Abstract
Effects of vibration on human vocal fold extracellular matrix composition and the resultant tissue viscoelastic properties are difficult to study in vivo. Therefore, an in vitro bioreactor, simulating the in vivo physiological environment, was explored. A stress-controlled commercial rheometer was used to administer shear vibrations to living tissues at stresses and frequencies corresponding to male phonation, while simultaneously measuring tissue viscoelastic properties. Tissue environment was evaluated and adjustments made in order to sustain cell life for short term experimentation up to 6 h. Cell nutrient medium evaporation, osmolality, pH, and cell viability of cells cultured in three-dimensional synthetic scaffolds were quantified under comparably challenging environments to the rheometer bioreactor for 4 or 6 h. The functionality of the rheometer bioreactor was demonstrated by applying three vibration regimes to cell-seeded three-dimensional substrates for 2 h. Resulting strain was quantified throughout the test period. Rheologic data and cell viability are reported for each condition, and future improvements are discussed.
INTRODUCTION
Empirical observation of cellular response to vocal fold vibration is difficult to study in humans. An alternative is to grow a tissue surrogate from vocal fold derived cells in vitro and expose it to vibration force fields that are comparable to human phonation in a bioreactor. A bioreactor is a system that simulates physiological environments for the creation, physical conditioning and testing of cells, tissues, precursors, support structures, and organs in vitro (Barron et al., 2003). Freed and Vunjak-Novakovic (2000) outlined that to be functional, a bioreactor should be capable of performing at least one of four functions: (1) establish a uniform distribution of cells on a three-dimensional (3-D) scaffold, (2) maintain gas and nutrient concentrations amenable to cell growth, (3) provide fluid circulation in order to move nutrients into and remove waste out of the growing tissue, and (4) expose developing tissue to physical stimuli.
Unique challenges of vocal fold tissue bioreactor design not only include the framework laid down by Freed and Vunjak-Novakovic but also include quantifying physical stimuli and measuring real-time viscoelastic tissue properties. These additional functions are necessary for accurate simulation of typically occurring phonation forces, and for examining biomechanical contributions of cellular and extracellular matrix (ECM) response to specific forces. It is important to find connections between macroscopic and microscopic vocal fold biomechanics. Viscoelastic properties of vocal fold tissues are macroscopic frequency-dependent quantities that directly affect mucosal wave form, phonation threshold pressure, and fundamental frequency (Chan and Titze, 2006; Perlman and Durham, 1991; Titze and Durham, 1991; Titze, 1988; Titze and Hunter, 2004). It is therefore essential to accurately determine the rheologic character of engineered vocal fold tissues while they are being tested or conditioned in a bioreactor. Characterization of microscopic cellular properties such as protein and gene expression, cell orientation, and molecular architecture, after the same exposures, can then help to better understand their contributions to biomechanical vocal fold vibration. Aims of the present investigation were to improve cell viability conditions of a cell-seeded three-dimensional substrate in a rheometer bioreactor, to deliver three different vibrational loading conditions where both input stress and output strain of the tissue were quantified, and to determine rheologic and cell viability response of three-dimensional cellularized tissues after vibration.
BIOREACTOR REQUIREMENTS
To date, some but not all of the stipulated bioreactor requirements have been met for the rheometer bioreactor used in the present study.
Uniform cell distribution
The first function of a bioreactor, to generate uniform cell distribution in a three-dimensional scaffold, is at this time a reflection of the scaffold. There is no expectation of substantial tissue development while in a bioreactor for experiments lasting several hours. Instead, cells and ECM should inhabit a synthetic substrate for several weeks while in an incubator. During this time mechanical properties of the scaffold should remain stable, and similar to vocal fold tissues, and the scaffold should support cell growth and protein production.
Tecoflex® (TFX) (Lubrizol Advanced Materials, Inc) is a nonbiodegradable, medical grade polymer. It was used in the present study, because TFX had similar properties to vocal fold tissues and was well characterized as biocompatible. TFX has been used for synthetic vascular grafts, in vitro tissue engineering studies (Klemuk et al., 2001; Mulder et al., 1998; Titze et al., 2005; Titze et al., 2004a; Vara et al., 2005; Webb et al., 2003), and has been fabricated in many shapes including electrospinning for controlled microfiber formation and increased cellular matrix formation (Andrews et al., 2008; Andrews and Hunt, 2008; Mulder et al., 1998). When TFX was fabricated as a 60%–70% porous scaffold with pore diameters of 66 μm or larger, its Young’s modulus was similar to linearly functioning vocal fold ligament (20–30 kPa) (Min et al., 1995) and remained constant up to 100% strain (Webb et al., 2003). When this same material was seeded with tracheal fibroblast cells, as was done in the present study, cells distributed homogeneously and stained abundantly positive for collagen I, vimentin, and fibronectin. Fluorescein diacetate and ethidium homodimer staining indicated cell viability and no evidence of necroses up to 4 weeks in culture (Webb et al., 2003).
Gas, nutrient, and fluid exchange
Direct environmental measurements in the rheometer bioreactor are needed. Evaporation of the cell nutrient medium was a unique challenge when using a stress-controlled rheometer to vibrate living tissues (Titze et al., 2004b). Up to 22% of cell nutrient medium evaporated in 3 h and about half evaporated at 8 h with most fibroblasts being nonviable.
Use of a sealed incubator into which a bioreactor is placed is a common method for controlling gases and humidity, but this is not feasible for the rheometer bioreactor. The rheometer itself is large, measuring approximately 50 cm tall, 30 cm wide, and 30 cm deep, has purified air lines, water lines, and digital cables connected to peripheral devices, and cannot be moved without performing extensive calibrations. The user must also have access to stationary and moving parts for mounting, dismounting, raising, and lowering the plate. Because of these circumstances, our goal for the present investigation was to evaluate the extent to which the mounted cup, along with a newly designed acrylic cover, could sustain an adequate cell environment.
Osmotic pressure and pH change are indicators of medium evaporation and cell environment adequacy. Osmolality is the ratio of moles of solute to kilograms of solvent. De Zengotita, Schmelzer, and Miller (2002) found that increased osmolality, evaluated at 320–476 mOsm∕kg, decreased hybridoma cell proliferation and increased apoptotic cell death. Potter and DeMarse (2001) reported that changes greater than 50 mOsm∕day resulted in neural cell culture death. Osmolality of fresh cell nutrient medium from the Titze et al. (2004b) study, was 300.3 mOsm∕kg (not published). After 3 h, the osmolality would have been 464.6 mOsm∕kg, a value near the limits of the De Zengotita et al. study and exceeding Potter and DeMarse’s maximum osmolality change by 26 times.
