DNA polymerases are crucial for maintaining the fidelity of genetic information encoded into DNA, and failure to repair aberrant bases in damaged DNA strands is notoriously implicated in oncogenesis. During base excision repair (BER),1,2 DNA polymerase β (pol β) inserts the correct deoxynucleoside triphosphate (dNTP), replacing an excised damaged or mismatched nucleoside residue, with release of pyrophosphate. The smallest eukaryotic cellular DNA polymerase, pol β is an error-prone enzyme that is tightly down-regulated in normal somatic cells, but often overexpressed in human tumors, and has been the subject of extensive studies examining its roles in BER2 and cancer.3
Probes of molecular interactions with nucleic acid polymerases can be created by designed modifications in the structures of natural dNTPs. Although such analogues may be usefully modified in their nucleoside moieties to create active site probes, as shown for example by the recent work of Kool,4 changes to the triphosphate group are of particular interest because this group is the locus of chemical transformation catalyzed in the polymerase active site. Replacement of the Pα-O-Pβ bridging oxygen by a carbon atom (CXY) will prevent hydrolysis, whereas a Pβ-CXY-Pγ modification will alter the leaving group properties, depending on the nature of substituents X and Y, while conferring resistance5 to dephosphorylation. The introduction of these substituents potentially may also enable entirely new bonding (or repulsive) active site interactions, not present with the natural nucleoside triphosphate, and thus could inform inhibitor design seeking to exploit pol β as a drug target.
Recently, a series of unfluorinated (X,Y = H, 1) and fluorinated (X,Y = F, 2; X,Y = H,F, 3 (R) or F,H 4 (S)) β,γ-methylenebisphosphonate analogues of dGTP (Chart 1) and related compounds were used to examine leaving group effects on pol β catalysis and fidelity.6 These analogues are substrates of the polymerase, but release an X,Y-methylenebisphosphonate in place of the natural pyrophosphate leaving group. Several β,γ-CXY dNTP analogues were previously investigated as inhibitors of different DNA polymerases and viral reverse transcriptases,7 but the structures of the putative complexes formed during turnover were not determined. In previous studies, the CHX analogues (or more generally, CXY analogues where X ≠ Y) have been used as the diastereomer mixtures always obtained synthetically when the substituted methylenebisphosphonate is coupled to a dNMP, and their potential for a stereospecific interaction with the polymerase active site has received little or no attention.
Chart 1.
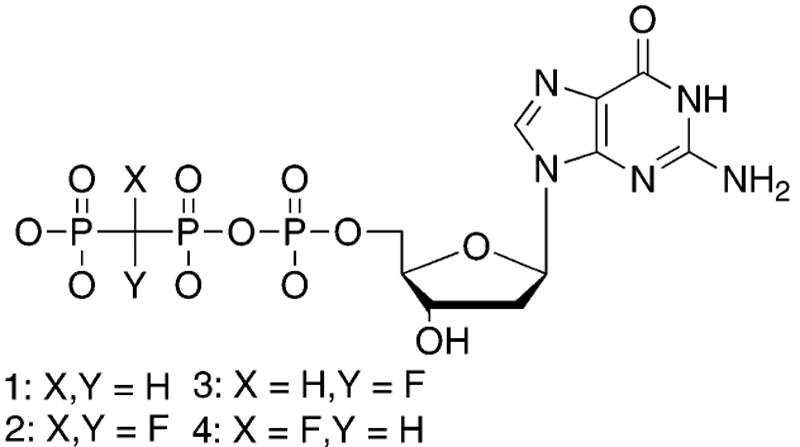
β,γ-CXY Analogues of dGTP (Charges Omitted)
In this Communication, we report X-ray crystallographic studies and computer docking simulations of terminal ddNMP primer-template pol β complexes with dGTP β,γ-fluoromethylenebisphosphonate analogues 3 and 4, leading to the first demonstration of stereospecificity in dNTP β,γ-CHX analogue-enzyme complex formation.
(R/S)-β,γ-CHF-dGTP (3/4) (and also the corresponding CH2 and CF2 analogues) were prepared by DCC-mediated conjugation of the morpholidate8,9 of dGMP (5) in anhydrous DMSO with the tributylammonium salt of the appropriate methylenebisphosphonic acid, followed by two-stage preparative HPLC to achieve a high degree of purity with respect to contaminating nucleotides. The (mono- and difluoromethylene)bisphosphonic acids10,11 (6b,7b) were prepared using the more convenient12 Selectfluor (8) in place of perchloryl fluoride10 to synthesize the intermediate fluorinated esters (6a,7a)10,11 from tetraisopropyl methylenebisphosphonate. In consistency with an early study of the mixed (R/S)-β,γ-CHF analogues of ATP,13 at pH 10 or higher, 3 and 4 could be individually detected and quantified (1:1) by their overlapping but resolvable 19F NMR resonances, exhibited as a pair of ddd with δ -218.61 and -218.77 ppm.
