Abstract
Context
Depressive symptoms predict adverse cardiovascular outcomes in patients with coronary heart disease, but the mechanisms responsible for this association are unknown.
Objective
To determine why depressive symptoms are associated with an increased risk of cardiovascular events.
Design and Participants
The Heart and Soul Study is a prospective cohort study of 1017 outpatients with stable coronary heart disease followed up for a mean (SD) of 4.8 (1.4) years.
Setting
Participants were recruited between September 11, 2000, and December 20, 2002, from 12 outpatient clinics in the San Francisco Bay Area and were followed up to January 12, 2008.
Main Outcome Measures
Baseline depressive symptoms were assessed using the Patient Health Questionnaire (PHQ). We used proportional hazards models to evaluate the extent to which the association of depressive symptoms with subsequent cardiovascular events (heart failure, myocardial infarction, stroke, transient ischemic attack, or death) was explained by baseline disease severity and potential biological or behavioral mediators.
Results
A total of 341 cardiovascular events occurred during 4876 person-years of follow-up. The age-adjusted annual rate of cardiovascular events was 10.0% among the 199 participants with depressive symptoms (PHQ score ≥10) and 6.7% among the 818 participants without depressive symptoms (hazard ratio [HR], 1.50; 95% confidence interval, [CI], 1.16–1.95; P=.002). After adjustment for comorbid conditions and disease severity, depressive symptoms were associated with a 31% higher rate of cardiovascular events (HR, 1.31; 95% CI, 1.00–1.71; P=.04). Additional adjustment for potential biological mediators attenuated this association (HR, 1.24; 95% CI, 0.94–1.63; P=.12). After further adjustment for potential behavioral mediators, including physical inactivity, there was no significant association (HR, 1.05; 95% CI, 0.79–1.40; P=.75).
Conclusion
In this sample of outpatients with coronary heart disease, the association between depressive symptoms and adverse cardiovascular events was largely explained by behavioral factors, particularly physical inactivity.
Depression has long been recognized as a risk factor for the development of cardiovascular disease in healthy patients, for recurrent events in patients with established cardiovascular disease, and for adverse outcomes after coronary bypass graft surgery.1–6 Depression is also a risk factor for the development of heart failure and for adverse outcomes in patients with existing heart failure.7–10 In a recent survey of 245 404 adults from 60 countries, patients with comorbid depression reported worse overall health than those with asthma, diabetes, arthritis, or cardiovascular disease alone.11 Based on the results of this study, the World Health Organization highlighted the detrimental effects of depression on medical illnesses as 1 of its 10 most important global public health statistics for 2007.12
Despite the substantial body of evidence demonstrating a strong link between depression and cardiovascular disease, the explanation for this association remains unclear. Several candidate mechanisms have been suggested as potential mediators, including smoking, lack of exercise,13 medication non-adherence,14,15 worse underlying cardiac disease severity,16 lower heart rate variability,17 antidepressant toxicity,18 enhanced activity of the hypothalamic pituitary axis,19 greater catecholamine levels,20 dietary factors, lowomega-3 fatty acid levels,21 increased serotonin and platelet activation,22 and inflammatory processes.23,24 However, the extent to which these proposed mechanisms explain the increased risk of cardiovascular events in depressed patients is unknown.
Understanding how depression leads to cardiovascular events is necessary for developing interventions to decrease the excess cardiovascular morbidity and mortality associated with depression. In a prospective cohort study of 1017 participants with stable coronary disease, we evaluated the extent to which the association of depressive symptoms with subsequent cardiovascular events was explained by differences in comorbid conditions, cardiac disease severity, use of antidepressant medications, and potential biological and behavioral mediators.
METHODS
The primary goal of the Heart and Soul Study was to determine why depression is associated with an increased risk of cardiovascular events in outpatients with stable coronary heart disease.25 We used administrative databases to identify outpatients with documented coronary artery disease at 2 Department of Veterans Affairs Medical Centers (San Francisco VA Medical Center and the VA Palo Alto Health Care System, California), 1 university medical center (University of California, San Francisco), and 9 public health clinics in the Community Health Network of San Francisco.
Patients were eligible to participate if they had at least 1 of the following: a history of myocardial infarction, angiographic evidence of at least 50% stenosis in 1 or more coronary vessels, prior evidence of exercise-induced ischemia by treadmill or nuclear testing, a history of coronary revascularization, or a diagnosis of coronary artery disease documented by an internist or cardiologist. Between September 11, 2000, and December 20, 2002, a total of 1024 participants were enrolled: 240 from the public health clinics, 346 from the university medical center, and 438 from the VA medical centers.
All participants completed a baseline examination that included an interview, fasting blood draw, psychiatric interview, questionnaire, echocardiogram, exercise treadmill test, 24-hour ambulatory electrocardiogram, and 24-hour urine collection. Of the 1024 participants who completed the baseline examination, we were not able to contact 7 (<1%) during the follow-up period, leaving 1017 for this analysis. Our protocol was approved by the appropriate institutional review boards, and all participants provided written informed consent.
Depressive Symptoms
We assessed depressive symptoms using the 9-item Patient Health Questionnaire (PHQ),26 a self-report instrument that measures the frequency of depressive symptoms corresponding to the 9 Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV)27 criteria for depression. The PHQ has demonstrated excellent validity when compared with a structured diagnostic interview for depression in patients with coronary artery disease.28 Participants indicated the frequency of experiencing each symptom during the prior 2 weeks; the items were scored as no points for not at all, 1 point for several days, 2 points for more than half the days, or 3 points for nearly every day. We evaluated depressive symptoms as a continuous variable (range, 0–27), as a categorical variable (≤4, >4-<10, 10-<15, 15-<20, and ≥20), and as a dichotomous variable using the standard cut point of 10 or higher.26 There was no difference in the mean (SD) PHQ score between the 1017 participants included in the analysis (5.2 [5.5]) and the 7 participants lost to follow-up (3.6 [1.0]; P=.43).
We also ascertained the presence of major depressive disorder (past month, past year, or lifetime) using the Computerized Diagnostic Interview Schedule for the DSM-IV (C DIS-IV).29 Computerized versions of the Diagnostic Interview Schedule have previously demonstrated acceptable validity and reliability.30,31 Participants with a major depressive episode in the past month were informed of this diagnosis, instructed to discuss their symptoms with their primary care physician, and provided a list of local resources. Of the 2 participants who did not complete the C DIS-IV, one had a PHQ score of 10 or higher, and the other had a PHQ score of 0.
Baseline Cardiac Disease Severity and Risk Factors
All participants underwent resting echocardiography using an Acuson Sequoia Ultrasound System (Siemens, Mountain View, California). We obtained standard 2-dimensional views and performed planimetry with a computerized digitization system to determine left-ventricular ejection fraction. We categorized participants as having diastolic dysfunction if their mitral inflow ratio of peak early-to-late diastolic filling velocity was more than 0.75 and if the velocity time integral in their pulmonary vein was greater during diastole than during systole.32 Fasting venous blood samples were drawn to determine low- and high-density lipoprotein cholesterol levels.