Potential of hydrogen (pH) of the nutrient medium used in the Titze et al. (2004b) study, a measure of adequacy of gas supplementation, rose from 7.2 to 8.69 in 3 h. This indicates a lack of CO2 gas exchange within the medium despite CO2 exposure at the upper surface of the medium. Hepes (N-2-hydroxyethylpiperazine-N′-2-ethenesulphonic acid), a widely used organic buffer, is resistant to rapid pH change. Buffer strength for cell culture applications is usually in the range of 10–20 mM, but concentrations can vary depending on the cell nutrient medium used and can be toxic if concentrations are too high (Freshney, 2000). In the Titze et al. (2004b) study, cell viability improved by including 1.5 mM of Hepes buffer in the medium, but pH was 7.71, still high after 2.5 h ambient air exposure, and quantitative osmolality and cell viability results were not reported (Titze et al., 2004b).
Vibrational stresses quantified
Force application to tissues in a bioreactor is quite common, but quantifying vibrational stresses has been attempted in very few bioreactors (Bacabac et al., 2006; Tanaka et al., 2003; Titze et al., 2004b; Titze et al., 2005; Titze et al., 2004a). Bacabac and colleagues determined regions of quasi-steady-state dynamic flow in a parallel plate flow bioreactor but the bioreactor was limited to 5–30 Hz vibrations. Tanaka used a compression bioreactor that delivered 1 second periodic compression, white noise compression of 0–50 Hz, or a combination of the two at 3000 and 300 μstrain, respectively (Tanaka et al., 2003). Responding movement of the osteoblast seeded collagen was not reported. Titze and colleagues developed a bioreactor for vocal fold tissues that delivered low frequency (<5 Hz) elongation and high frequency (up to 100 Hz) transverse movement to engineered tissue ribbons (Titze et al., 2004a). Analytical calculations of standing waves matched fairly well with experimental displacements (Titze et al., 2005). Shear strain maxima were 0.1–0.2 up to 70 Hz and 0.05 at 130 Hz, with a strain gradient along the length of the ribbon. This shear strain and frequency is at the lower boundary of physiologic vocal fold vibration. Instrumental limitations precluded larger strain delivery, and viscous and elastic tissue measurements, while in the bioreactor, were still not feasible.
The rheometer bioreactor used in the present investigation is capable of delivering vibrational shear strains observed during vocal fold movement and directly measuring viscoelastic properties. A commercially built rheometer, the Bohlin CVO 120 (Malvern Instruments, Worcestershire, UK), exposes developing tissue to physical stimuli and measures rheologic properties. A custom designed stainless steel cup mounts directly onto the base plate of the rheometer so that the upper plate and cell-seeded substrate are submerged in nutrient medium. The upper plate then makes contact with the substrate and the torque motor is programmed to apply the desired shear stress or strain. The rheometer bioreactor is capable of delivering 1–2 Hz shear strains, on the time scale of pitch change, or dynamic shear strains at low speaking frequencies, currently limited to 150 Hz. Titze, Klemuk, and Gray (2004b) determined that a range of strains is possible depending on four parameters: shear elastic modulus of the sample G′, sample radius R, sample thickness h, and system inertia I. These four terms shift the location of the motor∕plate∕sample (i.e., system) resonant frequency. This frequency is defined as the maximum strain per N m torque. It occurs when measuring properties of viscoelastic solids. The present study aimed to take advantage of the large strain potential at the system resonant frequency when exposing engineered substrates to vocal fold-like amplitudes and frequencies. By increasing radius and decreasing thickness, we hypothesized that this resonant frequency would be in the desired low sonic frequency range and that strains of near 0.5 could be administered to a living tissue.
Rheologic measurement in the rheometer bioreactor
Viscoelastic measurement of tissues is a required function of a vocal fold tissue bioreactor. The hypothesis is that cellular and extracellular response to vibration exposure, as is experienced in the vocal fold, will be reflected in rheologic change to the tissue as a whole. Work from several research groups support this hypothesis in terms of both passive and active environments. Cells had different shapes, elastic properties of the ensuing tissue, and mechano-transduction when viscoelastic properties of their seeded substrate were varied (Discher et al., 2005; Engler et al., 2006; Takai et al., 2004). Cells also became more or less stiff depending on the frequency, amplitude, or duration of the applied force (Janmey and McCulloch, 2007; Matthews et al., 2006). The present study shows that reliable rheologic properties of engineered vocal fold tissues can be measured in the bioreactor, a functionality that allows for future investigations of cellular mechanical response to vibration.
METHODS FOR MEDIUM EVALUATION
Fibroblast cell culture on 3-D porous Tecoflex® substrates
Established human tracheal scar fibroblast cell lines (T31) were obtained from the Division of Otolaryngology—Head and Neck Surgery, Department of Surgery, University of Utah, Salt Lake City. This cell line, originating from a 16 year old male, was shown to be phenotypically stable through passage 10 (Thibeault et al., 2002), but was always used in lower passages, usually passage five to prevent senescence effects. Tracheal scar fibroblast cells were used rather than vocal fold fibroblasts, because the vocal fold fibroblasts were found to be phenotypically unstable at low passages (Thibeault et al., 2002).
Elastomeric TFX substrates, made from TFX S-80, were supplied by Dr. Patrick Tresco at the Keck Tissue Engineering Center, University of Utah with dimensions of less than 1 mm thick, and 1 mm×1 mm squares or 2 mm×2 mm squares. The substrates were sterilized by cold ethylene oxide treatment. They were exposed overnight to 20 μg∕ml fibronectin solution in Dulbecco’s Phosphate Buffered Saline (PBS), then washed and placed in a petri dish. Cells were passaged, dissociated by trypsin treatment, counted in a hemocytometer, and resuspended at a concentration of 2×106 cells∕ml. Cells were uniformly seeded onto each scaffold, as was described by Webb et al. (2003), by releasing two aliquots of cell suspension from one end to the other at constant speed with a pipette. The substrates were incubated 30 min at 37 °C, turned over, and another aliquot of cell suspension seeded in the same manner on the opposite side. Substrates were incubated for 30 min, and two more aliquots were introduced to the top surface in the same manner. The final concentration was 1.0×105 cells and 5.0×105 cells per matrix for the smaller and larger squares, respectively. Substrates were transferred to a fresh petri dish and cultured for 1–2 weeks with periodic exchange of the nutrient medium. Experiments commenced when cells were elongated across the scaffold pores, as observed under indirect microscopy.
Experimental conditions
Cell viability and medium characteristics were evaluated for six Hepes concentrations ranging from inadequate to excessive, and under two less than ideal environmental conditions. For the first environmental condition, Hepes concentrations of 0, 4.5, 13, 22, and 30 mM were included in cell nutrient media, seeded substrates were placed in the bioreactor stainless steel cup, and were immersed in a given conditional medium, all of which contained gentamicin and fungizone to guard against contaminants. A specially designed acrylic lid was used in all experiments. The lid diameter matched that of the cup, rested flatly on the top of the cup, and included a 2 mm lip around the perimeter to keep it in place. The lid also had a 15 mm diameter hole in the center so that, during a rheometer bioreactor experiment, contact between the moving rheometer shaft and the lid was avoided. The covered cup was then set in a waterbath environment for 4 h. For the second environmental condition, the 0 and 22 mM Hepes concentrations were repeated and additional concentrations of 0.89, 43, and 82 mM were included in cell nutrient media to better detect upper and lower limits of Hepes concentration in terms of pH and cell viability. Cell-seeded substrates were placed in the bioreactor cup, were immersed in the different media, covered with the same acrylic cover, and allowed to set in a laminar flow hood for a slightly longer period of time (6 h) to determine the cellular impact of an extra 2 h. Measurements of pH (Orion Triode pH electrode and model 420A benchtop pH meter, Thermo Fisher Scientific, Waltham, MA) were made at the start and end of each experiment. Osmolality (Advanced Micro-Osmometer model 3300, Advanced Instruments, Inc, Norwood, MA) was measured at the start of every experiment and at the end of the 4 h experiments.