For protein crystallography, human pol β was overexpressed in E. coli and purified as described previously.14 The double-stranded DNA substrate consisted of a 16-mer template (5′-CCGACCGCGCATCAGC-3′), a complementary 9-mer primer (5′-GCTGATGCG-3′), and a 5-mer downstream oligonucleotide (5′-pGTCGG-3′), thus creating a two-nucleotide gap with annealed primer. Addition of ddCTP terminates the primer and creates a one-nucleotide gap. The 1:1 mixture of 3 and 4 was added to a solution of the preformed protein-DNA complex.
The crystal structure of the resulting complex was resolved at 2.1 Å. The analogue was found in the enzyme active site position normally occupied by dGTP, and its configuration overall was a good match for that of the natural substrate. However, despite both CHF stereoisomers being present at very similar concentrations in the crystallizing mixture, electron density was only observed for a single fluorine atom in the complex, corresponding to the (R)-CHF stereoisomer (3) (Figure 1). In contrast, in a comparable crystallography experiment, the corresponding β,γ-CHCl analogue stereoisomers populated the DNA pol active site about equally (Pedersen, L. C., personal communication). Exclusion of the (S)-β,γ-CHF analogue is not the result of an unattainable binding conformation, because the crystal structure of the corresponding CF2-analogue complex6 superimposes well with that of the (R)-stereoisomer.
Figure 1.
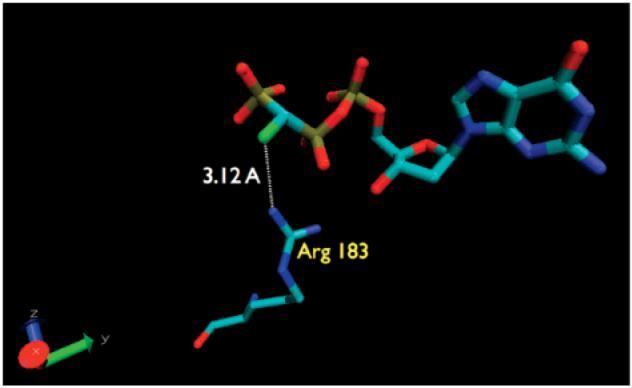
Detail from X-ray crystal active-site structure of DNA pol β:DNA complex soaked with a 1:1 mixture of 3 and 4.
Thus, in the absence of an dominant steric factor, asymmetric polarization induced by the F substituent presumably influences 3 versus 4 binding specificity electrostatically.15 If we assume that the limit for detection of fluorine electron density at the (S)-F position corresponds to a S/R ratio of roughly 1:4 or less, then a stereospecific interaction on the order of 0.8 kcal/mol would suffice. The fluorine atom in the 3 complex is located 3.1 Å from an Arg183 guanidine N atom (Figure 1), raising the possibility that an unusual16 F•••H bonding interaction17 contributes to stabilizing the preferred stereoisomer within the desolvated and preorganized15,18 enzyme active site complex. Further studies are in progress to explore this possibility and alternative explanations.16b
Molecular docking calculations19 are often valuable in studying protein-ligand interactions. Prior to attempting the crystallography studies, we first carried out an exploratory ligand docking experiment based on the α,β-NH-dUTP-DNA-pol β structure (2FMS),20 using Autodock 3.0,21 substituting β,γ-CF2-dTTP for the dUTP analogue. Docking runs predicted a preferred β,γ-CF2 triphosphate chain orientation similar to that of natural dNTPs, but placing one of the two diastereotopic F atoms close to Arg183 in the active site environment. Redocking of 3 using our recently available β,γ-CF2-dGTP-DNA-pol β structure (2ISO)6 revealed a clustering of solutions placing the F atom within bonding proximity of the Arg183, whereas with 4 such an interaction was less favored. An overlay of the nucleoside moieties and triphosphate backbones of 1, 2, and 3 within the DNA pol β complexes (X-ray crystallographic data) reveal them to be substantially congruent, confirming that introduction of the F atom(s) does not perturb the overall fit of the substrate to the active site and that the F atom positions are similar in 2 and 3.