Potential Biological Mediators
Three-channel 24-hour ambulatory Holter electrocardiography was used to assess heart rate variability,33 including the SD of 5-minute mean NN intervals and the natural log of very low frequency power. We collected 24-hour urine samples to measure norepinephrine and cortisol excretion. Norepinephrine was assessed by gas chromatography-mass spectrometry at the Associated Regional and University Pathologists, Inc (Salt Lake City, Utah). Cortisol was analyzed using radioimmunoassay or (due to a change at the laboratory) high-performance liquid chromatography/tandem mass spectrometry.19,20
We used high-pressure liquid chromatography with electrochemical detection to assay whole blood serotonin levels. High-sensitivity C-reactive protein levels were measured using the Roche (Indianapolis, Indiana) Integra assay in the first 229 participants and (due to a change in the laboratory) the Beckman Extended Range (Galway, Ireland) assay in the remaining samples.34 Blood levels of 2 omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid, were measured by capillary gas chromatography as the percentage composition of total fatty acid methyl esters in the red blood cell membranes.35
Potential Behavioral Mediators
Smoking and alcohol use were determined by self-report questionnaire. To assess medication adherence, we asked, “In the past month, how often did you take your medications as the doctor prescribed?” Possible responses were all of the time (100%), nearly all of the time (90%), most of the time(75%), about half the time(50%), or less than half the time (<50%). We defined medication non-adherence as taking prescribed medications 75% or less of the time.36 We chose to measure self-reported medication adherence for several reasons. First, self-reported medication adherence has been validated as are liable predictor of health outcomes, including blood pressure control,37 hospitalization for heart failure,38 serum drug concentrations,39 and cardiovascular events.36,40 Second, in a study of hypertensive patients taking hydrochlorothiazide, self-reported medication adherence was more strongly correlated with qualitative urinary hydrochlorothiazide levels, changes in serum potassium, and decreases in blood pressure than was pill count adherence.41 Third, pharmacy refills are often provided for 90 days at a time, and thus pharmacy data would not have allowed us to assess the association of current (past month) adherence with depression. Fourth, Medication Event Monitoring System (MEMS) caps have their own limitations42 because the number of cap openings does not necessarily reflect the number of pills ingested by the patient.
To assess physical activity, we asked, “Which of the following statements best describes how physically active you have been during the last month, that is, done activities such as 15 to 20 minutes of brisk walking, swimming, general conditioning, or recreational sports?” Participants chose from 1 of the following 6 categories: not at all active, a little active(1–2 times per month), fairly active (3–4 times per month), quite active (1–2 times per week), very active (3–4 times per week), or extremely active(≥5timesperweek). Self-report has been shown to be a reliable, valid, and accurate method of assessing physical activity.43–46 In particular, single-response items have demonstrated excellent construct validity.45–47 Participants who reported that they were not at all or a little active were considered physically inactive.
As an objective measure of physical fitness, participants also completed an exercise treadmill test according to a standard Bruce protocol at the baseline examination. Those who were unable to continue the standard Bruce protocol were switched to slower settings and encouraged to exercise for as long as possible. Exercise capacity was calculated as the total number of metabolic equivalent tasks achieved. Treadmill exercise capacity is a gold-standard measure of physical fitness and is highly correlated with self-reported physical activity.43,46,48–50
Other Patient Characteristics
Age, sex, race, educational achievement, and medical history were determined by self-report questionnaire. We measured height and weight, and calculated body mass index. Participants were instructed to bring their medication bottles to their appointment, and study personnel recorded all current medications. Medications were categorized using Epocrates Rx (San Mateo, California).
Cardiovascular Events
Between the baseline examination and the last day of follow-up on January 12, 2008, we conducted annual telephone follow-up interviews with participants (or their proxy), asking specifically about hospitalization for “heart trouble.” For any reported event, medical records, electrocardiograms, death certificates, and coroner’s reports were retrieved and reviewed by 2 independent blinded adjudicators. In the event of disagreement, the adjudicators conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator as necessary.
Outcome events were heart failure, myocardial infarction, stroke, transient ischemic attack, or death. Heart failure was defined as hospitalization for a clinical syndrome involving at least 2 of the following: paroxysmal nocturnal dyspnea, orthopnea, elevated jugular venous pressure, pulmonary rales, third heart sound, and cardiomegaly or pulmonary edema on chest radiography. These signs and symptoms must have represented a clear change from the usual clinical status.32 Nonfatal myocardial infarction was defined using standard criteria.51 Stroke was defined as a new neurological deficit not known to be secondary to brain trauma, tumor, infection, or other cause. Transient ischemic attack was defined as a focal neurological deficit(in the absence of head trauma)lasting more than 30seconds and no longer than 24 hours, with rapid evolution of the symptoms to the maximal level of deficitin less than 5minutes and with subsequent complete resolution. Death was determined by death certificates and coroner’s reports.
Statistical Analysis
We aimed to enroll 1000 patients, including an expected 200 with depression. Assuming a 25% cumulative event rate over 5 years among patients without depression, this provided 80% power in 2-sided tests with an α of 5% to detect a relative hazard for depression of 1.56, after accounting for loss of precision due to adjustment for confounders.52 Our testing was 2-sided because we were open to the possibility that depression would be associated with better outcomes, and we did not know whether adjustment for potential mediators would increase or decrease the effect of depressive symptoms on cardiovascular events; in addition, 2-sided tests require stronger evidence at any given α level to reject the null hypothesis.
Baseline differences in characteristics between participants with and without depressive symptoms were compared using t tests and χ2 tests. C-reactive protein was log transformed because it did not have a normal distribution. We estimated the risk of cardiovascular events associated with depressive symptoms (both as a continuous and a dichotomous variable) using Cox proportional hazards models. We also estimated the risk of cardiovascular events associated with the presence of major depressive disorder (based on C DIS-IV interview).
To evaluate whether a covariate changed the strength of association between depressive symptoms and cardiovascular events, we calculated the percentage change in the effect size (log hazard ratio [HR]) for depressive symptoms before and after adjustment for the potential confounder or mediator. To avoid any artifact due to different sample sizes between the 2 nested models, participants missing the covariate of interest were excluded. We sequentially considered demographic variables, comorbid conditions, cardiac disease severity, use of medications, potential biological mediators, and potential behavioral mediators.53 All variables that resulted in a more than 5% change in the effect size (log HR) for depressive symptoms were considered confounders or potential mediators and included in the final multivariable models,54 with the exception of “other antidepressants.” Adjusting for use of “other antidepressants” (eg, mirtazapine, venlafaxine, or bupropion) reduced the effect size for depressive symptoms on cardiovascular events by 8.8%. Although this met our a priori definition for potential mediation, we thought the reduction in effect size most likely occurred because use of “other antidepressants” was a marker of more severe or treatment-resistant depression. Therefore, use of other antidepressants was not included in the final models.
