Abstract
We studied the effect of ovariectomy and estrogen replacement on expression of adrenal angiotensin II AT1 and AT2 receptors, aldosterone content, catecholamine synthesis, and the transcription factor Fos-related antigen 2 (Fra-2). Ovariectomy increased AT1 receptor expression in the adrenal zona glomerulosa and medulla, and decreased adrenomedullary catecholamine content and Fra-2 expression when compared to intact female rats. In the zona glomerulosa, estrogen replacement normalized AT1 receptor expression, decreased AT1B receptor mRNA, and increased AT2 receptor expression and mRNA. Estrogen treatment decreased adrenal aldosterone content. In the adrenal medulla, the effects of estrogen replacement were: normalized AT1 receptor expression, increased AT2 receptor expression, AT2 receptor mRNA, and tyrosine hydroxylase mRNA, and normalized Fra-2 expression and catecholamine content. We demonstrate that the constitutive adrenal expression of AT1 receptors, catecholamine synthesis and Fra-2 expression are partially under the control of reproductive hormones. Our results suggest that estrogen treatment decreases aldosterone production through AT1 receptor downregulation and AT2 receptor upregulation. AT2 receptor upregulation and modulation of Fra-2 expression may participate in the estrogen- dependent normalization of adrenomedullary catecholamine synthesis in ovariectomized rats. The AT2 receptor upregulation and the decrease in AT1 receptor function and in the production of the fluid-retentive, pro-inflammatory hormone aldosterone partially explain the protective effects of estrogen therapy.
Keywords: Renin-angiotensin system, Angiotensin II AT1 receptors, Adrenal medulla, Adrenal cortex, Reproductive hormones, Transcription factors, Fra-2, Tyrosine hydroxylase, Aldosterone
Introduction
It is well-established that hyperactivity of circulating and local renin-angiotensin-systems (RAS) is important for the development of cardiovascular and renal disorders and diabetes [1]. Increased RAS function as a result of estrogen deficiency has been considered a major contributor to the increase in cardiovascular and renal disease in postmenopausal women [2]. Estrogen has complex and sometimes contradictory effects on the RAS, but the overall effect appears to be a reduction in RAS activity [3, 4]. This may partially explain the proposed, and controversial, protective effect of estrogen replacement in postmenopausal women [3, 4].
The adrenal gland contributes to the regulation of cardiovascular function [5], with local RAS systems located in the zona glomerulosa and medulla [6, 7]. Circulating and locally formed angiotensin II (Ang II), through AT1 receptor stimulation, is established as a fundamental mediator of synthesis and release of aldosterone in the adrenal zona glomerulosa and of catecholamines in the adrenal medulla [8–11]. In rodents, there are two AT1 receptor subtypes, AT1A and AT1B, which are pharmacologically identical but differently regulated [12, 13]. Both AT1A and AT1B receptors are expressed in the adrenal zona glomerulosa, with a predominance of AT1B receptors [12, 13]. AT1B receptors in the zona glomerulosa contribute to regulate aldosterone synthesis and release [10]. The adrenal medulla expresses only low levels of AT1A receptors and no AT1B receptors [7]. In this tissue, AT1 receptors control Ang II- and stress-induced catecholamine formation and release [14–16].
Excess aldosterone production contributes to blood pressure increase, enhances peripheral and central sympathetic activity [17, 18] and is an important inflammatory factor in the genesis of atherosclerosis [19]. Estrogen regulates adrenal gland function and the expression of adrenal RAS. In the adrenal gland, estrogen reduces AT1 receptor expression, and as a consequence, Ang II-induced aldosterone secretion is decreased [20, 21]. Conversely, most of the evidence indicates that estrogen enhances adrenomedullary catecholamine synthesis [22, 23].
In addition to AT1 receptors, the adrenal zona glomerulosa expresses substantial numbers of Ang II AT2 receptors and in the adrenal medulla, AT2 receptor concentration outnumbers that of AT1 receptors tenfold [7, 24]. The physiological functions of AT2 receptors have not clarified, but their stimulation has been proposed to balance and oppose that of AT1 receptor stimulation [25, 26]. While adrenomedullary AT2 receptors may participate in catecholamine formation and release in normal conditions and during stress [7, 27], their role in aldosterone formation and secretion remains controversial [8, 9].
We have previously reported that estrogen markedly upregulates AT2 receptor expression in kidneys of female rats and mice, a factor likely to contribute to the protective effect of estrogens in kidney disease [28, 29]. We asked whether estrogens influence the expression of AT2 receptors in the adrenal gland, an effect that might contribute to their reported beneficial effects on cardiovascular function. In the present study we administered estrogen to ovariectomized (OVX) rats to determine the effect of estrogens on adrenal gland Ang II receptors, aldosterone and catecholamine synthesis.
Materials and Methods
Animals
These studies used 8-week-old female Wistar Hanover rats (Taconic, Germantown, N.Y., USA). Groups of 6 or 7 control intact rats, and 12–16 OVX rats were kept under controlled conditions with free access to water and food, according to protocols approved by the NIMH Animal Care and Use Committee. Fourteen days after surgery, the OVX rats were randomly divided into two groups. One group was implanted subcutaneously with 17β-estradiol (1.7 mg/pellet – 60 days release; Innovative Research of America, Sarasota, Fla., USA), under pentobarbital anesthesia (30 mg/kg, i.p.). This treatment provided about 28 µg 17β-estradiol per rat per day, resulting in blood levels of 180 ± 25.5 pg/ml, as previously reported [29]. The other group was implanted subcutaneously with cholesterol placebo pellets (Innovative Research of America). Ten days after the pellets were implanted, all animals were killed by decapitation between 10:00 and 11:00 h and the adrenal glands were immediately removed. For measurement of aldosterone, catecholamines and Fos-related antigen 2 (Fra-2), adrenals were quickly dissected into cortex and medulla, and the tissues were frozen at −30°C by immersion in isopentane and stored at −80°C. For quantification of Ang II receptor binding by autoradiography and in situ hybridization determination of mRNA for Ang II receptors and tyrosine hydroxylase (TH) mRNA, whole adrenals were frozen and stored as above.
