Abstract
We previously demonstrated that oxidative stress subsequent to gastroesophageal reflux is an important driving force of esophageal adenocarcinoma (EAC) formation in the esophagogastroduodenal anastomosis (EGDA) rat model. The present study investigated the possible tumor inhibitory effects of two antioxidants, α-tocopherol (389 ppm and 778 ppm), N-acetylcysteine (NAC, 500 ppm and 1,000 ppm), and their combination (389 ppm and 500 ppm, respectively), as well as an antacid therapeutic agent, omeprazole (1,400 ppm). The rats were fed experimental diets two weeks after EGDA. All the animals were sacrificed 40 weeks after EGDA and the esophagi were harvested for histopathological examination. α-Tocopherol dose-dependently decreased the incidence of EAC (P=0.03), with 778 ppm α-tocopherol reducing the incidence of EAC to 59% (16/27) in comparison to 84% (26/31) in the control group (P=0.04). Supplementation of α-tocopherol also increased the serum concentration of α-tocopherol. NAC at 500 ppm and 1,000 ppm did not significantly decrease EAC incidence; however, the combination of α-tocopherol 389 ppm and NAC 500 ppm significantly reduced the incidence of EAC to 55% (15/27) (P=0.02). α-Tocopherol alone or in combination with NAC significantly reduced the number of infiltrating cells positively stained for 4-hydroxynonenal. Omeprazole showed only a slight non-significant inhibitory effect at the dose given. Our results suggest that supplementation with α-tocopherol inhibits the development of EAC in the rat EGDA model and similar inhibitory effect can be achieved when a lower dose of α-tocopherol is used in combination with NAC.
Keywords: esophageal adenocarcinoma, inhibition, α-tocopherol, N-acetylcysteine, omeprazole
Introduction
In the last thirty years, the incidence of esophageal adenocarcinoma (EAC) in the United States has increased 4 fold, at a rate of 4-10% annually.1, 2 Surgery remains the first choice in treatment, but even with improved techniques, the operative mortality rate is still around 10% and the 5 year survival rate is only 20%-30%.3 It is therefore important to understand the etiology and pathogenesis of this disease and to develop strategies for its prevention.
It is now clear that gastroesophageal reflux disease (GERD) and the formation of Barrett's esophagus (BE) are major risk factors for the development of EAC. Chronic GERD induces an adaptive metaplastic response in the squamous epithelium of the esophagus and transforms it into columnar epithelium marked by the appearance of glandular structures and goblet cells.4, 5 This type of pathological change is known as BE, and the risk of developing EAC in BE patients is 30 times higher than the general population.4
In order to mimic the pathological changes in human EAC, we developed a surgical procedure named esophagogastroduodenal anastomosis (EGDA), to produce gastroesophageal reflux and induce EAC in rats.6 This model is superior to the previously used esophagoduodenal anastomosis model in that it maintains the stomach functions and relatively normal nutritional status of the animals.7 The gastroesophageal reflux induce chronic inflammation of the proximal esophagus and increase the expression of the cyclooxygenase-2, 5-lipoxygenase, and leukotriene A4 hydrolase in the inflamed esophageal epithelia. Using this model we have demonstrated the chemopreventive activities of sulindac, celecoxib, zileuton, and bestatin.8-10 In this model reactive oxygen species (ROS) and reactive nitrogen species are also produced.11 These reactive species contribute to inflammation and cause oxidative damage to DNA, proteins, and membrane lipids.12 Both oxidative stress and aberrant arachidonic acid metabolism are believed to contribute to carcinogenesis in the esophagus.13
In theory, oxidative stress should be inhibited by antioxidants. Indeed, using the EDA model, we have previously demonstrated a mild inhibitory effect of α-tocopherol, but the results are confounded by the presence of selenium in some treatment groups.14 N-acetylcysteine (NAC) is an antioxidant and a precursor for the formation of glutathione 15. Glutathione has broad antioxidative activities and works by eliminating hydroperoxide through glutathione peroxidase-catalyzed reactions and by conjugating with electophilic reactive metabolites.16 In this study we investigated the possible protective effect of α-tocopherol, present at 5 and 10 times the level of the standard AIN93M diet, as well as the possible synergistic effect of its combination with NAC in the EGDA model.
In gastriesophageal reflux, the acidity of the gastric reflux is another irritating factor which contributes to the inflammation. Since PPI (proton pump inhibitor) treatment can efficiently control the GERD symptoms, it is expected that long term acid suppression may also prevent EAC development. But the role of the PPI treatment in esophageal adencarcinogenesis is still controversial.17 Some suggest that PPI has chemopreventive effect by inflammation relief and decreased epithelial proliferation;18, 19 while others suggest PPI may promote EAC by producing hypergastrinemia and allowing bile acid-induced mutagenesis in a neutral environment.20, 21 In the present study we addressed this issue using our rat surgical model.
Methods and Materials
Animals and treatment
Six-week-old male Sprague–Dawley rats from Taconic Farms (Germantown, NY) were housed three per cage, separated into eight groups, given water ad libitum, and maintained on a 12 h light/dark cycle, and allowed to acclimate for 2 weeks on respective diets prior to surgery. Food was withdrawn from one day before to one day after surgery. EGDA was performed according to the procedure described previously,6 which was approved by the Institutional Animal Care and Use Committee at Rutgers University (protocol no. 94-017). Basically, the procedure made two 1.5 cm incisions on the gastroesophageal junction and the duodenum on the anti-mesenteric border, and then these were anastomosed together with accurate mucosa to mucosa opposition. The EGDA animals were given iron dextran i.p. at 50 mg Fe/kg once every month to increase oxidative status, starting 4 weeks after surgery and continuing for the duration of the experiment.6 The animals were weighed weekly.