In model checking, we verified the log-linearity assumption for continuous variables by checking for improvement in fit after addition of quadratic and cubic terms. We verified the proportional hazards assumption of these models using log-minus-log survival plots and by checking for secular patterns in scaled Schoenfeld residuals. We used Wald tests to check for interactions of depressive symptoms with age, race, history of myocardial infarction, stroke, diabetes, left ventricular ejection fraction, smoking status, medication adherence, and physical activity in both age- and multivariable-adjusted models. Plots of estimated cumulative risk were generated using PROC PHREG followed by PROC GPLOT. Analyses were performed using SAS version 9.0 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Of the 1017 participants, 199 (19.6%) had depressive symptoms (PHQ ≥10). Compared with participants who did not have depressive symptoms, those with depressive symptoms were younger and less likely to be male (Table 1). They were more likely to smoke, to be less physically active, to be less adherent to medications, and to have a higher mean body mass index. Participants with depressive symptoms had more comorbid conditions, including history of myocardial infarction, heart failure, and diabetes. Depressive symptoms were also associated with greater antidepressant use, greater 24-hour urinary norepinephrine excretion and C-reactive protein levels, and lower omega-3 fatty acid levels.
Table 1.
Baseline Characteristics of 1017 Participants With Stable Coronary Heart Disease, by Depressive Symptoms (Patient Health Questionnaire Score of ≥10 vs <10)a
| Variable | Depressive Symptoms (n = 199) |
No Depressive Symptoms (n = 818) |
P Value |
|---|---|---|---|
| Demographic characteristics Age, mean (SD), y |
63 (12) | 68 (10) | <.001 |
| Male sex, No. (%) | 152 (76) | 682 (83) | .02 |
| White, No. (%) | 110 (55) | 500 (61) | .13 |
| High school graduate, No. (%) | 166 (83) | 718 (88) | .08 |
| Body mass index, mean (SD) | 29.2 (5.7) | 28.2 (5.2) | .02 |
| Comorbid conditions, No. (%) Hypertension |
151 (76) | 569 (70) | .07 |
| Myocardial infarction | 121 (62) | 423 (52) | .01 |
| Stroke | 33 (17) | 115 (14) | .34 |
| Revascularization | 109 (55) | 492 (60) | .21 |
| Congestive heart failure | 49 (25) | 130 (16) | .004 |
| Diabetes mellitus | 68 (34) | 197 (24) | .003 |
| Cardiac disease severity and risk factors Resting left ventricular ejection fraction, mean (SD), % |
61 (10) | 62 (10) | .06 |
| Diastolic dysfunction, No. (%) | 22 (13) | 94 (13) | .96 |
| Cholesterol, mean (SD), mg/dL Low-density lipoprotein |
105 (36) | 104 (33) | .58 |
| High-density lipoprotein | 44 (13) | 46 (14) | .02 |
| Medication use, No. (%) Aspirin |
151 (76) | 638 (78) | .52 |
| β-Blocker | 119 (60) | 472 (58) | .59 |
| Renin-angiotensin system inhibitor | 104 (52) | 420 (51) | .82 |
| Tricyclic antidepressant | 15 (8) | 29 (4) | .01 |
| SSRI | 38 (19) | 59 (7) | <.001 |
| Other antidepressant | 35 (18) | 44 (5) | <.001 |
| Potential biological mediators, mean (SD) Heart rate variability, SDANN, ms |
106 (34) | 110 (37) | .29 |
| Heart rate variability, ln VLF, ms2 | 6.28 (0.81) | 6.32 (0.88) | .67 |
| Serotonin among non-SSRI users, ng/mL | 120 (74) | 120 (67) | >.99 |
| Cortisol, μg/d | 36.0 (25.3) | 39.1 (30.3) | .23 |
| Norepinephrine, μg/d | 56 (33) | 51 (25) | .02 |
| Log C-reactive protein, mg/L | 0.89 (1.33) | 0.67 (1.30) | .04 |
| Omega-3 fatty acid levels, % DHA 3 EPA | 3.9 (2.2) | 4.2 (2.0) | .03 |
| Potential behavioral mediators Regular alcohol use, No. (%) |
55 (28) | 237 (29) | .69 |
| Smoking status, No. (%) Current |
67 (34) | 131 (16) | <.001 |
| Past | 74 (38) | 428 (53) | |
| Never | 54 (28) | 252 (31) | |
| Medication nonadherence | 29 (15) | 54 (7) | <.001 |
| Self-reported physical activity, No. (%) Not at all active |
59 (30) | 130 (16) | <.001 |
| A little active, 1–2 times/mo | 55 (28) | 127 (15) | |
| Fairly active, 3–4 times/mo | 33 (17) | 122 (15) | |
| Quite active, 1–2 times/wk | 13 (7) | 141 (17) | |
| Very active, 3–4 times/wk | 24 (12) | 193 (24) | |
| Extremely active, ≥5 times/wk | 14 (7) | 103 (13) | |
| Exercise capacity, MET | 6.5 (3.2) | 7.5 (3.3) | <.001 |
Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; ln VLF, natural log of very low frequency; MET, metabolic equivalent task; SDANN, SD of 5-minute mean NN intervals; SSRI, selective serotonin reuptake inhibitor; body mass index is calculated as weight in kilograms divided by height in meters squared.
SI conversion factors: To convert high-density and low-density lipoprotein to mmol/L, multiply by 0.0259.
Totals may not sum to their respective totals due to missing data, and percentages may not sum to 100 due to rounding.
A total of 341 cardiovascular events occurred during 4876 person-years with a mean (SD) of 4.8 (1.4) years’ follow-up. The age-adjusted annual rate of cardiovascular events was 10.0% among the 199 participants with depressive symptoms (PHQ ≥10) and 6.7% among the 818 participants without depressive symptoms (HR, 1.50; 95% confidence interval [CI], 1.16–1.95; P=.002; Table 2).
Table 2.
Cardiovascular Events During Follow-up, by Depressive Symptoms
| Events (Age-Adjusted Annual Rate), No. (%) |
||||
|---|---|---|---|---|
| Event | Depressive Symptoms (n = 199) |
No Depressive Symptoms (n = 818) |
Age-Adjusted Hazard Ratio (95% Confidence Interval) |
P Value |
| Heart failure | 36 (4.5) | 103 (2.4) | 1.87 (1.27–2.74) | .001 |
|
| ||||
| Myocardial infarction | 23 (2.9) | 80 (1.9) | 1.45 (0.91–2.33) | .12 |
|
| ||||
| Stroke or transient ischemic attack | 14 (1.7) | 33 (0.8) | 2.11 (1.12–3.99) | .02 |
|
| ||||
| All-cause mortality | 48 (5.5) | 190 (4.1) | 1.36 (0.99–1.87) | .06 |
|
| ||||
| Any of above outcomes | 73 (10.0) | 268 (6.7) | 1.50 (1.16–1.95) | .002 |
Potential Mediators
Several variables, including comorbid conditions, smoking status, medication adherence, physical activity, left ventricular function, and C-reactive protein level, met the criterion for potential confounding or mediation (Table 3). Most notably, adjustment for physical activity was associated with a 31.7% reduction in the strength of association between depressive symptoms and cardiovascular events. Variables that did not change the effect size for depressive symptoms by 5% or more included use of selective serotonin reuptake inhibitors or tricyclic antidepressants, heart rate variability, levels of serotonin and omega-3 fatty acids, and 24-hour excretion of norepinephrine and cortisol (Table 3).