Quantitative Autoradiography of Angiotensin II Receptor Types
To determine Ang II receptor binding, we cut 16-µm-thick sections from adrenal gland in a cryostat at −20°C, thaw-mounted the sections on poly-l-lysine-coated slides (LabScientific, Inc., Livingston, N.J., USA), dried them overnight in a desiccator at 4°C, and stored them at −80°C until use. To determine binding to selective Ang II receptor types, we incubated adjacent sections as described [30]. We determined the total binding to Ang II receptors by incubating the sections with 0.5 nm of [125 I]Sar1-Ang II (Bachem California, Inc., Torrance, Calif., USA) iodinated by the Peptide Radioiodination Service Center (University of Mississippi School of Pharmacy, University, Miss., USA), to a specific activity of 2,176 Ci/mmol). We determined the non-specific binding as the binding remaining after incubation of consecutive sections as above in the presence of 1 µM unlabelled Ang II (Bachem, Torrance, Calif., USA). Specific binding to Ang II receptors was the difference between total binding and non-specific binding. For selective binding to AT1 receptors, we incubated consecutive sections with 0.5 nm of [125 I]Sar1-Ang II in the presence of the selective AT1 receptor antagonist losartan (10 µm, from DuPont-Merck, Wilmington, Del., USA) or the selective AT2 receptor antagonist PD 123319 (1 µm, from Sigma, St. Louis, Mo., USA), chosen to give maximum specific displacement [30]. The number of AT1 and AT2 receptors was the binding displaced by the respective receptor antagonists. To quantify the autoradiographic images obtained after exposure to BioMax MR films (Eastman Kodak, Rochester, N.Y., USA), and development with GBX developer (Eastman Kodak) for 4 min followed by fixation, we compared the optical densities of the images with those generated by [14C] microscales (American Radiolabeled Chemicals, Inc., St. Louis, Mo., USA) as described [31]. This was followed by computerized microdensitometry using the NIH Image 1.61 program (NIMH, Bethesda, Md., USA). At least two sections at similar planes were studied per animal by investigators unaware of each animal’s treatment.
In situ Hybridization of TH mRNA
For in situ hybridization experiments, 16 µm-thick brain sections consecutive to those used for receptor binding were collected as above and stored at −80°C until assayed. We synthesized an antisense oligonucleotide of 48 bases as a probe for the rat TH cDNA sequence (Lofstrand Labs Ltd, Gaithersburg, Md., USA), localized in nt 1562–1609 [32]. We labeled the oligonucleotide using terminal deoxynucleotidyl transferase (GE Healthcare, Little Chalfont, Bucks., UK) to a specific activity of 3–4 × 108 dpm/µg. Each reaction was performed with 70 pmol of oligonucleotide in the presence of 70 µCi of [α-35S] ATP (GE Healthcare). The labeled oligonucleotide was separated from unincorporated nucleotides using MicroSpin G-25 columns (GE Healthcare). In situ hybridization of rat adrenal sections and post-hybridization washings were performed as described [33] in consecutive brain sections, one incubated with labeled antisense oligonucleotide (157 pmol/ml) and another with labeled oligonucleotide in the presence of a tenfold excess of unlabelled probe. After exposure to BioMax MR films, the films were then developed and quantified by comparison with 14C standards (American Radiolabeled Chemicals, Inc.) [31]. At least two sections at similar planes were studied per animal by investigators unaware of each animal’s treatment.
In situ Hybridization of Ang II Receptor Subtype mRNAs
To obtain rat AT1B and AT2 receptor-specific riboprobes, partial fragments of full-length cDNA were subcloned into the pBluescript KS+ vector (Stratagene, La Jolla, Calif., USA). The rAT1B cDNA was restricted with HindIII and EcoRI [34]. The restriction fragment of 398 bp (from nt 1832–2229) was prepared and ligated into the HindIII-EcoRI site of the vector. The fragment corresponded to the 3′ UTR of the gene. The rAT2 cDNA was restricted with XbaI and BglII [34] and the fragment of 375 bp (from nt 1478–1852) was prepared and ligated into the XbaI-BglII site of the vector. The fragment corresponded to the 3′ UTR of the gene. The subclones rAT1B and rAT2 were confirmed by DNA sequencing.
For in vitro transcription of the 35S-labeled antisense and sense (as a control) riboprobes, the subclones were linearized with HindIII or EcoRI for the rAT1B or with XbaI or EcoRI for the rAT2, and then with T3 or T7 RNA polymerase, respectively, were used in in vitro transcription reactions using an RNA labeling kit (GE Healthcare) as previously described [34]. The labeling of the ribo-probes was monitored with liquid scintillation counting. In a preliminary experiment, adrenal glands were used as positive controls.