For all cell counts, each Tecoflex®-seeded disk was trypsinized, released cells were centrifuged, resuspended, mixed with trypan blue, and counted using a hemocytometer. Viable cells excluded the dye and were transparent. Nonviable cells absorbed the dye and were blue in color. Viable cell fraction, defined as the ratio of counted cells with no dye uptake to total counted cells, was determined at 0 h for each condition, at 4 h for the waterbath conditions, and 6 h for the laminar flow hood conditions.
RESULTS AND DISCUSSION FOR MEDIUM EVALUATION
Initial viable cell fractions, pH, and osmolality readings were taken for each of the conditions so that the n value was fairly high and the result quite accurate. At time=0 h, mean viable cell fraction and standard error (SEM) were 0.91±0.02 (n=16), mean and standard error pH were 7.07±0.01 (n=18), and mean and standard error osmolality were 294±2 mOsm∕kg (n=5). A control seeded substrate was kept in a 37 °C humidified and 5% CO2 environment incubator with 0% Hepes in the medium for the 4 h experiment, and another control exposed to the same condition was kept in an incubator for the 6 h experiment. At the end of the two time periods, their pooled mean±SEM living fraction was 0.87±0.07 (n=2), mean±SEM pH was 7.2±0.1 (n=4), and mean±SEM osmolality was 297±2 mOsm∕kg (n=2). As expected, under standard cell culture growth conditions, where environmental carbon dioxide and humidity were controlled, living cell fractions reflected physiologically ideal pH and osmotic values.
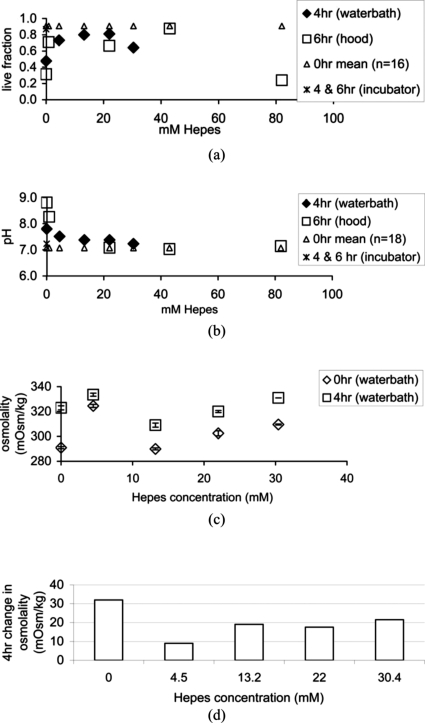
Cell viability, causative pH, and osmolality were then quantified under less ideal conditions, a temperature controlled waterbath, and a laminar flow hood. Figure 1a shows that Hepes concentrations of 0.89–43 mM maintained a 0.64–0.91 viable cell fraction for 4–6 h in a water bath or a laminar flow hood. Live fractions were 0.31–0.48 at the end of the experiment when no Hepes was used for either environmental conditions. When 82 mM Hepes was used the live fraction was 0.24. These values are much lower than the initial live fraction or the incubator control fractions, thus ruling out their use for rheometer bioreactor studies.
Figure 1.
Live cell fractions (a), as determined using trypan blue and hemocytometer counting, pH readings (b), and osmolality (c), as a function of Hepes concentration in cell culture medium. Environmental conditions were in a 37 °C waterbath for 4 h, in a laminar flow hood for 6 h, and in a 37 °C, 5% CO2, humidified incubator at 4 and 6 h. The 0 hour control was plotted across Hepes concentrations as a reference. (d) Osmolality change for five different Hepes concentrations.
Satisfactory Hepes concentration range further reduced when considering pH and osmolality measurements. Figure 1b shows Hepes concentrations 4.5–82 mM maintained a pH of 7.1–7.5, but the 0.89 mM concentration returned a pH of 8.26 thus eliminating this low concentration. The 82 mM concentration is not acceptable because of the very low cell viability shown in Fig. 1a. When considering osmolality readings, values increased regardless of the Hepes concentration after 4 h in the waterbath [Fig. 1c]. Change in osmolality during this time, shown in Fig. 1d, was less than 20 mOsm∕kg for the 4.5–22 mM Hepes conditions and reached a minimum change of 9 mOsm∕kg when 4.5 mM Hepes was used. Based on the Potter and DeMarse (2001) study with neural cells, osmolality changes greater than 8.3 mOsm∕kg in a 4 h period should be lethal. But when using 13.2 and 22 mM Hepes, both having osmotic changes twice the 8.3 value, human fibroblast cells exhibited maximum viability. For this reason, 4.5–22 mM Hepes concentrations were considered acceptable for less than ideal environmental conditions and for experiments lasting 4 h or less.
The two experimental environments each lacked elements of the ideal incubator condition. Both were equally deficient in atmospheric CO2, where levels were presumably the same as the atmosphere, less than 1%. The waterbath was humid, nonsterile, and temperature controlled at 37 °C. The laminar flow hood was dry, sterile, and room temperature at approximately 23 °C. With “waterbath” cell viability being higher than “hood” cell viability at both the 0% and 22% Hepes conditions, lack of sterility in the waterbath was most likely managed by gentimicin and fungizone added to the medium. Neither the engineered tissue nor the surrounding medium ever made contact with the water in the waterbath, so it is unlikely that direct contamination occurred in the experiment. Alternatively, the dry air, lower temperature, and 2 h extension of the test in the hood seemed to create a harsher environment for cells in the TFX scaffold.
Environmental conditions of the rheometer bioreactor were also less than ideal. Atmospheric CO2 levels were assumed to be low, humidity was that of the ambient environment, surfaces were wiped down with 70% ethanol for nominal sterility, and temperature was controlled at 37 °C. An additional complication was a steady flow of purified, dry air traveling through an air bearing around the motor chamber and exiting the chamber directly above the rheometer cup. The air flow allows the electromagnetic motor to float in a virtually frictionless environment for stress delivery and strain detection with high precision. Appliances or accessories making contact with the chuck or shaft from which the air originates, in an attempt to redirect airflow away from the cup, would compromise accurate motor function and possibly damage the instrument. The acrylic cover, placed on top of the cup, blocked some of the air flow from making contact with the cell environment. In order to compensate for additional medium evaporation due to the air flow, we tested medium exchange rates of every half hour and every 2 h over a 6 h period using 8.9 mM Hepes concentration in the medium. Pooled mean cell viability±SEM for both exchange conditions at 2 and 4 h was 0.77±0.005 (n=4). At 6 h, the viable cell fraction was 0.67±0.01 (n=2).