In conclusion, under crystallization conditions, 3 is preferentially bound from a 1:1 mixture of diastereomers 3 and 4 into a DNA-pol β complex, in which a polar CHF bond to Arg183 is spatially allowed. Docking simulations predicted this configuration to be more likely with 3 than with its S stereoisomer 4, which was not observed in the crystal complex. Substitution of a single fluorine atom at the bridging carbon atom of a β,γ-CH2-dNTP analogue, while offering the advantage pKa properties more closely mimicking those of the dNTP substrate,22 also may result in stereospecific binding to the targeted active site, determined by the CHF chirality.
Supplementary Material
Acknowledgment
Dedicated to Professor F. H. Westheimer, 1912-2007. We thank Dr. Kym Faull and Dr. Ron New for assistance with HRMS analysis. This research was supported by NIH Grant 5-U19-CA105010 and in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Supporting Information Available: Synthesis and characterization data for 1-7; crystallographic data for the complex of 3 with DNA pol β (PDB ID, 2PXI); computer docking results. This material is available free of charge via the Internet at http://pubs.acs.org.
Note Added in Proof: A PDB search for ligand C-F interactions with the guanidinium group of Arg has documented a number of examples underscoring the fluorophilic character of the Arg side chain: Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881-1886.
References
- (1).Barnes DE, Lindahl T. Annu. Rev. Genet. 2004;38:445–476. doi: 10.1146/annurev.genet.38.072902.092448. [DOI] [PubMed] [Google Scholar]
- (2).Beard WA, Wilson SH. Chem. Rev. 2006;106:361–382. doi: 10.1021/cr0404904. [DOI] [PubMed] [Google Scholar]
- (3).(a) Bergoglio V, Canitrot Y, Hogarth L, Minto L, Howell SB, Cazaux C, Hoffmann JS. Oncogene. 2001;20:6181–6187. doi: 10.1038/sj.onc.1204743. [DOI] [PubMed] [Google Scholar]; (b) Louat T, Servant L, Rols MP, Bieth A, Teissie J, Hoffmann JS, Cazaux C. Mol. Pharmacol. 2001;60:553–558. [PubMed] [Google Scholar]; (c) Servant L, Bieth A, Hayakawa H, Cazaux C, Hoffmann J-S. J. Mol. Biol. 2002;315:1039–1047. doi: 10.1006/jmbi.2001.5307. [DOI] [PubMed] [Google Scholar]; (d) Starcevic D, Dalal S, Sweasy JB. Cell Cycle. 2004;3:998–1001. [PubMed] [Google Scholar]; (e) Albertella MR, Lau A, O'Connor MJ. DNA Repair. 2005;4:583–593. doi: 10.1016/j.dnarep.2005.01.005. [DOI] [PubMed] [Google Scholar]; (f) Dalal S, Hile S, Eckert KA, Sun K, Starcevic D, Sweasy JB. Biochemistry. 2005;44:15664–15673. doi: 10.1021/bi051179z. [DOI] [PubMed] [Google Scholar]; (g) Sweasy JB, Lauper JM, Eckert KA. Radiat. Res. 2006;166:693–714. doi: 10.1667/RR0706.1. [DOI] [PubMed] [Google Scholar]
- (4).Kim TW, Delaney JC, Essigmann JM, Kool ET. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15803–15808. doi: 10.1073/pnas.0505113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).(a) Shipitsin AV, Victorova LS, Shirokova EA, Dyatkina NB, Goryunova LE, Beabealashvili RS, Hamilton CJ, Roberts SM, Krayevsky A. J. Chem. Soc., Perkin Trans. 1999;1:1039–1050. doi: 10.1080/15257779908041639. [DOI] [PubMed] [Google Scholar]; (b) Hamilton CJ, Roberts SM, Shipitsin A. Chem. Commun. 1998:1087–1088. [Google Scholar]
- (6).Sucato CA, Upton TG, Kashemirov BA, Batra VK, Martinek V, Xiang Y, Beard WA, Pedersen LC, Wilson SH, McKenna CE, Florian J, Warshel A, Goodman MF. Biochemistry. 2007;46:461–471. doi: 10.1021/bi061517b. [DOI] [PubMed] [Google Scholar]