All the diets were made by Research Diets, Inc. (New Brunswick, NJ). The AIN93M diet containing 77.8 ppm α-tocopherol (in the form of α-tocopherol acetate) was used as the basal diet. The supplemented diets contained 5 and 10-fold (389 ppm and 778 ppm) more α-tocopherol than that in the basal diet. The other diets were also AIN93M based and contained 500 ppm NAC, 1,000 ppm NAC, 389 ppm α-tocopherol plus 500ppm NAC, or 1,400 ppm omeprazole. The diets were stored at 4°C before use.
Tissue preparation
At the termination of the experiment, all the rats were anesthetized with CO2, and blood was collected by retro-orbital sinus bleeding. The rats were then killed by CO2 asphyxiation. The esophagus was removed, opened longitudinally and examined for gross abnormalities. The success of the EGDA procedure was evaluated by putting a probe through the gastroesophageal junction to the duodenum. If the probe could not pass through, the procedure was considered unsuccessful and the rat was considered as invalid. When a visible tumor was observed, the length, width and height were measured. The average of these values was used as the tumor diameter. Tumor volume was calculated by the formula: volume = 4/3πr3. The esophagus was cut longitudinally and fixed in 10% buffered formalin for 24 h and then transferred to 80% ethanol. The suture line was used as a reference to distinguish between the esophagus and duodenum. The formalin-fixed esophagus was swiss-rolled, processed, and embedded in paraffin. Five-micron sections were mounted onto glass slides, stained with hematoxylin and eosin, and used for pathological analyses.
Histopathology
Histopathological analysis was carried out on the first and 30th H&E-stained slides. EAC was diagnosed when neoplastic columnar epithelial cells invaded through the basement membrane. Neoplastic columnar cells were characterized by the partial loss of cell polarity and maturation, nuclear atypia, and an increase in mitotic figures.22
pH measurement
The gastric and duodenal contents of normal control rats and the gastric content of EGDA rats were collected in centrifuge tubes (Group A, n=8; Group B, n=24; and Group H, n=30). Samples were centrifuged at 10,000 rpm for 10 minutes. The supernatants were collected and the pH values were measured by a pH meter.
Analysis of fat-soluble vitamins
Serum samples (n=9 in group A and n=10 in other groups) were used for the measurement of retinol, α-tocopherol and γ-tocopherol by HPLC according to our previous method.14 In brief, fat-soluble vitamins were extracted from 150 μl of serum with ethanol and hexane. The hexane phase was dried, dissolved in a mixture of ethanol and acetonitrile (1:1 ratio), and analyzed by HPLC equipped with Supelco LC18 column (4.6×15 mm, 100 A; Bellefonte, PA). The nutrients were eluted isocratically using a mixture of ethanol:acetonitrile (1:1 ratio) and detected with a Waters 490 multi-wavelength detector (Waters-Millipore, Milford, MA) at wavelength settings of 300, 325, and 450 nm (for tocopherols, retinoids, and carotenoids, respectively).
4-hydroxynonenal (4-HNE) immunohistochemistry
The tissue sections were dewaxed in xylene, and rehydrated in a gradient of ethanol to distilled water. After quenching endogenous peroxidase activity with 3% hydrogen peroxide, tissue sections were incubated in normal sera (10% normal horse serum) to minimize non-specific binding. Monoclonal 4-HNE antibody (Cosmo Bio Co., LTD, Cat# MHN-020P, 1:50) was applied at 4°C overnight. Tissue sections were then incubated with secondary biotin conjugated antibody incubation at room temperature for 30 minutes. An avidin–biotin peroxidase complex (Vector Laboratories, Cat# PK-7200) was then applied, and the staining was visualized with diaminobenzidine (Vector Laboratories, Cat# SK-4100). The sections were counterstained with Mayer's hematoxylin. Positively stained cells were counted in 3 high magnification fields (hpf, ×400) around the metaplastic lesion and the average cell numbers were calculated.
Statistical analysis
The effects on tumor incidence were analyzed by the Fisher's exact test. The dose-response effect was analyzed by extended Mantel-Haenszel χ2 test. The tumor volume data were analyzed by the Mann–Whitney test. Fat-soluble vitamin data and 4-HNE results were analyzed by ANOVA and Tukey's post-hoc test. Other data were analyzed by the Student's t-test using the computer software Statview 4.2. All continuous numeric variables were expressed as Mean ±SD.
Results
General observations
The animals tolerated the surgical procedure, with an overall survival rate of 85%. We started the experiment with 251 EGDA rats and 9 non-operated control rats. At the end of the experiment we had 211 valid surgical rats and 9 non-surgical control rats for analysis. All animals were sacrificed at 40 weeks after the surgery. There were 26 invalid rats due to unsuccessful surgery in which the anastomosis opening was blocked after healing. And another 14 rats died during the experiment. The average body weight of EGDA rats was 8% lower than that of the non-operated control rats, but was not statistically significant. Treatment with α-tocopherol, NAC, and omeprazole did not significantly affect the body weight of the rats.