To perform in situ hybridization, sections were fixed in 4% paraformaldehyde for 10 min, and acetylated for 10 min in 0.1 m triethanolamine HCl, pH 8.0, containing 0.25% acetic anhydride. Sections were then dehydrated in alcohols, and air-dried. Each section was covered with 150 µl hybridization buffer containing 50% formamide, 0.3 m NaCl, 2 mm EDTA, 20 mm Tris (pH 8.0), 1 × Denhardt’s solution, 10% dextran sulfate, 100 µg/ml salmon sperm DNA, 250 µg/ml yeast tRNA, 150 mm dithiothreitol, 0.1% SDS, and 40,000 cpm/µl sense or antisense probe. Sections were hybridized overnight at 54°C, treated with 40 µg/ml ribonuclease A (Sigma) for 30 min, and washed in sodium chloride/sodium citrate with increasing stringency. After a final wash in 0.1 × standard saline citrate at 65°C for 60 min, sections were dehydrated through alcohols and exposed to Hyperfilm-3H (GE Healthcare) along with 14C-labeled microscales (American Radiolabeled Chemicals, Inc.) for 1–2 days. Films were developed as described above. The intensities of the hybridization signals were quantified as nanocuries per milligram tissue equivalent by measuring optical film densities using the NIH Image 1.61 program after calibration with the 14C-labeled microscales [31]. Non-specific hybridization was analyzed using sense (control) probes.
Determination of Fra-2 by Western Blot Analysis
After dissection of the adrenal cortex and medulla, tissues were homogenized on ice in buffer containing 10 mm Tris, pH 7.4, 1% SDS and a protease inhibitor cocktail (Roche Applied Science, Mannheim, Germany). The homogenate was centrifuged at 18,000 g for 5 min at 4°C. The protein concentration was determined by the Bradford procedure (Bio-Rad, Hercules, Calif., USA) in sample aliquots. Proteins were then fractionated by 10% SDS-polyacrylamide gel electrophoresis and transferred to a PVDF membrane (Millipore Corp., Bedford, Mass., USA). The membranes were blocked with 5% non-fat dried milk (Bio-Rad) in Tris-buffered saline (100 mm Tris-Cl, pH 7.5, and 100 mm NaCl) containing 0.1% Tween 20 (TBS-T) for 1 h. They were then incubated with anti-Fra-2 rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif., USA) at a dilution of 1:200 in TBS-T containing 5% dried milk at 4°C overnight. After washing the membranes with TBS-T, the membranes were incubated with horseradish peroxidase-conjugated donkey anti-rabbit IgG (GE Healthcare) for 1.5 h at a dilution of 1:2,000 in the blocking buffer. The proteins were visualized with the chemiluminescence system (GE Healthcare) and exposure to Kodak X-OMAT film. The amount of each protein was quantified using a Microsoft-based image processing system with Scion Image software (Scion Corp., Frederick, Md., USA) using actin to correct for differences in protein loading.
Determination of Adrenal Catecholamines by HPLC
We measured norepinephrine (NE) and epinephrine (E) content in homogenates of adrenal medulla. The adrenal medullas were homogenized in 0.3 n perchloric acid and centrifuged, and the supernatant was stored at −80°C until measured by high-performance liquid chromatography with electrochemical detection (HPLC) after partial purification by adsorption on alumina [35].
Aldosterone Determinations
Whole adrenal glands were homogenized in 0.3 n perchloric acid and centrifuged 15 min at 4°C at 1,800 g. Clear supernatants from the homogenates were stored at −80°C until assayed. A commercial RIA kit (MP Biomedicals, Solon, Ohio, USA) was used to determine aldosterone concentrations following the manufacturer’s recommended protocols as described earlier [35]. The intra-assay coefficient of variation was 4.5%
Statistics
We used one-way ANOVA followed by post-hoc analysis using the Tukey’s multiple comparisons test to assess the significance of differences among groups. p <0.05 was considered statistically significant. All values were expressed as the mean ± SEM; n = 5–7 in each group. All statistics were performed using GraphPad Prism 3.0 (GraphPad Software, Inc., San Diego, Calif., USA).
Results
Effects of Ovariectomy and Estrogen Treatment on Angiotensin II AT1 and AT2 Receptor Expression and mRNA in the Adrenal Zona Glomerulosa
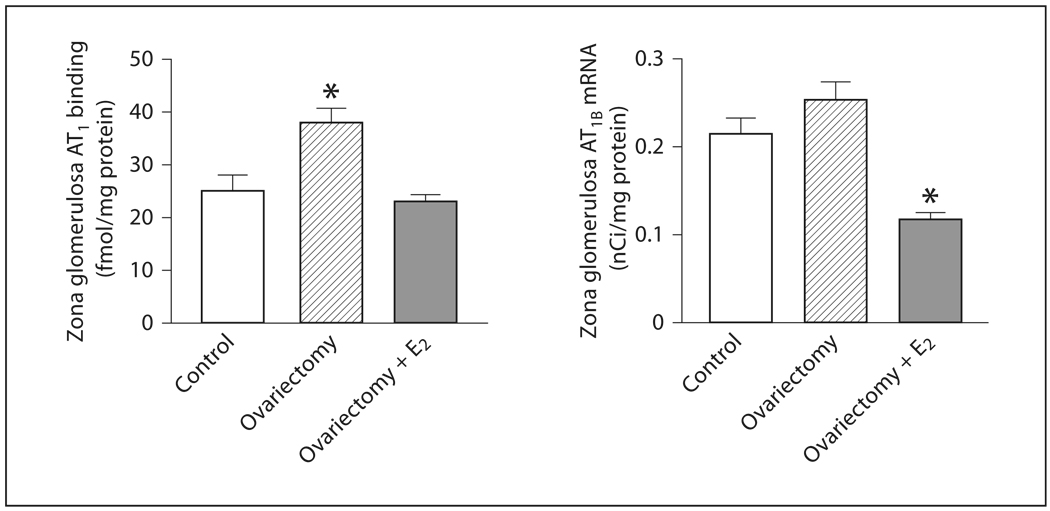
In the zona glomerulosa, AT1 receptor binding increased significantly in OVX rats when compared to non-operated control female rats (fig. 1, fig 2). There was a tendency for the expression of AT1B mRNA to increase in OVX rats when compared to non-operated females, but the change did not reach statistical significance (fig. 1). Estrogen treatment of OVX rats reduced AT1 receptor binding, normalizing AT1 receptor expression to values similar to those found in control rats, and significantly reduced AT1B mRNA expression below the levels found in OVX or control rats (fig. 1, 2).