Cell viability in the rheometer bioreactor could be improved in future studies by considering gas tensions. Hypoxia is a potential condition in the rheometer bioreactor, because tissues are compressed. Hypoxia occurs when oxygen tension in tissues is 5–15 mmHg O2 rather than normal levels of 30–90 mmHg O2 (Allen and Bhatia, 2003). The condition leads to anaerobic metabolism and reduction of mRNA translation, protein synthesis, and gene expression (Harris, 2002). Obradovic and colleagues found that gas exchange and medium replacement, three times per week, was adequate for generating a dense, well distributed cartilage tissue in a rotating bioreactor over a 5 week period, whereas no gas exchange over the same time period generated a sparse and poorly distributed cartilage tissue (Obradovic et al., 1999). Our bioreactor design is not yet capable of maintaining tissues for this length of time. Based on these results, oxygen tension and medium nutrients, with the control of pH, for short term vibration dosing experiments of the present study were most likely adequate and trypan blue viability was sufficient for detecting lethal responses to the environment. Future improvements of the rheometer bioreactor, in order to conduct experiments lasting days to weeks, will require gas tension and metabolic measures.
SEEDED SUBSTRATES SUBJECTED TO THREE VIBRATION REGIMES
Instrument calibration
Instrument calibration procedures were performed in order to reduce systematic error. Temperature was set with a water-circulating temperature control unit ±0.5 °C. Its accuracy was confirmed using a Cannon DSR temperature probe (State College, PA). Concentricity and parallelism of the rheometer, was calibrated by Malvern Instruments technicians and by the first author on site as per manufacturer instructions. Trueness of machined plates was determined by The University of Iowa Medical Instruments staff. Potential errors in the empirically determined motor inertia value, phase error, and gap calibration were also accounted for (Klemuk, 2007). The motor inertia value and phase variabilities were each ±3%. Gap calibrations returned Newtonian viscosity measurement variabilities of ±7%. Total variability attributed to these calibrations is approximately ±15%.
Compression determination
Preliminary observations while measuring the viscoelastic properties of TFX substrates using a stress-controlled rheometer showed a significant lack of adherence between the sample and rheometer plates (Klemuk et al., 2001). For the present investigation, the moving stainless steel parallel plate was bead-blasted in order to diminish slippage while still being able to sterilize the plate. 400grit wet∕dry sandpaper was adhered in the bottom of the cup rather than cost prohibitive sandblasting. The sandpaper surface was rinsed with 70% ethanol and allowed to air dry prior to experimentation. Sample compression was also observed to reduce slippage and measurement variability, but possible drawbacks included changes to the substrate rheology and adverse cell viability. To evaluate sample compression, 30 mm diameter TFX disks were prepared in the same manner prior to cell seeding and allowed to incubate in cell nutrient medium for 48 h without cells. Thickness was determined using the rheometer and a 30 mm diameter plate. The plate was lowered onto a sample with a nominal gap of 1.00 mm. Gap setting was reduced incrementally until the normal force strain gauge reading rose, indicating that sample contact was attained. Sample thickness was then recorded as the gap setting and subsequent compressions, 5%, 10%, 20%, and 40%, were converted to the appropriate gap. Amplitude sweeps were also performed at each level of compression to select driving amplitude in the linear viscoelastic (LVE) region. LVE region was maintained for strains of 0.05 or less. Frequency sweeps were performed on each TFX disk at each sample compression. Input torque, output displacement, and phase were used to calculate linear viscoelastic properties.
Cell viability was also tested under varying compression conditions. T31 cells were seeded onto TFX substrates as described earlier. Each disk was placed in the rheometer-mounted cup (Bohlin CVO 120) and subjected to 0%, 10%, 20%, or 70% compression with a 30 mm parallel plate. Compression was applied for 6 h. Cell viability was measured by trypan blue staining.
Tissue preparation and vibration regimes
TFX substrates, having a 45 mm diameter, were seeded with T31 tracheal fibroblast cells as specified earlier and allowed to grow for approximately 2 weeks. Medium environment during the vibration experiments included the upper limit of Hepes, 22 mM, gentamicin and fungizone to protect against contamination, and a 50% medium exchange every 30 min. A sterilized, bead-blasted 45 mm diameter stainless steel plate and 400grit wet∕dry sandpaper were used and sample compression was 10%, as determined by compression studies. Each substrate was subjected to one of three different vibration regimes for 2 h in the rheometer bioreactor where frequency, amplitude, and duty ratio (the on and off cycling of vibration) were specified. Using rheometer software, amplitude was controlled and resulting tissue strain was measured. Peak stresses were adjusted at the beginning of a given experiment so that resulting tissue strains were near 0.5 for each of the vibration regimes. Duty ratio was also controlled using the rheometer software. A 25% duty ratio cycled vibration on for 45 s and off for 135 s, and the 75% duty ratio cycled vibration on for 135 s and off for 45 s. These duty ratios were selected to test the machine’s ability to deliver two different duty ratios rather than constant vibration, as was done in other studies. The three vibration conditions were 1 Hz 25% duty ratio to test for minimal tissue disruption, high frequency 25% duty ratio, and high frequency 75% duty ratio to test for maximal tissue disruption.
High frequency for a given sample was at or near the resonant frequency of the motor∕plate∕sample system, the frequency at which maximum strain occurs with the least amount of torque. The resonant angular frequency ω0 for an undamped oscillator is written in terms of the shear elastic modulus G′, the sample radius R, the sample thickness h, and the motor and plate inertia I:
| (1) |
This is the same as Eq. (12) in Titze et al. (Titze et al., 2004b). Resonant frequency of the motor∕plate∕sample system was determined using Eq. (15) from Titze et al. (2004b). A frequency sweep was performed prior to initiating the vibration experiment at strains in the LVE region: 0.01–0.015. The resulting G′ and G″ values at each frequency ω were then inserted into the following equation, and magnitude of strain per N m torque MAG[γ∕T] was calculated.
| (2) |
Using values from Eq. 2, each vibration condition was subsequently performed on three different substrates.
No-vibration controls in the bioreactor were seeded substrates measuring 30 mm in diameter (n=3). Controls were subjected to 10% compression for 2 h, were not vibrated, and viscoelastic data were subsequently collected at 0.01–100 Hz. Each substrate was then cut into smaller pieces. For each substrate piece, cells were trypsanized, centrifuged, mixed with trypan blue, and counted using a hemocytometer.