Table 3.
Change in the Strength of Association Between Depressive Symptoms and Cardiovascular Events (Expressed as the Percent Change in the Age-Adjusted Log Hazard Ratio) After Adjustment for Potential Confounders and Mediators
| Covariate | Change in Effect Size After Adjustment, %a |
|---|---|
| Demographic characteristics Male sex |
3.1 |
| White | 0.5 |
| High school graduate | −2.5 |
| Body mass index | 0.7 |
| Comorbid conditions Hypertension |
−1.1 |
| Myocardial infarction | −7.8a |
| Stroke | −5.4a |
| Revascularization | −0.4 |
| Congestive heart failure | −15.7a |
| Diabetes mellitus | −8.4a |
| Cardiac disease severity and risk factors Left ventricular ejection fraction |
−19.0a |
| Diastolic dysfunction | −2.7 |
| Low-density lipoprotein cholesterol level | −0.8 |
| High-density lipoprotein cholesterol level | −4.4 |
| Medication use Aspirin |
−2.7 |
| β-Blocker | −0.1 |
| Renin-angiotensin system inhibitor | −0.7 |
| Tricyclic antidepressant | −3.1 |
| SSRI | −1.3 |
| Other antidepressant | −8.8a |
| Potential biological mediators Heart rate variability, SDANN |
4.5 |
| Heart rate variability, ln VLF | 4.1 |
| Serotonin level among nonusers of SSRIs | −1.5 |
| Cortisol excretion | 0.5 |
| Norepinephrine excretion | −0.5 |
| Log C-reactive protein level | −11.3a |
| Omega-3 fatty acid levels | −1.5 |
| Potential behavioral mediators Regular alcohol use |
−0.5 |
| Smoking | −10.9a |
| Medication nonadherence | −5.3a |
| Self-reported physical activity | −31.7a |
Abbreviations: ln VLF, natural log of very low frequency; SDANN, SD of 5-minute mean NN intervals; SSRI, selective serotonin reuptake inhibitor.
Changes of more than 5% were considered as biologically or clinically important, and (with the exception of other antidepressant use) included in subsequent multivariate regression models.
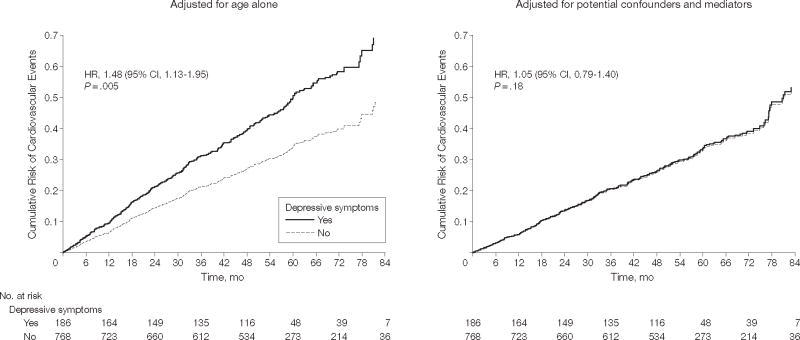
When adjusted for comorbid conditions and cardiac disease severity, depressive symptoms remained associated with a 31% greater rate of cardiovascular events (HR, 1.31; 95% CI, 1.00–1.71; P=.04). Additional adjustment for C-reactive protein attenuated this association (HR, 1.24; 95% CI, 0.94–1.63; P=.12; Table 4). After further adjustment for potential behavioral mediators, including physical inactivity, there was no longer a significant association between depressive symptoms and cardiovascular events (Table 4, Figure 1). Substituting exercise capacity (for self-reported physical activity) resulted in a similar reduction in the effect size for depressive symptoms (HR, 0.96; 95% CI, 0.70–1.31; = .79). In the final model, physical inactivity was associated with a 44% greater rate of cardiovascular events (HR, 1.44; 95% 1.14–1.82; P=.002), adjusting for depressive symptoms, comorbid conditions, left ventricular ejection fraction, C-reactive protein, smoking, and medication adherence (Table 5).
Table 4.
Association Between Baseline Depressive Symptoms (Patient Health Questionnaire Score ≥10 vs >10) and Subsequent Cardiovascular Events, With Sequential Adjustment for Potential Confounders and Mediators
| Hazard Ratio (95% Confidence Interval) |
|||||
|---|---|---|---|---|---|
| Adjusted Variablesa | Heart Failure | Myocardial Infarction | Stroke or Transient Ischemic Attack | Death | Any Event |
| Age per 10-y increase | 1.87 (1.27–2.74) | 1.45 (0.91–2.33) | 2.11 (1.12–3.99) | 1.36 (0.99–1.87) | 1.50 (1.16–1.95) |
|
| |||||
| History of myocardial infarction, stroke, diabetes, and heart failure | 1.63 (1.10–2.42) | 1.32 (0.82–2.12) | 1.89 (1.00–3.59) | 1.23 (0.89–1.70) | 1.37 (1.05–1.79) |
|
| |||||
| Left ventricular ejection fraction per 10% increase | 1.57 (1.06–2.33) | 1.26 (0.78–2.02) | 1.80 (0.95–3.42) | 1.15 (0.83–1.59) | 1.31 (1.00–1.71) |
|
| |||||
| Inflammation, log C-reactive protein per SD increase | 1.48 (0.99–2.20) | 1.17 (0.72–1.92) | 1.55 (0.78–3.07) | 1.05 (0.75–1.47) | 1.24 (0.94–1.63) |
|
| |||||
| Smoking status | 1.46 (0.98–2.17) | 1.09 (0.66–1.80) | 1.43 (0.71–2.90) | 1.01 (0.72–1.42) | 1.20 (0.91–1.58) |
|
| |||||
| Medication adherence | 1.45 (0.97–2.16) | 1.07 (0.65–1.78) | 1.31 (0.64–2.67) | 1.00 (0.71–1.41) | 1.18 (0.89–1.56) |
|
| |||||
| Physical activity | 1.18 (0.78–1.80) | 0.98 (0.58–1.64) | 1.47 (0.70–3.11) | 0.87 (0.61–1.23) | 1.05 (0.79–1.40) |
Each model includes the variables from the preceding row so that the final model includes all the variables listed in this table.
Figure 1. Cumulative Risk of Cardiovascular Events.
Data are stratified by depressive symptoms before and after adjustment for health behaviors in 954 participants with complete data. The adjusted hazard ratio (HR) differs slightly from Table 4 because 63 patients with incomplete data were excluded from the analysis. CI indicates confidence interval.
Table 5.