Fig. 1.
Effects of ovariectomy and estrogen treatment on AT1 binding and AT1B mRNA in the rat zona glomerulosa. AT1 binding was measured by quantitative autoradiography. Consecutive sections were incubated with [125I]Sar1-Ang II in the presence or absence of losartan to determine binding to AT1 receptors, and AT1B mRNA was determined in additional consecutive sections by in situ hybridization, as described in Materials and Methods. E2 indicates treatment with 17β-estradiol. Data are means ± SEM, n = 5–7 for each group. * p <0.05 compared to all other groups.
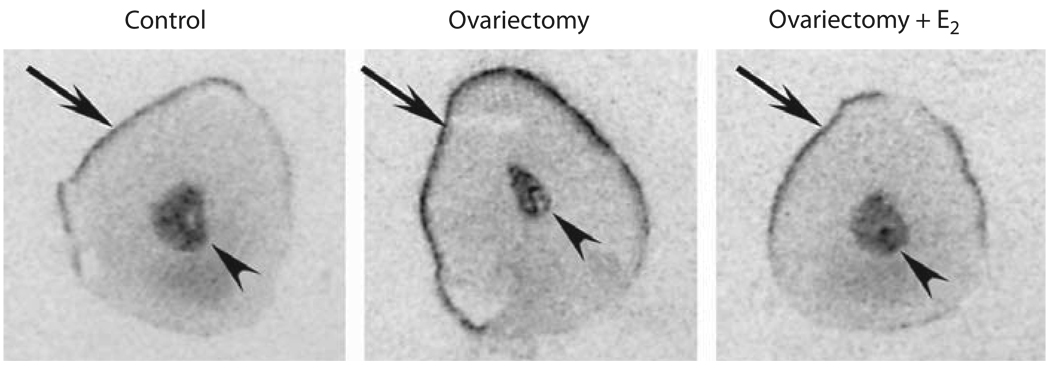
Fig. 2.
Effects of ovariectomy and estrogen treatment on AT1 binding in the rat adrenal gland. Figures represent typical autoradiograms to show specific binding to AT1 receptors. E2 indicates treatment with 17β-estradiol. Sections were incubated with [125I]-Sar1-Ang II in the presence of PD 123319 to determine binding to AT2 receptors, as described in Materials and Methods. Arrows point to the adrenal zona glomerulosa, arrowheads to the adrenal medulla.
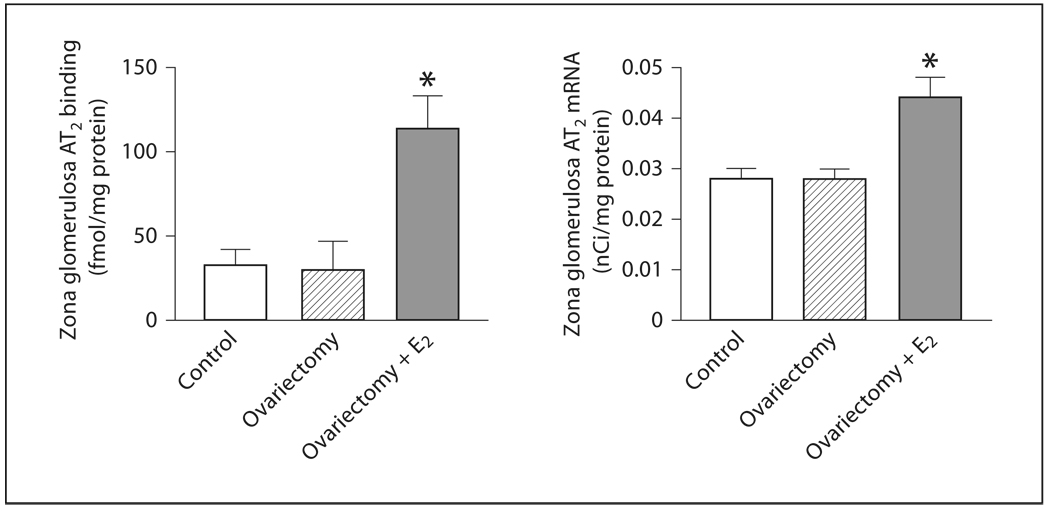
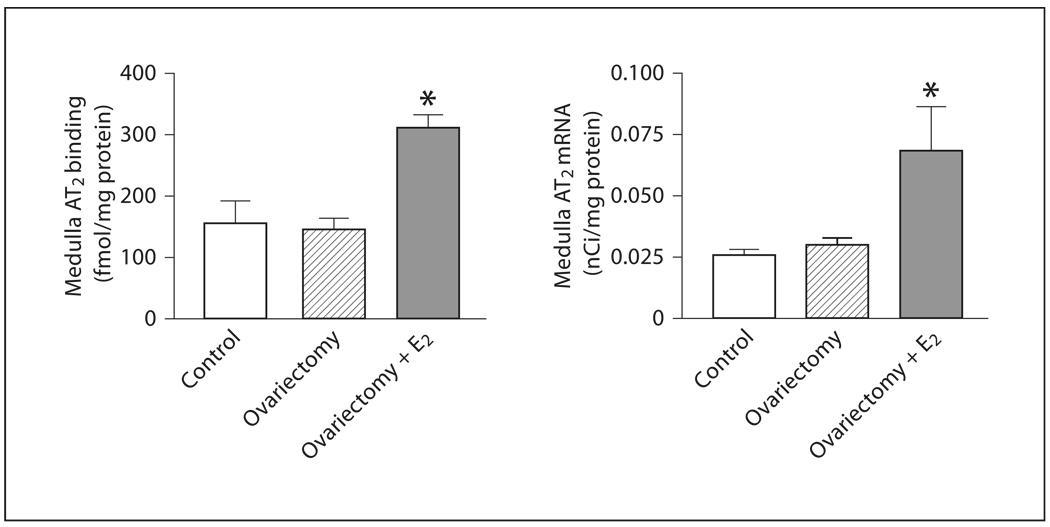