Fat soluble vitamin levels
EGDA rats had lower serum α-tocopherol, γ-tocopherol, and retinol levels than the non-operated control (Table 1), but the differences were not statistically significant. Supplementation with 389 ppm or 778 ppm α-tocopherol increased the serum α-tocopherol concentration by 57% (from 30.7 μmol/L to 48.2 μmol/L) or 79.8% (to 55.2 μmol/L, p<0.05), respectively. Meanwhile, γ-tocopherol level was decreased by more than 80% (from 0.76 μmol/L to 0.12-0.13 μmol/L, p<0.05). The serum concentration of retinol was not affected by α-tocopherol supplementation. NAC treatment seemed to decrease γ-tocopherol levels, but the difference was not statistically significant. When NAC was administered together with α-tocopherol, the serum concentration of α-tocopherol appeared higher than the group treated with NAC alone, but the difference was not statistically significant. Retinol remained almost the same regardless the treatment. Omeprazole did not significantly affect the serum levels of the three nutrients.
Table 1.
Serum concentrations of α-tocopherol, γ-tocopherol and retinol at 40 weeks after EGDA
| Group | Diet | α-tocopherol (μmol/L) |
γ-tocopherol (μmol/L) |
Retinol (μmol/L) |
|---|---|---|---|---|
| A | AIN93M (77.8 ppm α-tocopherol)(non-operated control) | 41.1±8.1 | 1.21±0.7 | 1.97±0.63 |
| B | AIN93M (77.8 ppm α-tocopherol) | 30.7±10.9 | 0.76±0.81 | 1.65±0.53 |
| C | AIN93M-5×T (389 ppm α-tocopherol) | 48.2±23.2 | 0.13±0.23* | 1.69±0.69 |
| D | AIN93M-5×T (778 ppm α-tocopherol) | 55.2±17.7* | 0.12±0.16* | 1.61±0.47 |
| E | AIN93M + 500 ppm NAC | 27.0±8.2 | 0.34±0.47 | 1.76±0.28 |
| F | AIN93M + 1,000 ppm NAC | 30.6±6.7 | 0.58±0.31 | 1.61±0.37 |
| G | AIN93M-5×T(389 ppm α-tocopherol) + 500 ppm NAC | 49.4±28.0 | 0.18±0.24* | 1.49±0.39 |
| H | AIN93M+1,400 ppm omeprazole | 25.2±4.5 | 0.24±0.25 | 1.76±0.53 |
Group A, n=9; other groups, n=10
Compared with group B,
P<0.05
Effects on tumorigenesis
In the EGDA model, the tumors developed were well-differentiated mucinous adenocarcinomas at the squamocolumnar junction (Figure 1). Most of the rats had only one esophageal tumor per rat, but 13 rats had two tumors each. We did not observe any cases with more than 2 tumors. The neoplastic tissue invaded the muscle layer and formed a massive mucinous tumor at the distal part of esophagus. The mucin secreted by the tumor cells formed mucin pools of various sizes. There was more stromal tissue than neoplastic tissue in the tumor. Neoplastic cells formed glandular structure in different sizes surrounded by stromal tissue. At the edge of the tumor we observed intestinal metaplasia in squamous epithelium (Figure 1, D). The squamous epithelium was transformed into columnar epithelium which contained mucin secreting goblet cells.
Figure 1.
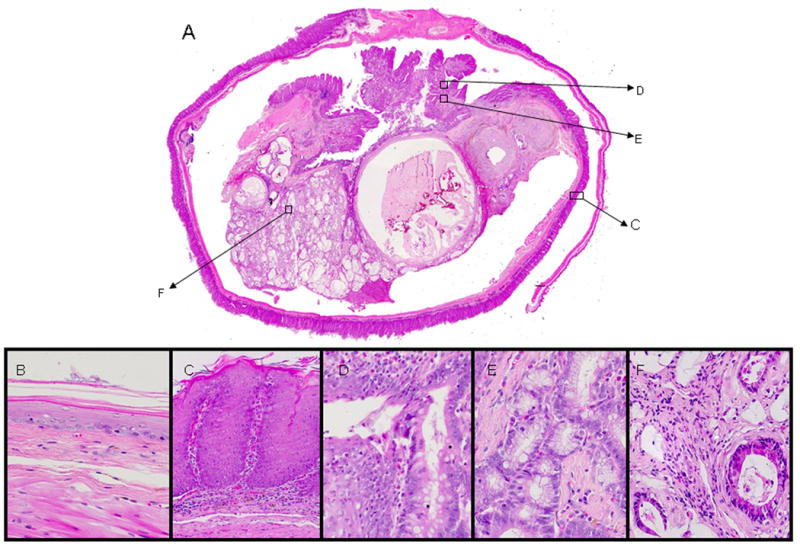
Histopathology of EAC in EGDA rats (HE staining). A. an invasive tumor in distal part of esophagus (25×) showing different lesions which are shown in larger magnifications in C-F; B. normal esophageal epithelium (400×); C. hyperplastic esophageal epithelium (200×); D. metaplastic lesion of esophageal epithelium (400×); E. dysplastic lesion of esophageal epithelium (400×); F. neoplastic gland (400×).