RESULTS AND DISCUSSION FOR THREE VIBRATION REGIMES
Compression effects to TFX rheology and cell viability
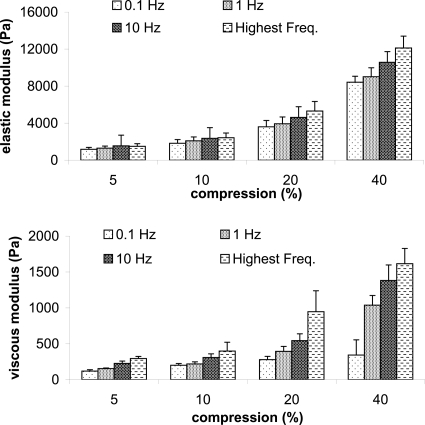
Compression directly affects rheologic properties of porous TFX substrate, saturated with cell nutrient medium. Displayed in Fig. 2 are mean elastic and viscous moduli (n=3) at four different frequencies. Magnitudes increased with increasing compression, where the largest increase occurred from 20% to 40% compression. For this compression doubling, G′ and G″ more than doubled for every frequency with only one exception: G″ at 0.1 Hz. The least rheologic increase occurred with a 5% to 10% compression increase, where values were about half again as much at 10% compression as compared to 5% compression. G′ variability (SEM∕mean×100) was, on average, ±18%, ±20%, ±19%, ±10% as compression increased, and G″ variability was ±11%, ±18%, ±20%, and ±26% as compression increased. Within a given compression, gap settings were within 0.2 mm for each of the three samples. If the gap had been kept constant rather than percent compression, 20% compression data from samples 1 and 2 would have been averaged with 40% compression data of the third sample, all having a gap of 0.5 mm. In that case, mean elastic and viscous moduli for the 20% compression would nearly double and average variabilities of G′ and G″ would be ±42% and ±45%, respectively.
Figure 2.
Mean and standard error of elastic and viscous moduli (n=3) as a function of TFX substrate compression at four frequencies. Highest frequency was 35, 43, 67, and 70 Hz for 5%, 10%, 20%, and 40% compression, respectively.
A natural log fit y=a ln x+b to mean elastic moduli versus frequency showed that the slope a steadily increased with increasing compression as well (Table 1). Viscous moduli of medium-saturated TFX also increased with increasing compression, but when fitting the dynamic moduli η′=G″∕ω to a power law function y=axb (Table 2), the declination rates were about the same. The steeper elastic moduli slopes with increasing compression may indicate a change in proportional solid to liquid presence, or changes in transient hydrogen bonding and van der Waals forces. With increased compression, relative positions of hard and soft molecular chains within the TFX may have clumped, causing the structure to become stiffer. It is unlikely that compression or rheology broke chemical bonds. If the substrate had torn, it would be more likely that property values decrease, which they did not. Repeated rheological measures on the same sample and scanning electron microscopy imaging would help in the future to determine the cause of change. TFX is described as an elastomer where, under tension, it behaves as an elastic solid for up to 100% strain. We therefore expect that viscosity originated from the cell nutrient medium and hydrophilic interactions between the medium and the substrate. This is supported by magnitude increases in viscosity without a noticeable change in the declination rate. As compression increases, free medium, having the viscosity near water, leaves the porous structure. Remaining medium molecules are too tightly bound to the substrate to exit but probably soften the substrate structure and give it a more viscous property.
Table 1.
Coefficients a and b and corresponding coefficient of determination R2 for natural log curve fit y=a ln x+b to each mean elastic modulus data set as a function of frequency.
| Elastic modulus fits for the following conditions | y=a ln x+b | |||
|---|---|---|---|---|
| a | b | R2 | ||
| TFX compressionconditions | 5% | 58.59 | 1331 | 0.869 |
| 10% | 97.42 | 2078 | 0.944 | |
| 20% | 204.7 | 4053 | 0.971 | |
| 40% | 604.5 | 9217 | 0.984 | |
| Cell-seeded TFXpre-vibration at 10%compression | Bioreactor control | — | — | — |
| 1 Hz 25% duty ratio | — | — | — | |
| High freq. 25% duty ratio | 152.6 | 2960 | 0.980 | |
| High freq. 75% duty ratio | 172.7 | 3459 | 0.983 | |
| Cell-seeded TFXpost-vibration at 10%compression | Bioreactor control | 165.2 | 3433 | 0.969 |
| 1 Hz 25% duty ratio | 156.2 | 2994 | 0.986 | |
| High freq. 25% duty ratio | 166.8 | 3192 | 0.987 | |
| High freq. 75% duty ratio | 205.8 | 3846 | 0.989 | |
Table 2.
Coefficients a and b and corresponding coefficient of determination R2 for power law curve fit y=axb to each mean dynamic viscosity data set as a function of frequency.
| Dynamic viscosity fits for the following conditions | y=axb | |||
|---|---|---|---|---|
| a | b | R2 | ||
| TFX compressionconditions | 5% | 26.56 | −0.8523 | 0.994 |
| 10% | 40.39 | −0.9034 | 0.996 | |
| 20% | 67.29 | −0.8587 | 0.998 | |
| 40% | 154.8 | −0.8682 | 0.992 | |
| Cell-seeded TFXpre-vibration at 10%compression | Bioreactor control | — | — | — |
| 1 Hz 25% duty ratio | — | — | — | |
| High freq. 25% duty ratio | 54.48 | −0.8987 | 0.998 | |
| High freq. 75% duty ratio | 62.40 | −0.8894 | 0.998 | |
| Cell-seeded TFXpost-vibration at 10%compression | Bioreactor control | 63.32 | −0.9024 | 0.999 |
| 1 Hz 25% duty ratio | 59.28 | −0.9333 | 0.996 | |
| High freq. 25% duty ratio | 62.24 | −0.9176 | 0.998 | |
| High freq. 75% duty ratio | 74.58 | −0.909 | 0.999 | |
Another effect of compression is an increase in the upper frequency at which valid rheologic data occur. The highest frequency for each compression was 35, 43, 67, and 70 Hz for 5%, 10%, 20%, and 40% compression, respectively. This high frequency limit was determined by locating the system resonant frequency using the original torque and displacement signal, and then doubling that frequency (Titze et al., 2004b). In the case of 40% compression, a doubling of the resonant frequency was 116, but G′ and G″ values above 70 Hz were in rapid decline, an indicator of an inertially controlled system. A 10%–20% compression boosts frequency a bit over the 5% compression and material properties are still similar. A 10%–20% compression also holds the sample in place when high strains are applied. When no compression and smooth plates were used in preliminary studies, high strains on TFX caused the material to roll up and be ejected from between the plates. The system resonant frequency also increases when using a larger diameter plate and sample, as in the vibration experiments.
The resulting viable cell fractions were 0.94, 0.82, 0.85, and 0.53 for 0%, 10%, 20%, and 70% compression respectively. The 0% compression result was in line with the baseline cell viability reported in Fig. 1, the 10% and 20% compression viabilities were similar, and the 70% compression was not conducive to cell survival. It was concluded that a 10%–20% sample compression was acceptable, and every sample should be compressed the same amount because of the strong rheologic dependence on compression.