There was no difference in AT2 receptor expression or mRNA in OVX rats when compared to controls (fig. 3, 4). However, estrogen treatment produced a significant enhancement of both AT2 receptor protein and mRNA expression (fig. 3, 4).
Fig. 3.
Effects of ovariectomy and estrogen treatment on AT2 binding and mRNA in the rat zona glomerulosa. AT2 binding was measured by quantitative autoradiography in consecutive sections, as described in Materials and Methods. AT2 mRNA was determined by in situ hybridization as described in Materials and Methods. E2 indicates treatment with 17β-estradiol. Data are means ± SEM, n = 5–7 for each group. * p <0.05 compared to all other groups.
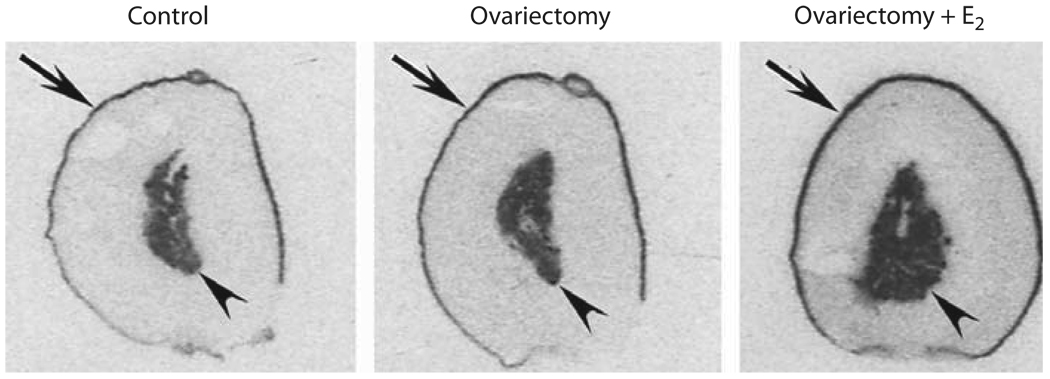
Fig. 4.
Effects of ovariectomy and estrogen treatment on AT2 binding in the rat adrenal gland. Figures represent typical auto-radiograms to show specific binding to AT2 receptors, determined by quantitative autoradiography as described in Materials and Methods. E2 indicates treatment with 17β-estradiol. Arrows point to the adrenal zona glomerulosa, arrowheads to the adrenal medulla.