The standard EGDA procedure induced EAC in 84% of the rats. Supplementation of α-tocopherol to 5 and 10 times of the AIN93M diet level (389 and 778 ppm, respectively) decreased the EAC incidence rate in a dose-dependant manner (P=0.03 for trend) (Table 2, Figure 1). Supplementation with NAC, at 1,000 ppm, did not significantly decrease tumor incidence. However, the combination of low doses of NAC (500 ppm) and α-tocopherol (389 ppm) produced a significant decrease in tumor incidence compared to the surgical control group. Omeprazole did not significantly decrease tumor incidence. We also measured the volume of visible tumor in all the groups (Figure 2). High-dose α-tocopherol (Group D), the combination (Group G) and omeprazole (Group H) appeared to decrease the number of tumors over 1,000 mm3, yet without statistical significance. Tumors larger than 2,500mm3 were not observed in these groups.
Table 2.
Tumor incidence of EGDA rats at 40 weeks after the surgery.
| Group | Diet | Treatment | Incidence Rate |
|---|---|---|---|
| A | AIN93M (77.8 ppm α-tocopherol) | Non-operated control | (0/9) |
| B | AIN93M (77.8 ppm α-tocopherol) | EGDA | 84% (26/31) |
| C | AIN93M-5×T (389 ppm α-tocopherol) | EGDA | 61% (20/33) |
| D | AIN93M-10×T (778 ppm α-tocopherol) | EGDA | 59% (16/27)* |
| E | AIN93M + 500 ppm NAC | EGDA | 78% (25/32) |
| F | AIN93M + 1,000 ppm NAC | EGDA | 68% (19/28) |
| G | AIN93M-5×T(389 ppm α-tocopherol) + 500 ppm NAC | EGDA | 55% (15/27)* |
| H | AIN93M + 1,400 ppm omeprazole | EGDA | 64% (21/33) |
Compared with group B,
P<0.05, Fisher's exact test
Group B, C and D showed dose-response effect (Extended Mantel-Haenszel chi square test, P=0.03).
Figure 2.
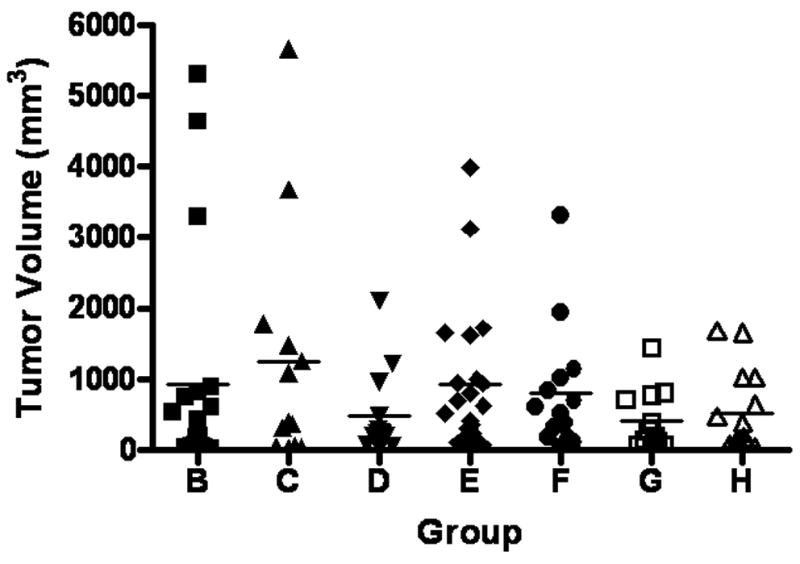
Tumor volume in EGDA rats. High dose α–tocopherol (group D), the combination (group G) and omeprazole (group H) appear to decrease the number of large tumors. Tumors larger than 2,500 mm3 were not observed in these groups. The average volume of visible tumors did not show any difference of statistical significance.
In order to evaluate the physiological effects of omeprazole in the surgical rats, we measured the pH value of the gastric and duodenal juice of the non-operated control (Group A), as well as the mixed duodenal and gastric juice in the surgical control (Group B) and omeprazole treated group (Group H). We found that, as expected, the surgery-induced refluxate was more acidic than the duodenal juice and more alkaline than the gastric contents of the non-operated control. It suggested that surgery successfully produced a mixed reflux of gastric and duodenal contents. With omeprazole treatment, the pH value of the refluxate was higher than that in the surgical control (Figure 3), suggesting that omeprazole suppressed acid secretion in EGDA rats.
Figure 3.
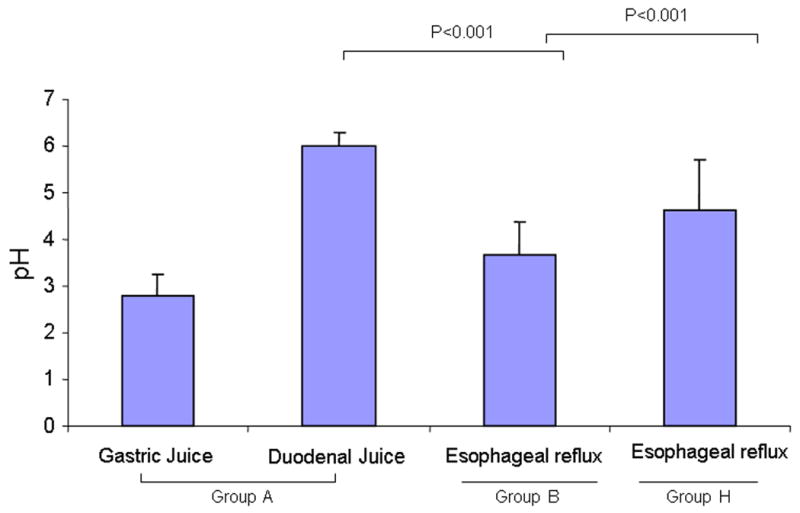
pH values of gastric and duodenal contents of EGDA rats. The gastric and duodenal contents of normal control rats (Group A), the refluxate of EGDA rats (Group B) and omeprazole-treated EGDA rats (Group H) were collected and centrifuged. The pH of the supernatant was measured by a pH meter.