Accurate rheological data collection is a critical component for evaluating engineered vocal fold tissues in a bioreactor. Biological tissues, composites of solid and liquid such as biocompatible substrates in cell nutrient medium, and dispersions used for vocal fold injection, are all problematic when collecting their rheologic data with a stress-controlled rheometer. Sample slippage is an unresolved problem in the field of rheology, has been observed by the current authors, and remains a difficult problem for complex material measurement (Klemuk, 2007; Sanchez-Reyes and Archer, 2003; Tapadia et al., 2006). Sample compression results in the present study lead to the particular conclusion that TFX sample should be exposed to the same compression, 10%–20% rather than the same gap, because of compression dependencies and variations in substrate thickness. But the results also suggest that any porous biocompatible scaffold or biological tissue, where free water or medium can escape from pores and between parallel plates, should be evaluated in a similar way for the exact same reasons.
Quantifying vibration stimulation
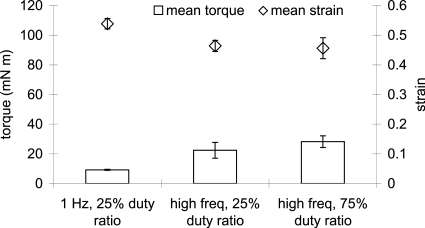
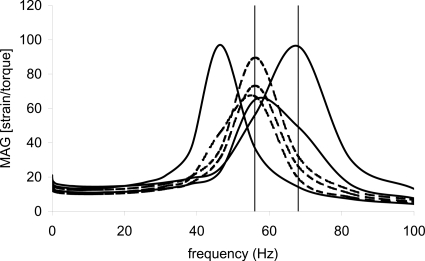
Experimental results up to this point were now put to the test. T31 seeded TFX scaffolds were immersed in 22 mM Hepes gentamicin fungizone-added cell nutrient medium, pre-cut as a large diameter disk of 45 mm, and subjected to 10% compression using a bead-blasted parallel plate with sandpaper cup bottom. After calculating and viewing response curves using Eq. 2 and Excel software, vibration frequencies were chosen at or near the peak response frequency. For the 25% duty ratio condition, one sample vibrated at 62 Hz and two at 56 Hz. Peak response frequencies were 46, 56, and 68 Hz, respectively. For the 75% duty ratio condition, one sample vibrated at 68 Hz and two at 56 Hz. Peak response frequency was 56 Hz for each sample. Figure 3 shows mean±SEM applied torque for each vibration condition (n=3) and the mean resulting strain±SEM (n=3) reported by the rheometer software. A total of 30–40 points were recorded for each sample during the 2 h test. Torque was 10–35 mN m for all conditions—well below the maximum torque capacity of the rheometer, 120 mN m. The mean ratio of strain to torque for the 1 Hz condition was more than twice that of the mean ratio of the 56–68 Hz conditions, 58.6 and 21.6 (N m)−1, respectively. This was an unexpected result. System response curves calculated with Eq. 2 prior to high frequency vibration experiments indicated that 1–7 times more strain should have been achievable at or near the resonant frequency compared to 1 Hz vibration (Fig. 4). Later, system response was calculated using the raw torque and strain signal. The peaks were slightly shifted but there still should have been the 1–7 times more strain available at the higher frequency vibration regimes.
Figure 3.
Mean applied torque and responding mean strain±SEM (n=3) of fibroblast-seeded TFX scaffold for three vibration conditions: 1 Hz and 25% duty ratio, high freq (62, 56, and 56 Hz) 25% duty ratio, and high frequency (68, 56, and 56 Hz) 75% duty ratio.
Figure 4.
Motor∕plate∕sample response, strain per N m torque, as a function of frequency for the high frequency samples. Solid curves are for 25% duty ratio samples and dashed curves are for 75% duty ratio samples.
A nonplausible explanation is that vertical standing waves developed in the sample. If the gap h is sufficiently small with respect to the sample viscosity η, density ρ, and frequency f, vertical standing waves will not develop within the sample, thus satisfying the gap loading condition. Schrag (1977) and later Ferry (1980) wrote the criterion as
| (3) |
Assuming a density of 1 g∕cm3 and complex viscosity of 10 Pa s and 4 Pa s at 56 Hz and 150 Hz, respectively, for seeded TFX, the largest gap of 0.86 mm in the present investigation is 39 times smaller and 12 times smaller than the right hand side of Eq. 3 at 56 Hz and 150 Hz, respectively. According to Schrag (1977), these both satisfy the gap loading criterion for “reasonable approximations.” Instead, it is suspected that the increased torque at higher frequency is due to slippage between the sample and the bead-blasted plate and 400grit sandpaper covered cup base. Preliminary slippage studies showed that a lower grit sandpaper (i.e., rougher sandpaper), reduced rheologic variability of TFX measurements (Klemuk, 2007). It was not used, however, because particles might have infiltrated the sample during vibration experiments and affected biological outcomes. Despite this potential slip effect, the desired strains of 0.4–0.5 were achieved using about of the available torque. These strains occurred at 56–68 Hz, frequencies much lower than the 150 Hz limit of the Bohlin CVO electromagnetic torque motor. It is therefore likely that large strains will be attainable at 150 Hz. In this study we did not customize rheometer controls beyond what is available to the typical rheometer user. More advanced controls will extend large amplitude vibrations up to 250 Hz in future investigations. Improvements to strain limits for vibration stimulation will include the use of more adherent, prototypic biomaterials that remain mechanically stable but eventually degrade, and use of alternative hard surface treatments for proper adhesion.
Cell viability under vibration
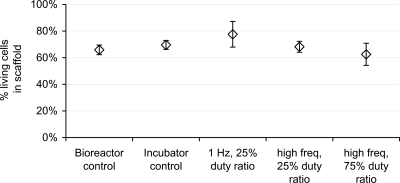
Viable cell fractions for vibration regime studies were comparable across all conditions, as shown in Fig. 5. The bioreactor control and the 1 Hz 25% duty ratio each had 10 samples, and the incubator control, the high frequency 25% duty ratio, and the high frequency 75% duty ratio each had five samples. All error bars corresponded to standard error, where standard deviation was normalized to the square root of n. The range of mean values was 0.63–0.78 and standard error bars overlapped for all conditions. Viability was similar to the 6 hour laminar flow hood, 22 mM condition (0.66) shown in Fig. 1, leading to the conclusion that viability was not affected by vibration conditions. Results from the present study indicate suitability and continued use of the rheometer bioreactor for tissue engineering studies.
Figure 5.
Mean viable cell fractions (SEM), as determined using trypan blue and hemocytometer, at the completion of each 2 h test condition.