Fully Adjusted Model of the Association Between Baseline Depressive Symptoms and Subsequent Cardiovascular Events (Heart Failure, Myocardial Infarction, Stroke, Transient Ischemic Attack, or Death) in 954 Participants With Complete Data on Covariates
| Variable | HR (95%Confidence Interval) | P Value |
|---|---|---|
| Depressive symptomsa | 1.05 (0.79–1.40) | .75 |
| Age per 10-y increase | 1.72 (1.52–1.94) | <.001 |
| Medical history Myocardial infarction |
1.06 (0.83–1.34) | .64 |
| Diabetes | 1.76 (1.38–2.25) | <.001 |
| Stroke | 1.31 (0.98–1.75) | .07 |
| Heart failure | 1.27 (0.96–1.67) | .09 |
| Left ventricular ejection fraction per 10% decrease | 1.40 (1.26–1.56) | <.001 |
| Log C-reactive protein per SD increase | 1.27 (1.13–1.43) | <.001 |
| Current smoking | 1.39 (1.17–1.66) | <.001 |
| Medication nonadherence | 1.90 (1.31–2.76) | <.001 |
| Physical inactivityb | 1.44 (1.14–1.82) | .002 |
A score of 10 or higher on the Patient Health Questionnaire.
Not at all or a little vs fairly, quite, very, or extremely active.
Alternative Approaches to Classifying Depression
We observed a dose-response relation between increasing depressive symptom scores and cardiovascular events (Figure 2). When adjusted for age, co-morbid conditions, and left ventricular ejection fraction, each SD (5.5-point) increase in depressive symptom score was associated with a 15% greater rate of cardiovascular events (HR, 1.15; 95% CI, 1.03–1.28; P=.01). Adjusting for log C-reactive protein minimally attenuated this relation (HR, 1.13; 95% CI, 1.02–1.26; P=.02), and further adjustment for smoking and non-adherence moderately diminished the association (HR, 1.09; 95% CI, 0.98–1.22; P=.12). Again, there was no association between depressive symptoms and cardiovascular events after adjustment for physical activity (HR, 1.03; 95% CI, 0.92–1.16; P=.53). We found no evidence that the association between depressive symptom score and cardiovascular events varied by age, race, history of myocardial infarction, stroke, diabetes, left ventricular ejection fraction, smoking, medication adherence, or physical activity (all P values for interaction ≥0.1).
Figure 2. Age-Adjusted Annual Rate of Cardiovascular Events by Depressive Symptom Score in 1017 Participants.
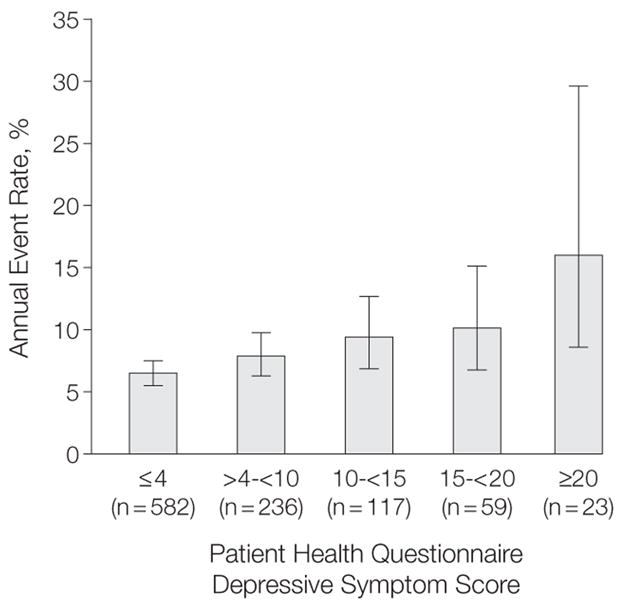
Events include heart failure, myocardial infarction, stroke, transient ischemic attack or death. χ2 P for trend<.001. Error bars indicate 95% confidence intervals.
Of the 1015 participants who completed the C DIS-IV for major depressive disorder, 120 had both current depressive symptoms (PHQ≥10) and major depressive disorder in the past month, 78 had depressive symptoms only, 102 had major depressive disorder only, and 715 had neither. In age-adjusted models, we did not observe any association between past month (HR, 1.08; 95% CI, 0.82–1.44; P=.56), past year (HR, 1.12; 95% CI, 0.86–1.46; P=.39), or lifetime (HR, 0.98; 95% CI, 0.78–1.23; P=.86) major depressive disorder and cardiovascular events.
COMMENT
In this prospective cohort study of more than 1000 outpatients with stable coronary heart disease, we found that participants with baseline depressive symptoms had a 50% greater rate of subsequent cardiovascular events (heart failure, myocardial infarction, stroke, transient ischemic attack, or death) than participants without depressive symptoms. After adjustment for comorbid conditions and cardiac disease severity, depressive symptoms remained associated with a 31% increased rate of cardiovascular events. Inflammation (as measured by C-reactive protein) explained a small part of this association. However, no significant association between depressive symptoms and cardiovascular events remained after adjustment for physical activity and other health behaviors. These findings raise the hypothesis that the increased risk of cardiovascular events associated with depression could potentially be preventable with behavior modification, especially exercise.
Given the relatively modest effects of traditional therapies on depressive symptoms in patients with heart disease,55–58 there is increasing urgency to identify interventions that not only reduce depressive symptoms but also directly target the mechanisms by which depression leads to cardiovascular events.59 Several large-scale studies have clearly documented that increased physical activity reduces cardiovascular events,60–62 but whether exercise interventions can eliminate the excess risk of cardiovascular events associated with depressive symptoms has not been studied. Patients with depressive symptoms are less likely to adhere to dietary, exercise, and medication recommendations,13–15 and poor health behaviors can lead to cardiovascular events.36,62 Exercise training can improve both depressive symptoms and markers of cardiovascular risk.63,64 It is therefore possible that the combination of exercise therapy plus antidepressant medication may reduce the risk of cardiovascular events in patients with depression.
Our study evaluated depression and physical activity at the same point in time, and thus we cannot determine whether physical inactivity was the cause or result of depression. Indeed, the association is almost certainly bidirectional because depression leads to physical inactivity,14,65–68 and physical inactivity exacerbates depression.69–72 This can result in a downward spiral in which depression and physical inactivity become mutually reinforcing. Likewise, treating depression can increase physical activity, and physical activity can elevate mood.64,73–75 To the extent that physical inactivity precedes depression, it would be acting as a confounder rather than as a mediator of the association between depression and cardiovascular events. Regardless of whether physical inactivity was the cause (a confounder) or the result (a mediator) of depressive symptoms, it appeared to account for almost half of the association between depressive symptoms and cardiovascular events in our sample. These findings raise the possibility that increased exercise may decrease the risk of cardiovascular events associated with depression.
Because we measured the potential behavioral mediators by self-report, it is possible that participants with depressive symptoms were more likely to underreport medication adherence and physical activity. We attempted to address this possibility by substituting exercise capacity (instead of self-reported physical activity) as an objective measure of physical fitness. Although exercise capacity may also have been influenced by worse underlying cardiac disease severity that was not otherwise accounted for in our models, adjusting for this gold standard measure of physical fitness resulted in the same findings as adjusting for self-reported physical activity.