4-HNE immunohistochemistry
In normal control esophageal tissue (Figure 4), we observed only non-specific background staining. We observed strong positive staining of 4-HNE adducts in the infiltrating macrophages and other inflammatory cells in surgical control samples (Figure 4), but not in lymphocytes and the nearby neoplastic or non-neoplastic epithelial cells. The strongest staining was observed at the metaplastic area near anastomosis, where many infiltrating inflammatory cells accumulated. Areas more distal to the anastomosis had fewer stained cells. In hyperplastic squamous tissue we only observed sporadical infiltration of positively stained cells. In tumor stromal tissue we did not observe many positively stained cells either. All the groups treated with antioxidants showed the same staining pattern as the surgical control group.
Figure 4.
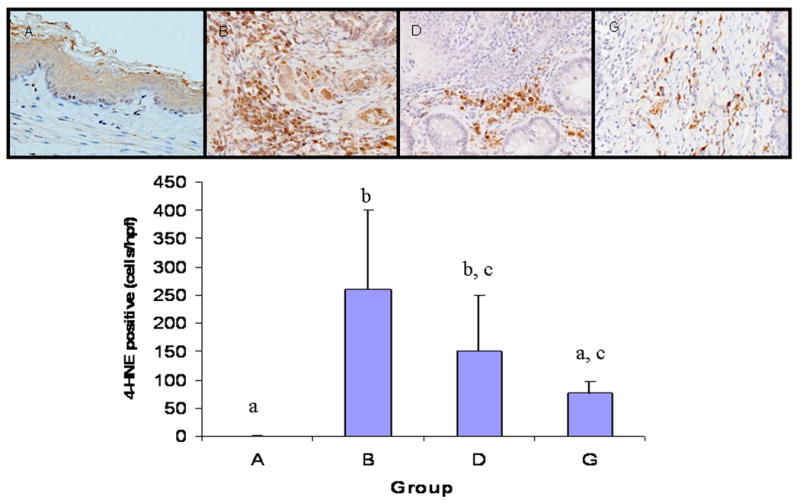
4-HNE immunohistochemistry. Monoclonal 4-HNE antibody was used to detect 4-HNE-His/Lys/Cys adducts. Positively stained cells were counted in 3 high magnification fields (×400) around the metaplastic lesion and the average numbers of positive cells were calculated. A. normal control group (n=5); B. surgical control group (n=9); D. α-tocopherol 778 ppm treatment group (n=9); and G. α-tocopherol 339 ppm plus NAC 500 ppm treatment group (n=9). α-Tocopherol alone or in combination with NAC significantly reduced the number of positively stained infiltrating inflammatory cells.
α-Tocopherol (778 ppm) treatment appear to decrease the positively stained cells compared with the surgical control (Figure 4), but the effect was not statistically significant. The combination of α-tocopherol (389 ppm) and NAC (500 ppm) significantly reduced the number of positively stained cells relative to the surgical control (Figure 4).
Discussion
The causative role of oxidative stress in esophageal adenocarcinogenesis has been observed in both human and animal studies.13 In patient samples, it has been reported that glutathione content is progressively decreased in the esophagitis-metaplasia-dysplasia-adenocarcinoma sequence, while myeloperoxidase activity is higher than in controls, plateauing at the stage of BE. Glutathione content is negatively correlated with DNA adducts.23 An oxidative DNA damage marker, 8-hydroxydeoxyguanosine, is significantly increased in the distal esophagus with Barrett's epithelium and high-grade dysplasia, as well as in EAC.24 Expression of manganese superoxide dismutase is significantly reduced in esophageal tissues of BE, low-grade dysplasia, high-grade dysplasia, and EAC when compared with normal esophagus.25 These findings indicate that oxidative stress is an important event in esophageal adenocarcinogenesis.
