from the bat on each trial. As such, the distance from the target to the bat ranged from 25 to 35 cm across trials. This span of distances prevented the bat from using a single, restricted time window to search for the target, and the resulting change in the aspect angle of the dipole prevented the bat from using a single decibel increment in amplitude or a single echo spectral shape to find the target (DeLong et al., 2008).
In the experiment, the height of the target in the foam was varied so that the profile of the target protruding above the surface of the foam disk, and thus the strength of its echo, could be varied systematically. The protrusion height of the target was either +12, +8, +4, or +2 mm. These target heights were presented in descending order on successive blocks of trials. The initial training criterion was set at >90% correct performance for the first condition (+12 mm), which took 150 trials. Each subsequent condition was run for a total of 150 trials. The protrusion height of the cylinders was decreased in steps from 12 mm to 8, 4, and 2 mm. At the end of the 2 mm condition, the initial 12 mm protrusion height was repeated as a control. In all of these conditions, the empty holes were present, too, and their reflections constituted a stable component of the acoustic backscatter that comprised the proximal stimuli. Thus, the bat received echoes from the target, holes, and rest of the foam panel. The question of interest was how high the target must protrude in order to be detected.
Echo measurements
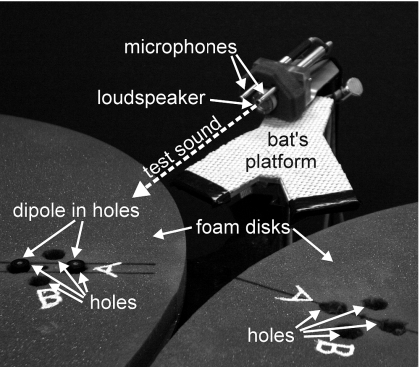
Figure 2 shows a photograph of the apparatus used to measure echoes from the clutter (holes) and target (dipole). A test signal consisting of a 1 ms long FM sweep (110–15 kHz) signal, similar to the bat’s echolocation emissions, was projected from an electrostatic loudspeaker (LTV Model EST-2; 20 mm diameter) aimed toward the target on one of the foam disks. The returning sounds (echoes) were recorded using two Bruel & Kjaer Model 4138 (“1∕4 in.”) condenser microphones separated by 24 mm. The two microphones, and their placement, were used to represent the bat’s two ears. The apparatus was originally constructed as part of a model of a bat’s head. The test signal was generated by a Tucker-Davis Model QDA2 digital-to-analog conversion and wave form memory board running in a Pentium-III computer. This analog electrical signal was amplified and mixed with 200 V polarization using a Krohn-Hite Model 7500 power amplifier before being delivered to the loudspeaker. Overall sound pressure 10 cm in front of the loudspeaker was 100 dB SPL. Signals from the microphones were amplified by 40 dB (Bruel & Kjaer Model 5935 Microphone preamplifier∕power supply), filtered to 15–100 kHz (Wavetek-Rockland Model 442 variable bandpass filter), and digitized at a sampling rate of 500 kHz and 12 bit accuracy by one of the analog-to-digital channels in a National Instruments PCI-6111e 2-channel analog-to-digital converter board.
Figure 2.
Echoes from the clutter (holes) and target (dipole cylinders) were measured by projecting a 1 ms long FM signal (sweeping from 110 to 15 kHz), similar to the bat’s echolocation emissions, from a 2 cm electrostatic loudspeaker and recording the echoes with two condenser microphones. To make the dipole echo measurements, the cylinders were placed in the foam holes with a specific protrusion height (12, 8, 4, or 2 mm) and the entire scene was ensonified to generate reflections.