- (7).(a) Arabshahi L, Khan NN, Butler M, Noonan T, Brown NC, Wright GE. Biochemistry. 1990;29:6820–6826. doi: 10.1021/bi00481a010. [DOI] [PubMed] [Google Scholar]; (b) Martynov BI, Shirokova EA, Jasko MV, Victorova LS, Krayevsky AA. FEBS Lett. 1997;410:423–427. doi: 10.1016/s0014-5793(97)00577-2. [DOI] [PubMed] [Google Scholar]; (c) Krayevsky A, Arzumanov A, Shirokova E, Dyatkina N, Victorova L, Jasko M, Alexandrova L. Nucleosides Nucleotides. 1998;17:681–693. doi: 10.1080/07328319808005209. [DOI] [PubMed] [Google Scholar]; (d) Alexandrova LA, Skoblov AY, Jasko MV, Victorova LS, Krayevsky AA. Nucleic Acids Res. 1998;26:778–786. doi: 10.1093/nar/26.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Moffatt JG, Khorana HG. J. Am. Chem. Soc. 1961;83:649–658. [Google Scholar]
- (9).Blackburn GM, Kent DE, Kolkmann F. Chem. Commun. 1981:1188–1190. [Google Scholar]
- (10).McKenna CE, Shen P-D. J. Org. Chem. 1981;46:4573–4576. [Google Scholar]
- (11).Blackburn GM, England DA, Kolkmann F. Chem. Commun. 1981:930–932. [Google Scholar]
- (12).(a) Marma MS, Khawli LA, Harutunian V, Kashemirov BA, McKenna CE. J. Fluorine Chem. 2005;126:1467–1475. [Google Scholar]; (b) Mohamady S, Jakeman DL. J. Org. Chem. 2005;70:10588–10591. doi: 10.1021/jo0518598. [DOI] [PubMed] [Google Scholar]
- (13).McKenna CE, Harutunian V. FASEB J. 1988;2:6148. [Google Scholar]
- (14).Beard WA, Wilson SH. Methods Enzymol. 1995;262:98–107. doi: 10.1016/0076-6879(95)62013-3. [DOI] [PubMed] [Google Scholar]
- (15).O'Hagan D, Rzepa HS. Chem. Commun. 1997:645–652. [Google Scholar]
- (16).(a) Howard JAK, Hoy VJ, O'Hagan D, Smith GT. Tetrahedron. 1996;52:12613–12622. [Google Scholar]; (b) Paulini R, Müller K, Diederich F. Angew. Chem., Int. Ed. 2005;44:1788–1805. doi: 10.1002/anie.200462213. [DOI] [PubMed] [Google Scholar]
- (17).(a) Mecozzi S, Hoang KC, Martin O. Abstract FLUO-047; 226th National Meeting of the American Chemical Society, New York; Sept. 7-11, 2003; Washington, DC: American Chemical Society; 2003. [Google Scholar]; (b) Mecozzi S. Abstract MEDI-467; 230th National Meeting of the American Chemical Society, Washington, DC; Aug. 28-Sept. 1, 2005; Washington, DC: American Chemical Society; 2005. [Google Scholar]
- (18).Shibakami M, Sekiya A. Chem. Commun. 1992:1742–1743. [Google Scholar]
- (19).(a) Vieth M, Hirst JD, Dominy BN, Daigler H, Brooks CL., III J. Comput. Chem. 1998;19:1623–1631. [Google Scholar]; (b) Bursulaya BD, Totrov M, Abagyan R, Brooks CL., III J. Comput. Mol. Design. 2004;17:755–763. doi: 10.1023/b:jcam.0000017496.76572.6f. [DOI] [PubMed] [Google Scholar]
- (20).Batra VK, Beard WA, Shock DD, Krahn JM, Pedersen LC, Wilson SH. Structure. 2006;14:757–766. doi: 10.1016/j.str.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) Goodsell DS, Morris GM, Olson AJ. J. Mol. Recognit. 1996;9:1–5. doi: 10.1002/(sici)1099-1352(199601)9:1<1::aid-jmr241>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]; (b) Morris GM, Goodsell DS, Huey R, Hart WE, Halliday S, Belew R, Olson AJ, The Scripps Research Institute Autodock User Guide. 2001 version 3.0.5. [Google Scholar]; (c) SPARTAN '02 for Windows. Wavefunction, Inc.; 2002. [Google Scholar]; (d) Li C, Xu L, Wolan DW, Wilson IA, Olson AJ. J. Med. Chem. 2004;47:6681–6690. doi: 10.1021/jm049504o. [DOI] [PubMed] [Google Scholar]
- (22).Berkowitz DB, Bose M. J. Fluorine Chem. 2001;112:13–33. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