Antioxidants have been studied as potential cancer chemopreventive agents. One population-based case-control study in Sweden suggest that subjects with a high intake of vitamin C, beta-carotene, and α-tocopherol have 40-50% reduced risk of EAC.26 We chose α-tocopherol and NAC as our EAC inhibitory agents in the present study based on the different antioxidative mechanisms these two presented. α-Tocopherol functions as lipid peroxidation inhibitor in cell membrane by its chain-breaking and free radical scavenger actions. In contrast, NAC is a small water soluble molecule directly providing SH-groups for adduction or oxidation, and is a precursor of glutathione.15 With possible future applications in mind, we chose these agents because of their low toxicity and low cost. α-Tocopherol alone or in combination with NAC showed significant inhibitory effect on EAC in our EGDA model. The highest doses in diet are equivalent to the commonly used supplementation dose of vitamin E and NAC on market (400 IU and 500 mg, respectively). The calculation is based on allometric scaling.27 For example, for a rat that consumes 20 g of diet daily, the diet contains 80 kcal and 20 mg NAC (for a diet containing 1,000 ppm NAC). The calorie-based dosage equals to 20 mg/80 kcal or 0.25 mg/ kcal. For a person with a caloric requirement of 2,000 kcal/day, this is equivalent to 0.25 × 2,000 = 500 mg/day. The daily dose of NAC ranges from 250 mg to 1,500 mg clinically for patients with chronic pulmonary diseases. Due to the extensive first-pass metabolism, oral administration of NAC results in low plasma and tissue levels, but plasma levels are dose dependent.15, 28 Considering the dose used on clinical trial, the doses of NAC used in this study are relatively low.29
α-Tocopherol supplementation significantly increased the serum level of α-tocopherol, but reduced the serum level of γ-tocopherol. This phenomenon is consistent with previous reports.30 The tumor inhibitory effect of α-tocopherol we observed in this study was dose-dependent with significant inhibition (from 84% to 59%) at the highest dose (778 ppm). NAC alone did not significantly reduce tumor incidence. The highest dose of NAC (1,000 ppm) achieved less inhibition than low dose α-tocopherol. In contrast to NAC treatment alone, the combination of 500 ppm NAC and 389 ppm α-tocopherol inhibited tumor incidence from 84% to 55%. It is possible that α-tocopherol played a major role in the combination treatment. As with many nutrients, the inhibitory effect of α-tocopherol shown in this study was not dramatic (less than 50% inhibition). However, the results suggest that the risk of EAC may decrease by increasing dietary α-tocopherol intake.
Omeprazole at 1,400 ppm reduced EAC incidence from 84% to 64% in the current study, which suggested a weak tumor inhibitory effect. We did not observe any unusual weight loss or high death rate in this group of animals. The pH data showed that omeprazole effectively inhibited acid secretion at the given dose. The data we showed here support our hypothesis that omeprazole does not promote EAC and may have weak tumor inhibitory effect. There have been concerns on the side effects of long term PPI treatment related side effect. Hypergastrinemia may induce epithelial proliferation, and bile acids in a neutral refluxate may induce DNA mutations in esophageal epithelium.20, 21 PPI treatment may also induce squamous re-epithelialization which covers more advanced malignancies in Barrett's glands located in submucosa.31, 32 Our data showed that PPI itself was not a strong chemopreventive agent. In order to achieve more pronounced chemopreventive effect, PPI may be applied in combination with other agents. The ongoing ASPECT Trial (Aspirin Esomeprazole Chemoprevention Trial) using PPI in combination with aspirin may answer this question.33
4-HNE is an extensively studied lipid peroxidation product, which is diffusible and can react with DNA bases and proteins.34 It is also an inducer of cyclooxygenase-2 and a mediator of oxidative stress.35, 36 It has been reported that enhanced lipid peroxidation and 4-HNE production may play a role in the recruitment of inflammatory cells.37 4-HNE has also been reported to form DNA adducts at codon 249 of the p53 gene and to inhibit nucleotide excision repair through interaction with cellular repair proteins.38, 39 In the present study, we found strong positive staining in the infiltrating inflammatory cells. We only found background staining in the nearby epithelial cells. This phenomenon may be explained by: 1) lipid peroxidation level is higher in the infiltrating inflammatory cells than the epithelial cells; 2) the method of immunohistochemistry we used is not sensitive enough to detect the 4-HNE adduct in the epithelial cells; 3) high level of 4-HNE in the infiltrating inflammatory cells may act on the nearby epithelial cells by diffusion.
In summary, our results lend support to the oxidative stress hypothesis of esophageal adenocarcinogenesis. α-Tocopherol alone or in combination with NAC significantly reduced tumor yield. The cancer preventive activities of tocopherols and NAC warrant further investigations.
Acknowledgments
This study was supported by NIH grant CA75683 and N01-CN-43309 as well as the shared facilities supported by NIEHS Center Grant ES05022 and NCI CCSG P30 CA72720. We are grateful to Dr. Joshua Lambert for his critical reading of the manuscript.
The abbreviations used
- BE
Barrett's esophagus
- EAC
esophageal adenocarcinoma
- EGDA
esophagogastroduodenal anastomosis
- GERD
gastroesophageal reflux disease
- 4-HNE
4-hydroxynonenal
- NAC
N-acetylcysteine
- PPI
proton pump inhibitor
- ROS
reactive oxygen species
Footnotes
- It is the first article to show a dose-dependent inhibitory effect of α-tocopherol and the combination of α-tocopherol and NAC on EAC formation;
- This article also demonstrate that omeprazole, a proton pump inhibitor, did not promote EAC formation, but it may have weak inhibitory activity.