To make the target echo measurements, the target was placed in the holes in one of the foam disks (Fig. 1). For each cylinder protrusion height (see above; 12, 8, 4, or 2 mm), the loudspeaker ensonified the target and the adjacent holes for 20 repetitions of the test signal while the signals recorded by the microphones were averaged to improve the signal-to-noise ratio. Similarly, the acoustic backscatter was measured for the holes alone and for the smooth surface of the foam disk without any holes. Echo measurements were also made for a flat target oriented perpendicular to the axis of sound propagation to show the reflection from a single point target [see Fig. 3C]. This was used as a reference for estimating the strength of echoes reflected by the target and by the holes. Custom software written in LABVIEW and MATLAB was used to window the acoustic backscatter to a time span of 250 μs, which is the time span for echoes returning from all four holes, plus the two reflections from the dipole itself, over an equivalent distance span of 4–5 cm. This software also controlled the production of test signals by the computer boards and automatically averaged the echoes. The digital files containing the averaged echoes were processed by additional MATLAB routines to display their spectra, spectrograms, and the output of a cross correlation or matched-filter receiver operating with the wave form reflected by the flat target as a replica. Use of the flat-target echo for the correlation replica eliminated the response characteristics of the loudspeaker and the microphones from measurements made on the echoes. To represent the true relative amplitudes of the reflections across different target heights, the cross correlation outputs were expressed as cross covariance; that is, without the normalization to a maximum value of +1.0 as is used for cross correlation. When the cross covariance functions are full wave rectified, differences in the magnitudes of these functions between the flat reference target and any of the stimulus targets directly indicate relative target strength.
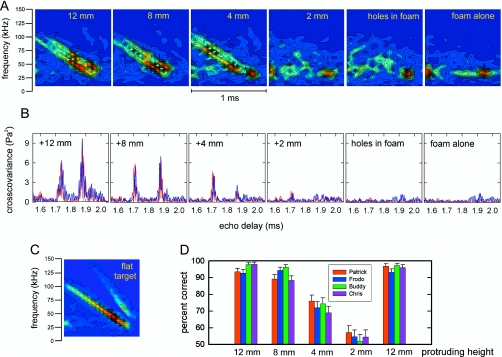
Figure 3.
(A) Spectrograms of reflections from the target at an orientation of 50° [see Fig. 1B] for different amounts of protrusion of the cylinders above the surface of the foam disk. The double sweeps in these spectrograms trace the separate reflections from the cylinders, which are oriented so their echoes arrive about 180 μs apart (see C for reference). (B) Output of matched filter (cross correlation receiver with full-wave-rectified plots) for the same series of reflections as in A. Red and blue curves trace outputs from left and right microphones (see Fig. 2). The twin peaks in the cross covariance curves register the separate reflections from the cylinders, while their heights have the same scale to indicate relative target strength. (C) Spectrogram for the specular reflection from a flat target oriented perpendicular to the sound from the loudspeaker (Fig. 2). The sweep pattern in C is for reference to the spectrograms in A, which show two closely spaced, overlapping sweeps from the two cylinders. The plots are labeled with the target’s protrusion height (12, 8, 4, or 2 mm and empty holes). The empty holes and the blank foam surface produce acoustic scattering (clutter) across the same time window as the echoes from the target. (D) Performance (percent correct responses) of four big brown bats in detection task with different amounts of cylinder protrusion. Performance closely mirrors visibility of the FM sweeps in the spectrograms (A) or the prominence of the peaks in the cross covariance curves (B).
RESULTS
Target echoes
Recordings of reflections from the target and from the holes yielded estimates of the timing and amplitude of individual scattered signals. For the cylinders, the strongest signals were returned from their front surfaces, while for the holes, these were returned from their back surfaces [Fig. 1C]. These echoes are illustrated in Figs. 3A, 3B. For reference, the test signal (1 ms FM sweep from 110 to 15 kHz) is represented by the spectrogram for the reflection from a flat target oriented perpendicular to the sound path [Fig. 3C]. In this single, specular reflection, which is essentially the same as the incident sound, note the strong first-harmonic sweep and the much weaker second harmonic due to the distortion by the electrostatic transducer. Figure 3A shows the spectrograms of the reflections from cylinders with protrusion heights of 12, 8, and 4 mm. These spectrograms show the principal acoustic feature of the dipole target to be a pair of strong specular reflections, as illustrated by the two closely spaced first-harmonic FM sweeps separated by roughly 200 μs for the 12 mm target height [Fig. 3B]. At the 8 mm target height, the two sweeps are still prominent, while at the 4 mm target height, the second of the two sweeps appears weaker than the first sweep in the spectrogram. Additional regions of energy in the spectrograms show the reflections from the holes in the foam disks and from the foam alone. Comparison of the spectrograms for the 2 mm target height with the spectrogram for the holes alone reveals no obvious sign of the presence of the cylinders at such a low protrusion height. The pattern of reflections returned by the holes themselves is similar, indicating that the specular reflections from the front of the cylinders has declined to an insignificant level relative to the clutter. When the smooth surface of the foam disk was ensonified without the holes or the cylinders, there still is some energy in the spectrogram, indicating that some of the clutter is due not specifically to the holes but to the foam disk itself on the hard Plexiglas surface.