Many cross-sectional studies have examined potential mediators of the association between depression and cardiovascular disease. However, only a few prospective studies have evaluated whether adjusting for any of these candidate mechanisms actually changes the effect size for depression on cardiovascular events. Carney et al17 found that low heart rate variability partially mediated the effect of depression on survival among 311 depressed patients after an acute myocardial infarction. In light of the present results, an intriguing possibility is that the mediating effects of heart rate variability may have been related to a less physically active lifestyle among the depressed participants. Vaccarino and colleagues24 reported that inflammatory biomarkers explained a small portion of the association between depression and cardiovascular disease incidence among 559 women with suspected coronary ischemia, but Empana et al76 found that adjustment for inflammatory markers did not change the strength of association between depressive symptoms and incident cardiovascular disease in a nested case-control study of 1005 healthy middle-aged men. In the present study, we found that adjusting for C-reactive protein was associated with a decrease in the strength of association between depressive symptoms and cardiovascular events. However, it is unclear whether inflammation was functioning as a mediator between depressive symptoms and cardiovascular events or as a marker of worse cardiac disease severity.
Others have raised the possibility that antidepressant toxicity may be responsible for the adverse cardiovascular outcomes associated with depressive symptoms. Cohen et al77 reported an excess risk of myocardial infarction in users of tricyclic antidepressants, and Sherwood et al18 found that use of antidepressant medication was associated with an increased risk of cardiovascular hospitalization or death in patients with heart failure. In contrast, Taylor and colleagues evaluated 2481 patients after experiencing a myocardial infarction and found that users of selective serotonin re-uptake inhibitors were at lower risk of death or recurrent myocardial infarction than nonusers.78 To evaluate the antidepressant toxicity hypothesis, we compared the effect size for depressive symptoms on cardiovascular events before and after adjustment for use of antidepressant medications. Although adjusting for use of selective serotonin reuptake inhibitors or tricyclic agents did not change the effect size for depressive symptoms, adjusting for use of “other antidepressants” reduced the effect size for depressive symptoms by 8.8%. One potential interpretation of these findings is that antidepressant toxicity may (at least partly) be responsible for the increased risk of cardiovascular events associated with depressive symptoms. However, a more likely explanation is that use of “other antidepressants” was a marker of more severe (or treatment-resistant) depression and that the effect size for depressive symptoms on cardiovascular events was reduced because adjusting for use of “other antidepressants” was tantamount to adjusting for depression severity. This interpretation is supported by previous studies identifying treatment-resistant depression as a high-risk subtype for cardiovascular events.59,79
We found that major depressive disorder as measured by the C DIS-IV was not associated with cardiovascular events, whereas depressive symptoms as measured by the PHQ were. It is possible that some participants may have felt more comfortable endorsing depressive symptoms on an anonymous questionnaire than in a face-to-face interview, making the interview a less accurate measure of depression. Assuming the interview was accurate, however, this discrepancy could inform our understanding of the mechanisms of association between depression and cardiovascular disease. An interview diagnosis of major depressive disorder was based on endorsing a minimum of 5 symptoms during the past month.
In contrast, the continuous PHQ-9 score captured both the number and frequency of depressive symptoms experienced in the past 2 weeks. Some patients with a major depressive episode in the past month may not have reported substantial depressive symptoms within the past 2 weeks. Likewise, some patients who did not meet interview criteria for major depressive disorder because they did not have 5 or more symptoms of depression may have had elevated PHQ-9 scores because they were experiencing fewer depressive symptoms more frequently or more recently than patients who did meet interview criteria. This would suggest that the frequency of depressive symptoms may be a stronger risk factor for physical inactivity and cardiovascular events than whether the patient meets criteria for major depressive disorder.
Our study has several strengths, including detailed assessments of depression and cardiac disease severity, careful measurement of potential biological and behavioral mediators, and comprehensive assessment of cardiovascular events. However, several limitations must also be considered. First, most of our participants were older men, and almost half were recruited from VA medical centers. Therefore, our results may not generalize to women or to other patient populations. Second, low physical activity and exercise capacity may have been the result of greater cardiac disease severity. We attempted to address this possibility by carefully measuring and adjusting for comorbid conditions and cardiovascular disease severity. However, no observational study can completely eliminate confounding, and it remains possible that the effect of physical inactivity on cardiovascular outcomes may have been influenced by worse underlying cardiac disease severity that was not otherwise accounted for in our models. Third, we did not assess dietary factors other than blood levels of omega-3 fatty acids. Finally, our study was limited to outpatients with stable coronary heart disease, and thus we cannot comment on the mechanisms of association between depression and cardiovascular outcomes in healthy populations or in patients following acute coronary syndrome.
In summary, we found that the association between depressive symptoms and cardiovascular events was largely explained by health behaviors, especially physical inactivity. These results suggest that the relationship between depression and cardiovascular events may be modifiable with behavioral interventions. The ongoing Understanding Prognostic Benefits of Exercise and Antidepressant Therapy (UPBEAT) study is comparing the effects of exercise vs antidepressant medication on depression and biomarkers of cardiovascular risk in patients with depressive symptoms and coronary heart disease.80 The longer-term goal is to identify an intervention that will improve both depression and cardiovascular disease outcomes. Regardless of whether physical inactivity causes depressive symptoms or vice-versa, increased activity has the potential to reduce the excess risk of cardiovascular events associated with depressive symptoms in patients with stable coronary heart disease.
Acknowledgments
Funding/Support: This study was supported by the VA Epidemiology (Merit Review) Program; the VA Health Research and Development Service Career Development Program; the National Heart, Lung, and Blood Institute (R01 HL079235); the American Federation for Aging Research (Paul Beeson Scholars Program); the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program); the Ischemia Research and Education Foundation; and the Nancy Kirwan Heart Research Fund.
Role of the Sponsor: The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosures: None reported.
Author Contributions: Dr Whooley had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Whooley, de Jonge, Carney, Feldman, Browner.
Acquisition of data: Whooley, Ali, Dowray, Na, Schiller.
Analysis and interpretation of data: Whooley, Vittinghoff, Otte, Moos, Carney, Na, Browner.
Drafting of the manuscript: Whooley, de Jonge, Otte, Moos, Ali, Dowray, Na.
Critical revision of the manuscript for important intellectual content: Whooley, de Jonge, Vittinghoff, Otte, Carney, Feldman, Schiller, Browner.
Statistical analysis: Whooley, Vittinghoff, Ali, Dowray, Na, Browner.
Obtained funding: Whooley, Otte, Moos, Schiller, Browner.
Administrative, technical, or material support: Whooley, de Jonge, Moos, Ali, Dowray, Na, Feldman.
Study supervision: Whooley, Schiller, Browner.