References
- 1.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17(1):2–9. doi: 10.1016/j.semradonc.2006.09.003. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17185192. [DOI] [PubMed]
- 2.Pera M, Manterola C, Vidal O, Grande L. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92(3):151–9. doi: 10.1002/jso.20357. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16299786. [DOI] [PubMed]
- 3.Casson AG, van Lanschot JJ. Improving outcomes after esophagectomy: the impact of operative volume. J Surg Oncol. 2005;92(3):262–6. doi: 10.1002/jso.20368. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16299792. [DOI] [PubMed]
- 4.Solaymani-Dodaran M, Logan RF, West J, Card T, Coupland C. Risk of oesophageal cancer in Barrett's oesophagus and gastro-oesophageal reflux. Gut. 2004;53(8):1070–4. doi: 10.1136/gut.2003.028076. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15247170. [DOI] [PMC free article] [PubMed]
- 5.Falk GW. Barrett's esophagus. Gastroenterology. 2002;122(6):1569–91. doi: 10.1053/gast.2002.33427. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12016424. [DOI] [PubMed]
- 6.Chen X, Yang G, Ding WY, Bondoc F, Curtis SK, Yang CS. An esophagogastroduodenal anastomosis model for esophageal adenocarcinogenesis in rats and enhancement by iron overload. Carcinogenesis. 1999;20(9):1801–8. doi: 10.1093/carcin/20.9.1801. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10469627. [DOI] [PubMed]
- 7.Goldstein SR, Yang GY, Curtis SK, Reuhl KR, Liu BC, Mirvish SS, Newmark HL, Yang CS. Development of esophageal metaplasia and adenocarcinoma in a rat surgical model without the use of a carcinogen. Carcinogenesis. 1997;18(11):2265–70. doi: 10.1093/carcin/18.11.2265. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9395230. [DOI] [PubMed]
- 8.Chen X, Wang S, Wu N, Sood S, Wang P, Jin Z, Beer DG, Giordano TJ, Lin Y, Shih WC, Lubet RA, Yang CS. Overexpression of 5-lipoxygenase in rat and human esophageal adenocarcinoma and inhibitory effects of zileuton and celecoxib on carcinogenesis. Clin Cancer Res. 2004;10(19):6703–9. doi: 10.1158/1078-0432.CCR-04-0838. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15475461. [DOI] [PubMed]
- 9.Chen X, Li N, Wang S, Wu N, Hong J, Jiao X, Krasna MJ, Beer DG, Yang CS. Leukotriene A4 hydrolase in rat and human esophageal adenocarcinomas and inhibitory effects of bestatin. J Natl Cancer Inst. 2003;95(14):1053–61. doi: 10.1093/jnci/95.14.1053. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12865451. [DOI] [PubMed]
- 10.Chen X, Li N, Wang S, Hong J, Fang M, Yousselfson J, Yang P, Newman RA, Lubet RA, Yang CS. Aberrant arachidonic acid metabolism in esophageal adenocarcinogenesis, and the effects of sulindac, nordihydroguaiaretic acid, and alpha-difluoromethylornithine on tumorigenesis in a rat surgical model. Carcinogenesis. 2002;23(12):2095–102. doi: 10.1093/carcin/23.12.2095. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12507933. [DOI] [PubMed]
- 11.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391(5):499–510. doi: 10.1007/s00423-006-0073-1. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16909291. [DOI] [PubMed]
- 12.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401(1):1–11. doi: 10.1042/BJ20061131. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17150040. [DOI] [PubMed]
- 13.Chen X, Yang CS. Esophageal adenocarcinoma: a review and perspectives on the mechanism of carcinogenesis and chemoprevention. Carcinogenesis. 2001;22(8):1119–29. doi: 10.1093/carcin/22.8.1119. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11470739. [DOI] [PubMed]
- 14.Chen X, Mikhail SS, Ding YW, Yang G, Bondoc F, Yang CS. Effects of vitamin E and selenium supplementation on esophageal adenocarcinogenesis in a surgical model with rats. Carcinogenesis. 2000;21(8):1531–6. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10910955. [PubMed]
- 15.Aitio ML. N-acetylcysteine -- passe-partout or much ado about nothing? Br J Clin Pharmacol. 2006;61(1):5–15. doi: 10.1111/j.1365-2125.2005.02523.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16390346. [DOI] [PMC free article] [PubMed]
- 16.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489–92. doi: 10.1093/jn/134.3.489. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14988435. [DOI] [PubMed]
- 17.Triadafilopoulos G. Proton pump inhibitors for Barrett's oesophagus. Gut. 2000;46(2):144–6. doi: 10.1136/gut.46.2.144. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10644301. [DOI] [PMC free article] [PubMed]
- 18.Triadafilopoulos G, Kaczynska M, Iwane M. Esophageal mucosal eicosanoids in gastroesophageal reflux disease and Barrett's esophagus. Am J Gastroenterol. 1996;91(1):65–74. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8561146. [PubMed]
- 19.Lao-Sirieix P, Roy A, Worrall C, Vowler SL, Gardiner S, Fitzgerald RC. Effect of acid suppression on molecular predictors for esophageal cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(2):288–93. doi: 10.1158/1055-9965.EPI-05-0528. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16492917. [DOI] [PubMed]
- 20.Feagins LA, Zhang HY, Hormi-Carver K, Quinones MH, Thomas D, Zhang X, Terada LS, Spechler SJ, Ramirez RD, Souza RF. Acid has antiproliferative effects in nonneoplastic Barrett's epithelial cells. Am J Gastroenterol. 2007;102(1):10–20. doi: 10.1111/j.1572-0241.2006.01005.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17266684. [DOI] [PubMed]