Figure 3B shows the output of a cross correlation or matched-filter receiver for the same reflections, as shown by the spectrograms in Fig. 3A. There are two curves in each plot, one for the left microphone (red) and the other for the right microphone (blue). (Here, no special significance is attached to the use of two microphones; they were part of the echo-measuring apparatus and both their outputs are illustrated.) The curves are plots for values of cross covariance to show the amplitudes of the echoes in units that can be directly compared across different target conditions. They are the full-wave-rectified cross covariance functions of the echoes, with the reflection from the flat target used as the correlation replica. Consequently, the locations of the peaks in Fig. 3B directly represent delay differences between reflections, and the heights of the peaks represent relative target strength. As in the case of the spectrograms in Fig. 3A, the cross covariance functions clearly register the locations of the two cylinders. (At an aspect angle of 40°–50°, the time separation of the reflections from the two cylinders should be about 180 μs.) For protrusion heights of 12, 8, and 4 mm, the twin peaks stand above the background returns from the holes in the foam and the foam itself, unambiguously revealing the presence of the target. However, for a cylinder protrusion height of 2 mm, the cross covariance peaks are comparable in height to the background peaks generated by the clutter, and the target’s presence is only minimally registered, if at all.
Based on the hypothesis that the bat’s detection of the target should depend on the relative strength of its echoes and possibly the presence of the two cylinders as a dipole, the acoustic measurements shown in Fig. 3 lead to the prediction that detection performance should be very good for protrusion heights of 12 and 8 mm, while performance should decline somewhat for a protrusion height of 4 mm and should vanish altogether for a protrusion height of 2 mm.
Detection performance in clutter
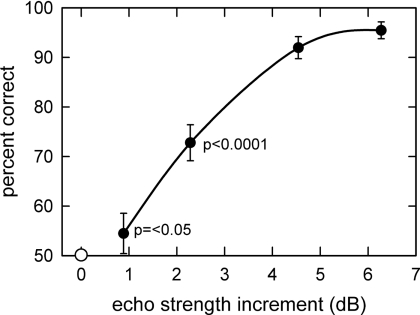
All four bats (“Patrick,” “Frodo,” “Buddy,” and “Chris”) detected the target embedded in foam with a protrusion height of 12 mm at levels exceeding the criterion of 90% correct responses. Figure 3D plots the percentage of correct responses achieved by each bat to facilitate comparison to the acoustic measurements of target strength. The bats’ performance varied as a function of protrusion height, with ∼90% correct at heights of 12 and 8 mm, ∼70%–75% correct at 4 mm, and ∼55% correct at a height of 2 mm. The performance of the bats on the second presentation of the 12 mm protrusion height returned to levels better than 90% correct responses, indicating that the bats did not undergo a loss in their trained response as a consequence of exposure to the more difficult conditions of 4 and 2 mm. The behavioral results mirror changes in the strength of echoes from the target relative to the strength of the clutter echoes [Figs. 3B, 3D]. Figure 4 plots the mean performance of the bats as a function of the reflective strength of the target relative to the clutter. Relative target strength was determined by summing the energy contained in the two 250 μs time windows centered on the peak of the autocovariance function for each cylinder [Fig. 3B]. Then, the energy in the same two time windows for the clutter was summed in the absence of the cylinders. The ratio of target energy to clutter energy was computed for each cylinder protrusion height and expressed in decibels. The curve in Fig. 4 traces the decline in the bats’ detection performance for different target conditions as the relative target strength (ratio of target energy to clutter energy in paired 250 μs windows) decreases with protrusion height. The bats’ performance was greater than chance levels (50%) when the target strength increment was greater than 1–2 dB (Fig. 4).