Effects of Ovariectomy and Estrogen Treatment on Adrenal Aldosterone
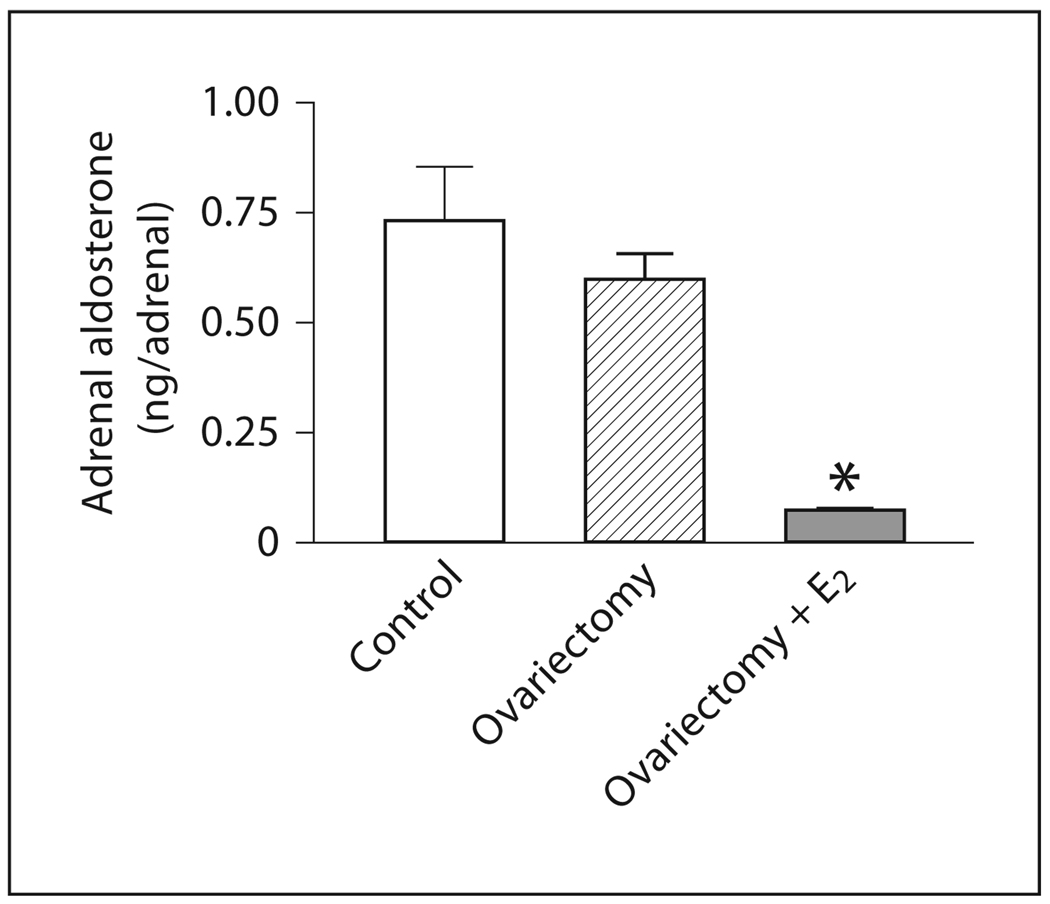
No significant differences in total adrenal aldosterone content were detected in OVX rats when compared to control rats (fig. 5). Estrogen treatment of OVX rats significantly decreased the adrenal aldosterone content (fig. 5).
Fig. 5.
Effects of ovariectomy and estrogen treatment on aldosterone content in adrenal zona glomerulosa. Aldosterone was measured by RIA. E2 indicates treatment with 17β-estradiol. Data are means ± SEM, n = 5–7 for each group. * p <0.05 compared to all other groups.
Effects of Ovariectomy and Estrogen Treatment on Angiotensin II AT1 and AT2 Receptor Expression and mRNA in the Adrenal Medulla
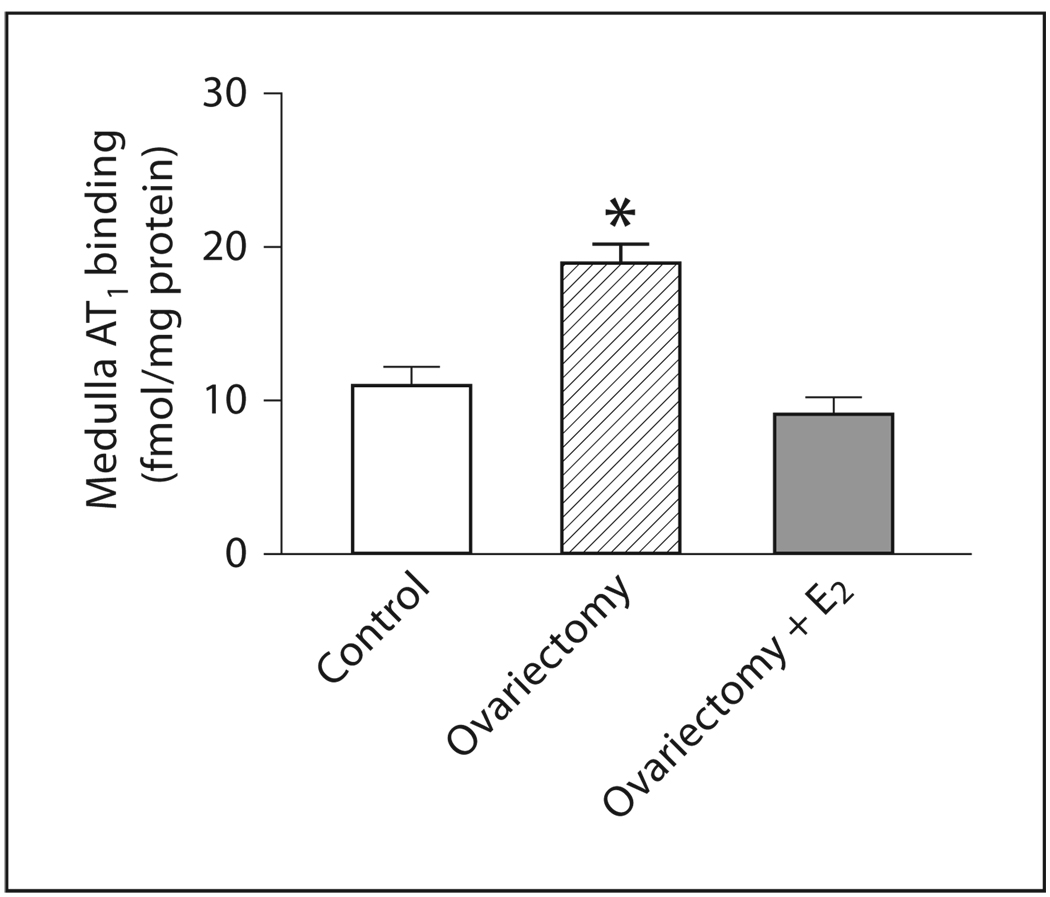
In the adrenal medulla, OVX produced a significant increase in AT1 receptor binding but no changes in AT2 receptor expression or mRNA when compared to controls (fig. 2, 4, 6, 7). Estrogen treatment of OVX rats decreased AT1 binding to levels similar to those of controls, and significantly increased expression of AT2 receptor binding and mRNA (fig. 2, 4, 6, 7).