References
- 1.Whooley MA. Depression and cardiovascular disease: healing the broken-hearted. JAMA. 2006;295(24):2874–2881. doi: 10.1001/jama.295.24.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borowicz L, Jr, Royall R, Grega M, Selnes O, Lyketsos C, McKhann G. Depression and cardiac morbidity 5 years after coronary artery bypass surgery. Psychosomatics. 2002;43(6):464–471. doi: 10.1176/appi.psy.43.6.464. [DOI] [PubMed] [Google Scholar]
- 3.Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305–315. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 4.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66(6):802–813. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 5.van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66(6):814–822. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 6.Wellenius GA, Mukamal KJ, Kulshreshtha A, Asonganyi S, Mittleman MA. Depressive symptoms and the risk of atherosclerotic progression among patients with coronary artery bypass grafts. Circulation. 2008;117(18):2313–2319. doi: 10.1161/CIRCULATIONAHA.107.741058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramson J, Berger A, Krumholz HM, Vaccarino V. Depression and risk of heart failure among older persons with isolated systolic hypertension. Arch Intern Med. 2001;161(14):1725–1730. doi: 10.1001/archinte.161.14.1725. [DOI] [PubMed] [Google Scholar]
- 8.Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–1856. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 9.Vaccarino V, Kasl SV, Abramson J, Krumholz HM. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Cardiol. 2001;38(1):199–205. doi: 10.1016/s0735-1097(01)01334-1. [DOI] [PubMed] [Google Scholar]
- 10.Williams SA, Kasl SV, Heiat A, Abramson JL, Krumholz HM, Vaccarino V. Depression and risk of heart failure among the elderly: a prospective community-based study. Psychosom Med. 2002;64(1):6–12. doi: 10.1097/00006842-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. World Health Statistics. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 13.Ruo B, Rumsfeld JS, Pipkin S, Whooley MA. Relation between depressive symptoms and treadmill exercise capacity in the Heart and Soul Study. Am J Cardiol. 2004;94(1):96–99. doi: 10.1016/j.amjcard.2004.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med. 2000;160(12):1818–1823. doi: 10.1001/archinte.160.12.1818. [DOI] [PubMed] [Google Scholar]
- 15.Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med. 2005;165(21):2508–2513. doi: 10.1001/archinte.165.21.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lett H, Ali S, Whooley M. Depression and cardiac function in patients with stable coronary heart disease: findings from the Heart and Soul Study. Psychosom Med. 2008;70(4):444–449. doi: 10.1097/PSY.0b013e31816c3c5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carney RM, Blumenthal JA, Freedland KE, et al. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Arch Intern Med. 2005;165(13):1486–1491. doi: 10.1001/archinte.165.13.1486. [DOI] [PubMed] [Google Scholar]
- 18.Sherwood A, Blumenthal JA, Trivedi R, et al. Relationship of depression to death or hospitalization in patients with heart failure. Arch Intern Med. 2007;167(4):367–373. doi: 10.1001/archinte.167.4.367. [DOI] [PubMed] [Google Scholar]
- 19.Otte C, Marmar CR, Pipkin SS, Moos R, Browner WS, Whooley MA. Depression and 24-hour urinary cortisol in medical outpatients with coronary heart disease: the Heart and Soul Study. Biol Psychiatry. 2004;56(4):241–247. doi: 10.1016/j.biopsych.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otte C, Neylan TC, Pipkin SS, Browner WS, Whooley MA. Depressive symptoms and 24-hour urinary norepinephrine excretion levels in patients with coronary disease: findings from the Heart and Soul Study. Am J Psychiatry. 2005;162(11):2139–2145. doi: 10.1176/appi.ajp.162.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frasure-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry. 2004;55(9):891–896. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Schins A, Hamulyak K, Scharpe S, et al. Whole blood serotonin and platelet activation in depressed post-myocardial infarction patients. Life Sci. 2004;76(6):637–650. doi: 10.1016/j.lfs.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 23.Frasure-Smith N, Lesperance F, Irwin MR, Sauve C, Lesperance J, Theroux P. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62(4):302–308. doi: 10.1016/j.biopsych.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 24.Vaccarino V, Johnson BD, Sheps DS, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute–Sponsored WISE study. J Am Coll Cardiol. 2007;50(21):2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 25.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290(2):215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroenke K, Spitzer RL, Williams JB. The PHQ-9 validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 28.McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease (data from the Heart and Soul Study) Am J Cardiol. 2005;96(8):1076–1081. doi: 10.1016/j.amjcard.2005.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robins LN, Helzer J, Croughan J, Ratcliff K. National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38(4):381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 30.Greist JH, Klein MH, Erdman HP, et al. Comparison of computer- and interviewer-administered versions of the Diagnostic Interview Schedule. Hosp Community Psychiatry. 1987;38(12):1304–1311. doi: 10.1176/ps.38.12.1304. [DOI] [PubMed] [Google Scholar]
- 31.Blouin AG, Perez EL, Blouin JH. Computerized administration of the Diagnostic Interview Schedule. Psychiatry Res. 1988;23(3):335–344. doi: 10.1016/0165-1781(88)90024-8. [DOI] [PubMed] [Google Scholar]
- 32.Ren X, Ristow B, Na B, Ali S, Schiller NB, Whooley MA. Prevalence and prognosis of asymptomatic left ventricular diastolic dysfunction in ambulatory patients with coronary heart disease. Am J Cardiol. 2007;99(12):1643–1647. doi: 10.1016/j.amjcard.2007.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gehi A, Mangano D, Pipkin S, Browner WS, Whooley MA. Depression and heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry. 2005;62(6):661–666. doi: 10.1001/archpsyc.62.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62(4):314–320. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali S, Garg S, Cohen B, Bhave P, Harris WS, Whooley M. Association between omega-3 fatty acids and depressive symptoms among patients with established coronary artery disease: data from the Heart and Soul Study. Psychother Psychosom. doi: 10.1159/000203118. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the Heart and Soul Study. Arch Intern Med. 2007;167(16):1798–1803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Ghali JK, Kadakia S, Cooper R, Ferlinz J. Precipitating factors leading to decompensation of heart failure: traits among urban blacks. Arch Intern Med. 1988;148(9):2013–2016. [PubMed] [Google Scholar]
- 39.Blumberg EJ, Hovell MF, Kelley NJ, Vera AY, Sipan CL, Berg JP. Self-report INH adherence measures were reliable and valid in Latino adolescents with latent tuberculosis infection. J Clin Epidemiol. 2005;58(6):645–648. doi: 10.1016/j.jclinepi.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166(17):1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 41.Haynes SG, Feinleib M, Kannel WB. The relationship of psychosocial factors to coronary heart disease in the Framingham Study, III: eight-year incidence of coronary heart disease. Am J Epidemiol. 1980;111(1):37–58. doi: 10.1093/oxfordjournals.aje.a112873. [DOI] [PubMed] [Google Scholar]