Figure 4.
Graph showing the mean performance of all four bats for different increments in target strength expressed in decibels relative to the target strength of the holes alone. Target strength is the energy reflected in a 250 μs integration-time window around each cylinder’s reflection delay [see Fig. 3B], expressed in decibels relative to the energy reflected by the holes in the same time window. The bat’s performance was significantly greater than chance (p<0.05) for echo increments greater than 1–2 dB.
DISCUSSION
These data show that big brown bats are able to detect an insect-sized target embedded in clutter. The bat’s task was to locate the target on either the left or right side, in a two-alternative forced-choice procedure, in the presence of additional reflecting structures (holes) common to both sides, which provided clutter. In psychological terms, this task could be described as either detection or discrimination; a distinction that is not important for the essential interpretation of the data. The bats’ performance was graded according to the height of the cylinders protruding above the clutter, or according to the increment in echo strength, beyond that of the clutter alone, expressed in decibels. It diverged from chance when the target plus the clutter returned echoes approximately 1–2 dB greater than echoes from the clutter alone. Note that the same 250 μs time window was used for specifying the strength of the echoes for the clutter and the target plus clutter. The simplest description of the result is that the bat locates the target on the left or right by perceiving the small increment in echo strength based on target protrusion height.
In this experiment, the colocalization of the target and clutter removed the cues from the distance separation between a target and clutter present in previous field experiments (Jensen, 2001; Moss et al., 2006; Rydell, 1998; Siemers and Schnitzler, 2004) that examined bats’ abilities to capture prey in clutter. Because the target and clutter were colocalized, the results indicate that the bats are able to perform a task with demands similar to that of a natural situation where they are detecting insect prey (e.g., crickets and katydids) that often do not fly at night, and must be captured from substrates such as the ground or vegetation.
The trial-to-trial partial rotation of the foam disks shifted the distance from the bat on the platform to the holes and the target over a span of about 25–35 cm. As a result, echoes from the target varied in delay over a range of 1.5–2.0 ms between trials. Moreover, the delays of echoes from the target, and from the two empty holes on the same side (left or right) as the target, shifted by up to 0.5 ms relative to the echoes from the four empty holes on the other side. These movements were apart from the left-right alternations of target position. The bat could not have found the target on the left or the right just by examining whether a fixed, narrow time window contained reflections. Instead, it had to examine a broader time window because the clutter echoes and target echoes could fall anywhere within the span of delay created by trial-to-trial rotation of the disks.
The echoes from the target arrive within 300 μs of the cluttering echoes from the holes (Fig. 3). Such temporal conjunction of target and clutter echoes creates the strongest interference (Simmons et al., 1989) and allows us to evaluate influence of clutter strength on detection performance without having to hypothesize a function describing the decay of clutter interference over time (important in situations where target and clutter echoes are significantly separated in time). Two earlier experiments (Masters and Jacobs, 1989; Troest and Møhl, 1986) tested detection abilities for phantom (electronically generated) targets. Because of the setups for these experiments, the bat received the target stimulus as well as competing echoes from the face of the loudspeaker used to broadcast the electronic echoes. For both experiments, the thresholds for the target stimulus were interpreted as potentially masked by echoes reflected by the loudspeaker. These loudspeaker clutter echoes arrived at delays considerably different than the test stimuli (2.9 ms earlier in Masters and Jacobs, 1989; 2.1 ms later in Troest and Møhl, 1986), and much longer than the time difference in the current experiment. Thus, the observed negative stimulus-to-clutter ratios estimated in these earlier studies might be expected because the clutter is so removed in time from the target stimuli, in which case the masking effect inherent in clutter interference would be mitigated (see Simmons et al., 1989). Neither of these studies provided an explanation for how to discount the strength of the clutter when it arrives at a different time than the test echoes. In view of the relatively high echo-detection thresholds obtained in those studies compared to the audiometric sensitivity of big brown bats for either tone bursts (Koay et al., 1997) or echoes (Kick, 1982), it is unclear whether those experiments actually examined the effects of clutter on echo detection.