Fig. 6.
Effects of ovariectomy and estrogen treatment on AT1 binding in the rat medulla. AT1 binding was measured by quantitative autoradiography in consecutive sections, as described in Materials and Methods. E2 indicates treatment with 17β-estradiol. Data are means ± SEM, n = 5–7 for each group. * p <0.05 compared to all other groups.
Fig. 7.
Effects of ovariectomy and estrogen treatment on AT2 binding and mRNA in the rat adrenal medulla. AT2 binding was measured by quantitative autoradiography as described in Materials and Methods. AT2 mRNA was determined by in situ hybridization as described in Materials and Methods. E2 indicates treatment with 17β-estradiol. Data are means ± SEM, n = 5–7 for each group. * p <0.05 compared to all other groups.
Effects of Ovariectomy and Estrogen Treatment on Adrenal Catecholamines, TH mRNA and Fra-2 Expression
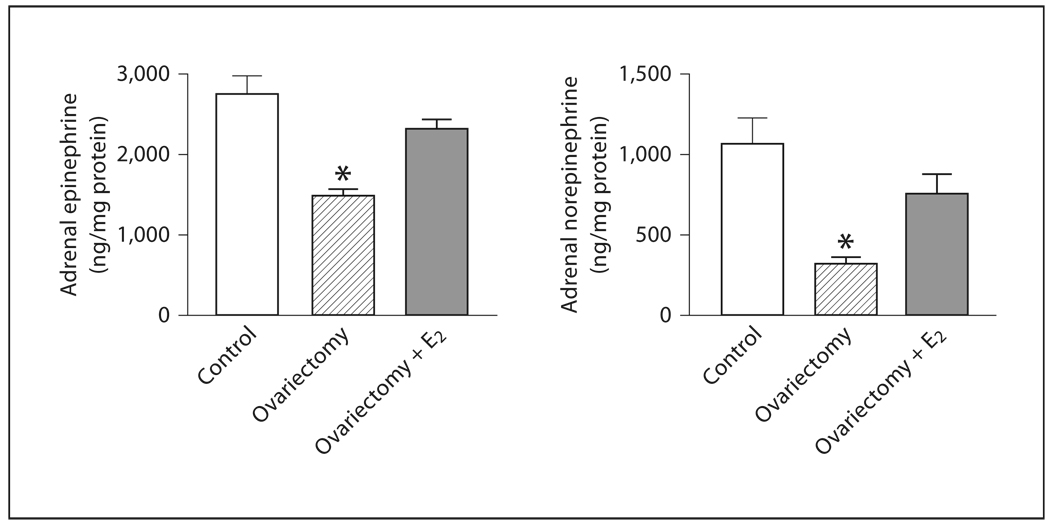
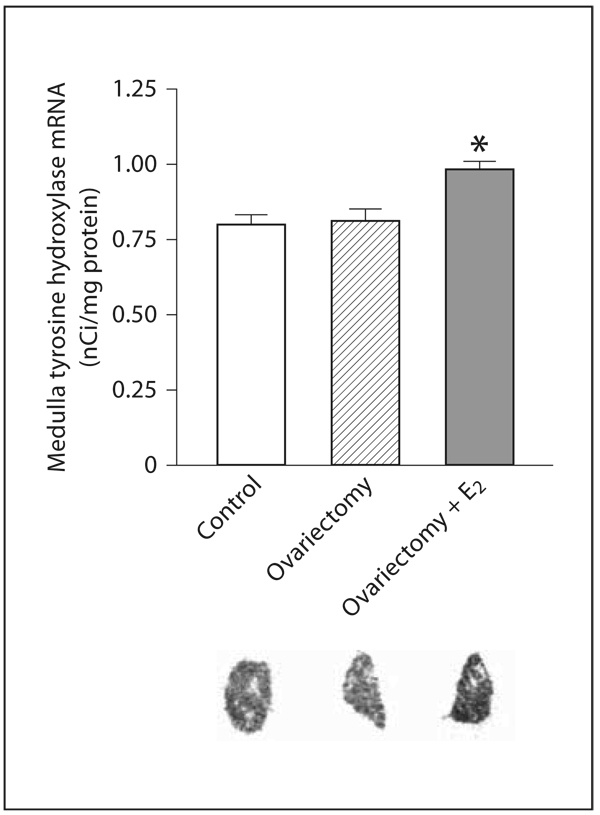
In the adrenal medulla, OVX significantly decreased E and NE content when compared to control rats, but did not affect the expression of TH mRNA (fig. 8, 9). Estrogen treatment of OVX rats increased E and NE levels, partially restoring these levels to those of controls (fig. 8). Additionally, TH mRNA expression was significantly increased by estrogen replacement to OVX rats (fig. 9).
Fig. 8.
Effects of ovariectomy and estrogen treatment on catecholamine content in the rat adrenal medulla. E and NE were measured by HPLC. E2 indicates treatment with 17β-estradiol. Data are means ± SEM, n = 5–7 for each group. * p <0.05 compared to all other groups.
Fig. 9.
Effects of ovariectomy and estrogen treatment on TH mRNA in the rat adrenal medulla. TH mRNA was determined by in situ hybridization as described in Materials and Methods. E2 indicates treatment with 17β-estradiol. Bars represent means ± SEM, n = 5–7 for each group. * p <0.05 compared to all other groups. The photographs are typical autoradiograms of in situ hybridization of TH in adrenal medulla.