- 42.Matsui D, Hermann C, Klein J, Berkovitch M, Olivieri N, Koren G. Critical comparison of novel and existing methods of compliance assessment during a clinical trial of an oral iron chelator. J Clin Pharmacol. 1994;34(9):944–949. doi: 10.1002/j.1552-4604.1994.tb04009.x. [DOI] [PubMed] [Google Scholar]
- 43.Bowles HR, FitzGerald SJ, Morrow JR, Jr, Jackson AW, Blair SN. Construct validity of self-reported historical physical activity. Am J Epidemiol. 2004;160(3):279–286. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]
- 44.Kurtze N, Rangul V, Hustvedt BE, Flanders WD. Reliability and validity of self-reported physical activity in the Nord-Trondelag Health Study: HUNT 1. Scand J Public Health. 2008;36(1):52–61. doi: 10.1177/1403494807085373. [DOI] [PubMed] [Google Scholar]
- 45.Ainsworth BE, Jacobs DR, Jr, Leon AS. Validity and reliability of self-reported physical activity status: the Lipid Research Clinics questionnaire. Med Sci Sports Exerc. 1993;25(1):92–98. doi: 10.1249/00005768-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Aadahl M, Kjaer M, Kristensen JH, Mollerup B, Jorgensen T. Self-reported physical activity compared with maximal oxygen uptake in adults. Eur J Cardiovasc Prev Rehabil. 2007;14(3):422–428. doi: 10.1097/HJR.0b013e3280128d00. [DOI] [PubMed] [Google Scholar]
- 47.Jackson AW, Morrow JR, Jr, Bowles HR, FitzGerald SJ, Blair SN. Construct validity evidence for single-response items to estimate physical activity levels in large sample studies. Res Q Exerc Sport. 2007;78(2):24–31. doi: 10.1080/02701367.2007.10599400. [DOI] [PubMed] [Google Scholar]
- 48.Knapik J, Zoltick J, Rottner HC, et al. Relationships between self-reported physical activity and physical fitness in active men. Am J Prev Med. 1993;9(4):203–208. [PubMed] [Google Scholar]
- 49.Kaleta D, Makowiec-Dabrowska T, Jegier A. Leisure-time physical activity, cardio respiratory fitness and work ability: a study in randomly selected residents of Lodz. Int J Occup Med Environ Health. 2004;17(4):457–464. [PubMed] [Google Scholar]
- 50.Vanhees L, Lefevre J, Philippaerts R, et al. How to assess physical activity? how to assess physical fitness? Eur J Cardiovasc Prev Rehabil. 2005;12(2):102–114. doi: 10.1097/01.hjr.0000161551.73095.9c. [DOI] [PubMed] [Google Scholar]
- 51.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 52.Schmoor C, Sauerbrei W, Schumacher M. Sample size considerations for the evaluation of prognostic factors in survival analysis. Stat Med. 2000;19(4):441–452. doi: 10.1002/(sici)1097-0258(20000229)19:4<441::aid-sim349>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 53.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 54.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 55.Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 56.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 57.Rees K, Bennett P, West R, Davey SG, Ebrahim S. Psychological interventions for coronary heart disease. Cochrane Database Syst Rev. 2004;(2):CD002902. doi: 10.1002/14651858.CD002902.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Melle JP, de Jonge P, Honig A, et al. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460–466. doi: 10.1192/bjp.bp.106.028647. [DOI] [PubMed] [Google Scholar]
- 59.de Jonge P, Honig A, van Melle JP, et al. Nonresponse to treatment for depression following myocardial infarction: association with subsequent cardiac events. Am J Psychiatry. 2007;164(9):1371–1378. doi: 10.1176/appi.ajp.2007.06091492. [DOI] [PubMed] [Google Scholar]
- 60.Bijnen FC, Caspersen CJ, Feskens EJ, Saris WH, Mosterd WL, Kromhout D. Physical activity and 10-year mortality from cardiovascular diseases and all causes: the Zutphen Elderly Study. Arch Intern Med. 1998;158(14):1499–1505. doi: 10.1001/archinte.158.14.1499. [DOI] [PubMed] [Google Scholar]
- 61.Franco OH, de Laet C, Peeters A, Jonker J, Mackenbach J, Nusselder W. Effects of physical activity on life expectancy with cardiovascular disease. Arch Intern Med. 2005;165(20):2355–2360. doi: 10.1001/archinte.165.20.2355. [DOI] [PubMed] [Google Scholar]
- 62.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 63.Blumenthal JA, Sherwood A, Babyak MA, et al. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: a randomized controlled trial. JAMA. 2005;293(13):1626–1634. doi: 10.1001/jama.293.13.1626. [DOI] [PubMed] [Google Scholar]
- 64.Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Penninx BW, Leveille S, Ferrucci L, van Eijk JT, Guralnik JM. Exploring the effect of depression on physical disability: longitudinal evidence from the established populations for epidemiologic studies of the elderly. Am J Public Health. 1999;89(9):1346–1352. doi: 10.2105/ajph.89.9.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stuck AE, Walthert JM, Nikolaus T, Bula CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48(4):445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 67.Ades PA, Savage PD, Tischler MD, Poehlman ET, Dee J, Niggel J. Determinants of disability in older coronary patients. Am Heart J. 2002;143(1):151–156. doi: 10.1067/mhj.2002.119379. [DOI] [PubMed] [Google Scholar]
- 68.Harris AH, Cronkite R, Moos R. Physical activity, exercise coping, and depression in a 10-year cohort study of depressed patients. J Affect Disord. 2006;93(1–3):79–85. doi: 10.1016/j.jad.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 69.Craft LL, Landers DM. The effect of exercise on clinical depression and depression resulting from mental illness: a meta-analysis. J Sport Exerc Psychol. 1998;20(4):339–357. [Google Scholar]
- 70.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 71.Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Penninx BW, Rejeski WJ, Pandya J, et al. Exercise and depressive symptoms: a comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. J Gerontol B Psychol Sci Soc Sci. 2002;57(2):P124–P132. doi: 10.1093/geronb/57.2.p124. [DOI] [PubMed] [Google Scholar]
- 73.North TC, McCullagh P, Tran ZV. Effect of exercise on depression. Exerc Sport Sci Rev. 1990;18:379–415. [PubMed] [Google Scholar]
- 74.Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322(7289):763–767. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 76.Empana JP, Sykes DH, Luc G, et al. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2005;111(18):2299–2305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- 77.Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med. 2000;108(1):2–8. doi: 10.1016/s0002-9343(99)00301-0. [DOI] [PubMed] [Google Scholar]
- 78.Taylor CB, Youngblood ME, Catellier D, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792–798. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- 79.Carney RM, Blumenthal JA, Freedland KE, et al. Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosom Med. 2004;66(4):466–474. doi: 10.1097/01.psy.0000133362.75075.a6. [DOI] [PubMed] [Google Scholar]
- 80.Blumenthal JA, Sherwood A, Rogers SD, et al. Understanding prognostic benefits of exercise and anti-depressant therapy for persons with depression and heart disease: the UPBEAT study rationale, design, and methodological issues. Clin Trials. 2007;4(5):548–559. doi: 10.1177/1740774507083388. [DOI] [PMC free article] [PubMed] [Google Scholar]