Detection of stationary targets colocalized with clutter on a substrate, as tested here, is different from the task faced by a flying bat completing an aerial interception of prey near background objects. Previous experiments that examined aerial interception of prey by bats in the vicinity of clutter found that bats do not successfully take targets within 10–20 cm of clutter (e.g., Jensen et al., 2001; Moss et al., 2006; Rydell, 1998; Siemers and Schnitzler, 2004). However, it is not clear that this 10–20 cm limit of proximity for successful interception is determined by perceptual limitations of echolocation. Perhaps instead there is a reluctance of flying bats to engage targets by flying close to obstacles for mechanical reasons (Aldridge and Rautenbach, 1987; Fenton, 1995; Neuweiler, 2000; Norberg and Rayner, 1987). Because the bats and target were stationary in the present experiment, there were no issues of maneuverability to obscure the measurement of perceptual sensitivity. However, this experiment was conducted in a laboratory under favorable psychoacoustic conditions. Given the rapid motion of bats in pursuit of prey near clutter, additional perceptual limitations might prevail in the field beyond those present in our laboratory experiment.
ACKNOWLEDGMENTS
This research was funded by NIH Grant No. R01-MH069633 and ONR Grant No. N00014-04-0415.
References
- Aldridge, H., and Rautenbach, I. L. (1987). “Morphology, echolocation, and resource partitioning in insectivorous bats,” J. Anim. Ecol. 56, 763–778. [Google Scholar]
- DeLong, C. M., Bragg, R., and Simmons, J. A. (2008). “Evidence for spatial representation of object shape by echolocating bats (Eptesicus foscus),” J. Acoust. Soc. Am. 123, 4582–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton, M. B. (1995). “Natural history and biosonar signals,” in Hearing by Bats, edited by Popper A. N. and Fay R. R. (Springer-Verlag, New York: ), pp. 37–86. [Google Scholar]
- Fullard, J. H., Ratcliffe, J. M., and Guignion, C. (2005). “Sensory ecology of predator-prey interactions: responses of the AN2 interneuron in the field cricket, Teleogryllus oceanicus to the echolocation calls of sympatric bats,” J. Comp. Physiol., A 191, 605–618. [DOI] [PubMed] [Google Scholar]
- Gellermann, L. M. (1933). “Chance orders of alternating stimuli in visual discrimination experiments,” J. Gen. Psychol. 42, 206–208. [Google Scholar]
- Griffin, D. R. (1958). Listening in the Dark (Yale University Press, New Haven, CT: /reprinted by Cornell University Press, Ithaca, NY, 1986). [Google Scholar]
- Hamr, J., and Bailey, E. D. (1985). “Detection and discrimination of insect flight songs by big brown bats (Eptesicus fuscus),” Biol. Behav. 10, 105–121. [Google Scholar]
- Jensen, M. E., Miller, L. A., and Rydell, J. (2001). “Detection of prey in a cluttered environment by the northern bat Eptesicus nilssonii,” J. Exp. Biol. 204, 199–208. [DOI] [PubMed] [Google Scholar]
- Kick, S. (1982). “Target-detection by the echolocating bat, Eptesicus fuscus,” J. Comp. Physiol. [A] 145, 432–435. [Google Scholar]
- Koay, G., Heffner, H. E., and Heffner, R. S. (1997). “Audiogram of the big brown bat (Eptesicus fuscus),” Hear. Res. 10.1016/S0378-5955(96)00208-0 105, 202–210. [DOI] [PubMed] [Google Scholar]
- Kurta, A., and Baker, R. H. (1990). “Eptesicus fuscus,” Mammalian Species 356, 1–10. [Google Scholar]
- Masters, W. M., and Jacobs, S. C. (1989). “Target detection and range resolution by the big brown bat (Eptesicus fuscus) using normal and time-reversed model echoes,” J. Comp. Physiol., A 166, 65–73. [Google Scholar]