Tissue rheology
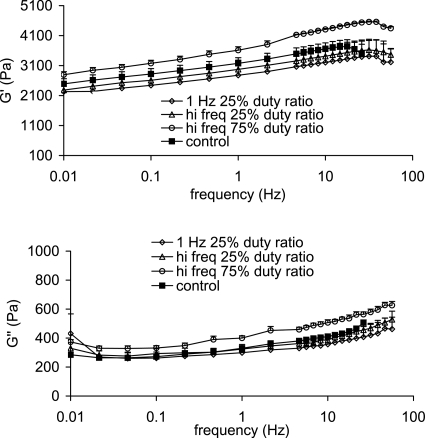
Viscoelastic properties through 50 Hz were attainable while living tissues were in the rheometer bioreactor before and after vibration experiments. Values were in the range of vocal fold related biomaterial properties and they were in the range of unseeded TFX substrate properties. Shear elastic and viscous moduli increased with increasing frequency with a G′ range of 2300–4600 Pa and a G″ range of 280–630 Pa across all vibration and control conditions (Fig. 6). Coefficients of natural log and power law fits to mean elastic moduli and to mean dynamic viscosities, respectively, were similar pre- and post-vibration and were coincident with unseeded TFX data at compressions between 10 and 20% (Tables 1, 2). G′ and G″ values did not remain constant before and after vibration. This is not a function of the machine but rather that of sample changes, and will be a topic to consider for a future study.
Figure 6.
Mean elastic moduli (top) and mean viscous moduli (bottom) of fibroblast-seeded TFX substrates after exposure to various vibration regimes. Error bars represent one standard error.
G′ and G″ magnitudes were to that of Cymetra and Radiesse, two biocompatible vocal fold dispersions typically injected into the lateral portion of the vocal fold to medialize tissue (Caton et al., 2007; Chan and Titze, 1999). G′ values were nearly an order of magnitude higher than hyaluronic acid based injectates, targeted for vocal fold mucosal injection, and cadaveric vocal fold mucosa. Dynamic viscosity values were 1–10 Pa s at 10 Hz and higher, corresponding to dynamic viscosities of two out of the ten male vocal fold mucosal samples from Chan and Titze (1999). G′ variability (SEM∕mean×100) was, on average, ±6.2%, ±15%, ±10%, ±1.6% for the control, 1 Hz, high frequency 25% duty ratio, and high frequency 75% duty ratio, respectively. G″ variability was ±1.4%, ±8.7%, ±4.2%, and ±1.5% as compression increased. The loss tangent G″∕G′ was also in an acceptable range, consistently 0.13. Upper frequency limits of rheologic measurement are only a current limitation. We have not extended to piezo motors in this paper, but with this technology, measurement and small amplitude vibration up to 1000 Hz will be possible.
SUMMARY AND CONCLUSION
Functionality of a rheometer as a bioreactor has been demonstrated for short term vibration experiments in the sonic region and with large strains. A stable biocompatible nondegradable substrate, known to support tracheal fibroblast cell growth, uniform distribution, and ECM production, was successfully seeded and exposed to three different vibration regimes. Cell fraction viability was maintained at approximately 0.7 by using 22 mM Hepes. Input stresses and output tissue deformation were quantified at 56–68 Hz and for strains of 0.4–0.5. Given the amount of torque used to generate the strains, it is likely that the rheometer can also generate the same strains at frequencies up to the instrument’s capacity of 150 Hz. Finally, rheologic measurements were made while in the bioreactor before and after vibration exposure, demonstrating a unique and important measuring capability of this rheometer bioreactor. Improvements to the cell environment are ongoing to increase experimental times from several hours to several days, and expanded technologies are being incorporated to increase vibration and measurement frequencies well into the sonic region.
ACKNOWLEDGMENTS
This work was supported by NIH Grant Nos. DC004224 and DC008047 from the National Institute on Deafness and Other Communication Disorders.
This paper includes material presented at the 6th ICVPB, Tampere, Finland, 6–9 August 2008.
References
- Allen, J. W., and Bhatia, S. N. (2003). “Formation of steady-state oxygen gradients in vitro,” Biotechnol. Bioeng. 82, 253–262. [DOI] [PubMed] [Google Scholar]
- Andrews, K. D., and Hunt, J. A. (2008). “Upregulation of matrix and adhesion molecules induced by controlled topography,” J. Mater. Sci.: Mater. Med. 19, 1601–1608. [DOI] [PubMed] [Google Scholar]
- Andrews, K. D., Hunt, J. A., and Black, R. A. (2008). “Technology of electrostatic spinning for the production of polyurethane tissue engineering scaffolds,” Polym. Int. 10.1002/pi.2317 57, 203–210. [DOI] [Google Scholar]
- Bacabac, R. G., Smit, T. H., Van Loon, J. J. W. A., Boulabi, B. Z., Helder, M., and Klein-Nulend, J. (2006). “Bone cell responses to high-frequency vibration stress: Does the nucleus oscillate within the cytoplasm?,” FASEB J. 20, 858–864. [DOI] [PubMed] [Google Scholar]
- Barron, V., Lyons, E., Stenson-Cox, C., McHugh, P. E., and Pandit, A. (2003). “Bioreactors for cardiovascular cell and tissue growth: Areview,” Ann. Biomed. Eng. 10.1114/1.1603260 31, 1017–1030. [DOI] [PubMed] [Google Scholar]
- Caton, T., Thibeault, S. L., Klemuk, S., and Smith, M. E. (2007). “Viscoelasticity of hyaluronan and nonhyaluronan based vocal fold injectables: Implications for mucosal versus muscle use,” Laryngoscope 117, 516–521. [DOI] [PubMed] [Google Scholar]
- Chan, R. W., and Titze, I. R. (1999). “Viscoelastic shear properties of human vocal fold mucosa: Measurement methodology and empirical results,” J. Acoust. Soc. Am. 10.1121/1.427947 106, 2008–2021. [DOI] [PubMed] [Google Scholar]
- Chan, R. W., and Titze, I. R. (2006). “Dependence of phonation threshold pressure on vocal tract acoustics and vocal fold tissue mechanics,” J. Acoust. Soc. Am. 10.1121/1.2173516 119, 2351–2362. [DOI] [PubMed] [Google Scholar]
- De Zengotita, V. M., Schmelzer, A. E., and Miller, W. M. (2002). “Characterization of hybridoma cell responses to elevated pCO2 and osmolality: Intracellular pH, cell size, apoptosis, and metabolism,” Biotechnol. Bioeng. 77, 369–380. [DOI] [PubMed] [Google Scholar]
- Discher, D. E., Janmey, P., and Wang, Y. (2005). “Tissue cells feel and respond to the stiffness of their substrate,” Science 10.1126/science.1116995 310, 1139–1143. [DOI] [PubMed] [Google Scholar]