- 21.Stamp DH. Bile acids aided by acid suppression therapy may be associated with the development of esophageal cancers in westernized societies. Med Hypotheses. 2006;66(1):154–7. doi: 10.1016/j.mehy.2005.04.045. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16125332. [DOI] [PubMed]
- 22.Su Y, Chen X, Klein M, Fang M, Wang S, Yang CS, Goyal RK. Phenotype of columnar-lined esophagus in rats with esophagogastroduodenal anastomosis: similarity to human Barrett's esophagus. Lab Invest. 2004;84(6):753–65. doi: 10.1038/labinvest.3700079. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15094711. [DOI] [PubMed]
- 23.Sihvo EI, Salminen JT, Rantanen TK, Ramo OJ, Ahotupa M, Farkkila M, Auvinen MI, Salo JA. Oxidative stress has a role in malignant transformation in Barrett's oesophagus. Int J Cancer. 2002;102(6):551–5. doi: 10.1002/ijc.10755. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12447994. [DOI] [PubMed]
- 24.Rasanen JV, Sihvo EI, Ahotupa MO, Farkkila MA, Salo JA. The expression of 8-hydroxydeoxyguanosine in oesophageal tissues and tumours. Eur J Surg Oncol. 2007;33(10):1164–8. doi: 10.1016/j.ejso.2007.03.003. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17467227. [DOI] [PubMed]
- 25.Hermann B, Li Y, Ray MB, Wo JM, Martin RC., 2nd Association of manganese superoxide dismutase expression with progression of carcinogenesis in Barrett esophagus. Arch Surg. 2005;140(12):1204–9. doi: 10.1001/archsurg.140.12.1204. discussion 09. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16365243. [DOI] [PubMed]
- 26.Terry P, Lagergren J, Ye W, Nyren O, Wolk A. Antioxidants and cancers of the esophagus and gastric cardia. Int J Cancer. 2000;87(5):750–4. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10925371. [PubMed]
- 27.Schneider K, Oltmanns J, Hassauer M. Allometric principles for interspecies extrapolation in toxicological risk assessment--empirical investigations. Regul Toxicol Pharmacol. 2004;39(3):334–47. doi: 10.1016/j.yrtph.2004.03.001. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15135212. [DOI] [PubMed]
- 28.Allegra L, Dal Sasso M, Bovio C, Massoni C, Fonti E, Braga PC. Human neutrophil oxidative bursts and their in vitro modulation by different N-acetylcysteine concentrations. Arzneimittelforschung. 2002;52(9):669–76. doi: 10.1055/s-0031-1299949. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12404881. [DOI] [PubMed]
- 29.van Zandwijk N, Dalesio O, Pastorino U, de Vries N, van Tinteren H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the EUropean Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J Natl Cancer Inst. 2000;92(12):977–86. doi: 10.1093/jnci/92.12.977. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10861309. [DOI] [PubMed]
- 30.Wolf G. How an increased intake of alpha-tocopherol can suppress the bioavailability of gamma-tocopherol. Nutr Rev. 2006;64(6):295–9. doi: 10.1111/j.1753-4887.2006.tb00213.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16808116. [DOI] [PubMed]
- 31.Tanaka K, Toyoda H, Kadowaki S, Hamada Y, Kosaka R, Yamanaka M, Imoto I. Use of proton pump inhibitors may cause squamous epithelial masking of intramucosal carcinoma in Barrett's esophagus. Endoscopy. 2007;39 1:E105–6. doi: 10.1055/s-2006-945174. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17440852. [DOI] [PubMed]
- 32.Shepherd NA. Barrett's oesophagus and proton pump inhibitors: a pathological perspective. Gut. 2000;46(2):147–9. doi: 10.1136/gut.46.2.147. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10644302. [DOI] [PMC free article] [PubMed]
- 33.Das D, Ishaq S, Harrison R, Kosuri K, Harper E, Decaestecker J, Sampliner R, Attwood S, Barr H, Watson P, Moayyedi P, Jankowski J. Management of Barrett's esophagus in the UK: overtreated and underbiopsied but improved by the introduction of a national randomized trial. Am J Gastroenterol. 2008;103(5):1079–89. doi: 10.1111/j.1572-0241.2008.01790.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18445097. [DOI] [PubMed]
- 34.Sayre LM, Lin D, Yuan Q, Zhu X, Tang X. Protein adducts generated from products of lipid oxidation: focus on HNE and one. Drug Metab Rev. 2006;38(4):651–75. doi: 10.1080/03602530600959508. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17145694. [DOI] [PubMed]
- 35.Uchida K, Kumagai T. 4-hydroxy-2-nonenal as a COX-2 inducer. Mol Aspects Med. 2003;24(4-5):213–8. doi: 10.1016/s0098-2997(03)00016-5. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12892999. [DOI] [PubMed]
- 36.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42(4):318–43. doi: 10.1016/s0163-7827(03)00014-6. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12689622. [DOI] [PubMed]
- 37.Dianzani MU. Lipid peroxidation and cancer. Crit Rev Oncol Hematol. 1993;15(2):125–47. doi: 10.1016/1040-8428(93)90052-6. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8117415. [DOI] [PubMed]
- 38.Hu W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, Chung FL, Tang MS. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23(11):1781–9. doi: 10.1093/carcin/23.11.1781. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12419825. [DOI] [PubMed]
- 39.Feng Z, Hu W, Tang MS. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: a possible mechanism for lipid peroxidation-induced carcinogenesis. Proc Natl Acad Sci U S A. 2004;101(23):8598–602. doi: 10.1073/pnas.0402794101. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15187227. [DOI] [PMC free article] [PubMed]