- Moss, C. F., Bohn, K., Gilkenson, H., and Surlykke, A. (2006). “Active listening for spatial orientation in a complex auditory scene,” PLoS Biol. 10.1371/journal.pbio.0040079 4, 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, C. F., and Surlykke, A. (2001). “Auditory scene analysis by echolocation in bats,” J. Acoust. Soc. Am. 10.1121/1.1398051 110, 2207–2226. [DOI] [PubMed] [Google Scholar]
- Neuweiler, G. (2000). The Biology of Bats (Oxford University Press, New York: ). [Google Scholar]
- Norberg, U. M., and Rayner, J. M. V. (1987). “Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy, and echolocation,” Philos. Trans. R. Soc. London, Ser. B 10.1098/rstb.1987.0030 316, 335–427. [DOI] [Google Scholar]
- Ratcliffe, J. M., and Dawson, J. W. (2003). “Behavioural flexibility: the little brown bat, Myotis lucifugus, and the northern long ear bat, M. septentrionalis, both glean and hawk prey,” Anim. Behav. 66, 847–856. [Google Scholar]
- Ratcliffe, J. M., Raghuram, H., Marimuthu, G., Fullard, J. H., and Fenton, M. B. (2005). “Hunting in unfamiliar space: echolocation in the Indian false vampire bat, Megaderma lyra, when gleaning prey,” Behav. Ecol. Sociobiol. 58, 157–164. [Google Scholar]
- Rydell, J. (1998). “Bat defense in lekking ghost swifts (Hepialus humuli), a moth without ultrasonic hearing,” Proc. R. Soc. London, Ser. B 265, 1373–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saillant, P. A., Simmons, J. A., Bouffard, F. H., Lee, D. N., and Dear, S. P. (2007). “Biosonar signals impinging on the target during interception by big brown bats, Eptesicus fuscus,” J. Acoust. Soc. Am. 10.1121/1.2714920 121, 3001–3010. [DOI] [PubMed] [Google Scholar]
- Schmidt, S., Hanke, S., and Pillat, J. (2000). “The role of echolocation in the hunting of terrestrial prey—new evidence for an underestimated strategy in the gleaning bat, Megaderma lyra,” J. Comp. Physiol., A 186, 975–988. [DOI] [PubMed] [Google Scholar]
- Schnitzler, H.-U., Moss, C. F., and Denzinger, A. (2003). “From spatial orientation to food acquisition in echolocating bats,” Trends Ecol. Evol. 10.1016/S0169-5347(03)00185-X 18, 386–394. [DOI] [Google Scholar]
- Siemers, B. M., and Schnitzler, H. U. (2004). “Echolocation signals reflect niche differentiation in five sympatric congeneric bat species,” Nature (London) 10.1038/nature02547 429, 657–661. [DOI] [PubMed] [Google Scholar]
- Simmons, J. A. (2005). “Big brown bats and June beetles: multiple pursuit strategies in a seasonal acoustic predator-prey system,” ARLO 10.1121/1.1985957 6, 238–242. [DOI] [Google Scholar]
- Simmons, J. A., Eastman, K. M., Horowitz, S. H., Farrell, M. J., and Lee, D. N. (2001). “Versatility of biosonar in the big brown bat, Eptesicus fuscus,” ARLO 10.1121/1.1352717 2, 43–48. [DOI] [Google Scholar]
- Simmons, J. A., Fenton, M. B., and O’Farrell, M. J. (1979). “Echolocation and pursuit of prey by bats,” Science 10.1126/science.758674 203, 16–21. [DOI] [PubMed] [Google Scholar]
- Simmons, J. A., Freedman, E. G., Stevenson, S. B., and Chen, L. (1989). “Clutter interference and the integration time for echoes in the bat, Eptesicus fuscus,” J. Acoust. Soc. Am. 10.1121/1.398693 86, 1318–1332. [DOI] [PubMed] [Google Scholar]
- Surlykke, A., and Moss, C. F. (2000). “Echolocation behavior of big brown bats, Eptesicus fuscus, in the field and the laboratory,” J. Acoust. Soc. Am. 10.1121/1.1315295 108, 2419–2429. [DOI] [PubMed] [Google Scholar]
- Troest, N., and Møhl, B. (1986). “The detection of phantom targets in noise by serotine bats; negative evidence for the coherent receiver,” J. Comp. Physiol., A 159, 559–567. [DOI] [PubMed] [Google Scholar]