- Engler, A. J., Sen, S., Sweeney, H. L., and Discher, D. E. (2006). “Matrix elasticity directs stem cell lineage specification,” Cell 126, 667–689. [DOI] [PubMed] [Google Scholar]
- Ferry, J. D. (1980). Viscoelastic Properties of Polymers, John Wiley & Sons, New York. [Google Scholar]
- Freed, L. E., and Vunjak-Novakovic, G. (2000). Tissue Engineering Bioreactors, in Principles of Tissue Engineering, edited by Lanza R. P., Langer R., and Vacanti J. (Academic Press, San Diego: ), pp. 143–156. [Google Scholar]
- Freshney, I. R. (2000). Culture of Animal Cells; A Manual of Basic Technique, Wiley-Liss, New York. [Google Scholar]
- Harris, A. L. (2002). “Hypoxia—A key regulatory factor in tumour growth,” Nat. Rev. Cancer 10.1038/nrc704 2, 38–47. [DOI] [PubMed] [Google Scholar]
- Janmey, P. A., and McCulloch, C. A. (2007). “Cell mechanics: Integrating cell responses to mechanical stimuli,” Annu. Rev. Biomed. Eng. 10.1146/annurev.bioeng.9.060906.151927 9, 1–34. [DOI] [PubMed] [Google Scholar]
- Klemuk, S. A. (2007). Methodological Improvements in Mechanical Measurement of Vocal Fold-Related Tissues, The University of Iowa, Iowa City, IA. [Google Scholar]
- Klemuk, S. A., Titze, I. R., and Gray, S. (2001). “Methodology for straining vocal fold tissues at audio frequencies,” J. Acoust. Soc. Am. 109, 2412. [Google Scholar]
- Matthews, B. D., Overby, D. R., Mannix, R., and Ingber, D. E. (2006). “Cellular adaptation to mechanical stress: Role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels,” J. Cell Biol. 119, 508–518. [DOI] [PubMed] [Google Scholar]
- Min, Y., Titze, I. R., and Alipour-Haghighi, F. (1995). “Stress-strain response of the human vocal fold ligament,” Ann. Otol. Rhinol. Laryngol. 104, 563–569. [DOI] [PubMed] [Google Scholar]
- Mulder, M. M., Hitchcock, R. W., and Tresco, P. A. (1998). “Skeletal myogenesis on elastomeric substrates: Implications for tissue engineering,” J. Biomater. Sci., Polym. Ed. 9, 731–748. [DOI] [PubMed] [Google Scholar]
- Obradovic, B., Carrier, R. L., Vunjak-Novakovic, G., and Freed, L. E. (1999). “Gas exchange is essential for bioreactor cultivation of tissue engineered cartilage,” Biotechnol. Bioeng. 63, 197–205. [DOI] [PubMed] [Google Scholar]
- Perlman, A., and Durham, P. (1991). In Vitro Studies of Vocal Fold Mucosa During Isometric Conditions, in Laryngeal Function in Phonation and Respiration, edited by Baer T. (Little, Brown, & Co, Boston: ), pp. 291–303. [Google Scholar]
- Potter, S. M., and DeMarse, T. B. (2001). “A new approach to neural cell culture for long-term studies,” J. Neurosci. Methods 10.1016/S0165-0270(01)00412-5 110, 17–24. [DOI] [PubMed] [Google Scholar]
- Sanchez-Reyes, J., and Archer, L. A. (2003). “Interfacial slip violations in polymer solutions: Role of microscale surface roughness 1,” Langmuir 10.1021/la0265326 19, 3304–3312. [DOI] [Google Scholar]
- Schrag, J. L. (1977). “Deviation of velocity gradient profiles from the “gap loading” and “surface loading” limits in dynamic simple shear experiments,” Trans. Soc. Rheol. 10.1122/1.549445 21, 399–413. [DOI] [Google Scholar]
- Takai, E., Costa, K. D., Shaheen, A., Hung, C. T., and Guo, X. E. (2004). “Osteoblast elastic modulus measured by atomic force microscopy is substrate dependent,” Annu. Rev. Biomed. Eng. 33, 963. [DOI] [PubMed] [Google Scholar]
- Tanaka, S. M., Li, J., Duncan, R. L., Yokota, H., Burr, D. B., and Turner, C. H. (2003). “Effects of broad frequency vibration on cultured osteoblasts,” J. Biomech. 10.1016/S0021-9290(02)00245-2 36, 73–80. [DOI] [PubMed] [Google Scholar]
- Tapadia, P., Ravindranath, S., and Wang, S. Q. (2006). “Banding in entangled polymer fluids under oscillatory shearing,” Phys. Rev. Lett. 10.1103/PhysRevLett.96.196001 96, 196001. [DOI] [PubMed] [Google Scholar]
- Thibeault, S. L., Li, W., Gray, S. D., and Chen, Z. (2002). “Instability of extracellular matrix gene expression in primary cell culture of fibroblasts from human vocal fold lamina propria and tracheal scar,” Ann. Otol. Rhinol. Laryngol. 111, 8–14. [DOI] [PubMed] [Google Scholar]
- Titze, I. R. (1988). “The physics of small-amplitude oscillation of the vocal folds,” J. Acoust. Soc. Am. 10.1121/1.395910 83, 1536–1552. [DOI] [PubMed] [Google Scholar]
- Titze, I. R., Broadhead, K., Tresco, P. A., and Gray, S. (2005). “Strain distribution in an elastic substrate vibrated in a bioreactor for vocal fold tissue engineering,” J. Biomech. 38, 2406–2414. [DOI] [PubMed] [Google Scholar]
- Titze, I., and Durham, P. L. (1991). Passive Mechanisms Influencing Fundamental Frequency Control, in Laryngeal function in phonation and respiration, edited by Baer, Sasaki, and Harris (Little, Brown and Company, Boston: ), pp. 304–319. [Google Scholar]
- Titze, I. R., Hitchcock, R. W., Broadhead, K., Webb, K., Li, W., Gray, S. D., and Tresco, P. A. (2004a). “Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses,” J. Biomech. 10.1016/j.jbiomech.2004.01.007 37, 1521–1529. [DOI] [PubMed] [Google Scholar]
- Titze, I. R., and Hunter, E. (2004). “Normal vibration frequencies of the vocal ligament,” J. Acoust. Soc. Am. 10.1121/1.1698832 115, 2264–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze, I. R., Klemuk, S. A., and Gray, S. (2004b). “Methodology for rheological testing of engineered biomaterials at low audio frequencies,” J. Acoust. Soc. Am. 10.1121/1.1631941 115, 392–401. [DOI] [PubMed] [Google Scholar]
- Vara, D. S., Salacinski, H. J., Kannan, R. Y., Bordenave, L., Hamilton, G., and Seifalian, A. M. (2005). “Cardiovascular tissue engineering: State of the art,” Pathol. Biol. (Paris) 53, 599–612. [DOI] [PubMed] [Google Scholar]
- Webb, K., Li, W., Hitchcock, R. W., Smeal, R. M., Gray, S. D., and Tresco, P. A. (2003). “Comparison of human fibroblast ECM-related gene expression on elastic three-dimensional substrates relative to two-dimensional films of the same material,” Biomaterials 10.1016/S0142-9612(03)00368-5 24, 4681–4690. [DOI] [PubMed] [Google Scholar]