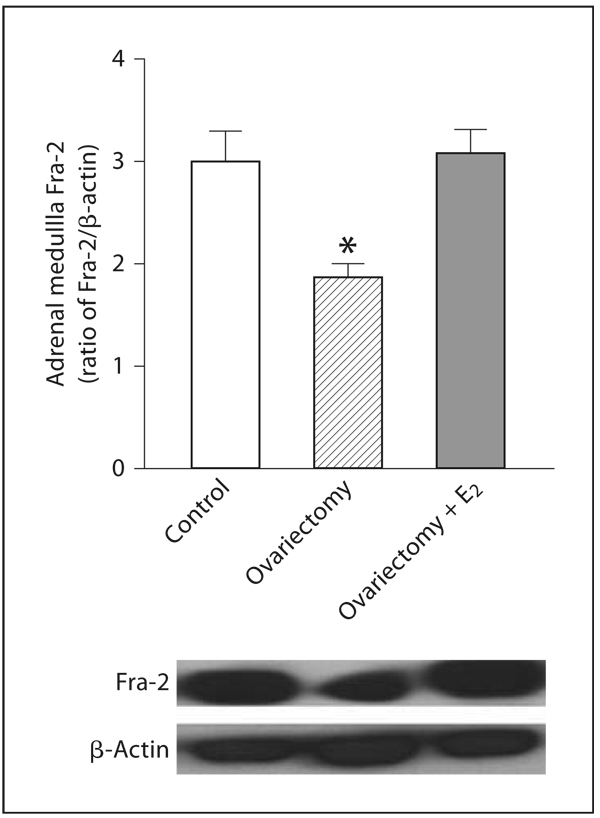
The expression of Fra-2 was significantly decreased in OVX rats when compared to control rats (fig. 10). Estrogen treatment of OVX rats increased Fra-2 expression to levels similar to those of controls (fig. 10).
Fig. 10.
Effects of ovariectomy and estrogen treatment on Fra-2 expression in rat adrenal medulla. Fra-2 was determined by Western blot using β-actin as a control, as described in Materials and Methods. Bars represent means ± SEM, n = 5–7 for each group. The photographs are representative X-ray film images of Fra-2 and β-actin detected after treatment of membranes with a chemiluminescent substrate. * p <0.05 compared to all other groups.
Discussion
We report a profound estrogen influence on the adrenal gland of female rats, including control of aldosterone and catecholamine synthesis and regulation of the transcription factor Fra-2 in the adrenal medulla. These effects may be the result of modulation of local RAS systems [6–8, 11, 36] because estrogen exerts a powerful regulatory effect on adrenal AT1 and AT2 receptors, as reported here.
We administered estrogen at a dose that increases its blood levels about twofold over those found during the ovulatory peak, levels similar to those found during pregnancy [37]. Our use of ovariectomy as well as estradiol treatment models addressed both endogenous and exogenous estrogen effects.
Ovariectomy increased AT1 receptor expression in the zona glomerulosa, in agreement with previous observations [20, 21, 38, 39] and in the adrenal medulla, effects reversed by estrogen treatment. In addition, estrogen treatment downregulated AT1B receptor mRNA in the zona glomerulosa. Our observations indicate that reproductive hormones control adrenal AT1 receptor transcription and expression.
Ovariectomy did not alter AT2 receptor binding or mRNA, suggesting that reproductive hormones may not be essential to maintain constitutive expression of adrenal AT2 receptors. On the other hand, estrogen treatment produced major increases in AT2 receptor expression and mRNA, in both the zona glomerulosa and the medulla. Receptor upregulation may be the consequence of altered estrogen/progesterone balance. Progesterone effects on adrenal AT2 receptors have not been studied. However, in the pituitary gland, progesterone does not alter the AT2 receptor expression induced after estrogen-induced hyperplasia [40]. AT2 receptor upregulation may also be a response to the high estrogen levels during our experiments, above those occurring during the estrous cycle but similar to those found during pregnancy. Significant AT2 receptor upregulation by estrogen has also been previously demonstrated in the human myometrium [41], and in the kidney of OVX rats and mice [28, 29], a finding recently confirmed in mice deficient in apolipoprotein E [42].
Estrogen treatment of OVX rats altered the ratio of expression of AT2/AT1 receptors in favor of AT2 expression in the zona glomerulosa and medulla. This observation agrees with the higher AT2/AT1 ratio seen in female rats [29, 43] and mice [28] compared to male rats or mice, respectively. The estrogen-dependent downregulation of AT1B receptor mRNA expression in the zona glomerulosa is similar to the downregulation of AT1B receptors in the pituitary gland [12, 44]. Possible mechanisms include modulation of receptor transcription through estrogen response elements [45] and/or the result of estrogen-induced decreased plasma K+ concentration [46, 47]. The AT2 receptor promoter does not contain estrogen response elements [48]. Estrogen may activate AT2 receptor transcription from alternative response elements such as the activator protein site (AP-1) [49, 50], by increased intracellular sodium concentration through estrogen inhibition of Na+,K+-ATPase [51, 52], a ouabain-sensitive mechanism [53] or by receptor activation potentiating its own expression, because AT2 receptors stimulate endogenous adrenal ouabain, and this inhibits Na+,K+-ATPase [54].
The present results support the hypothesis of a mutual influence between AT1 and AT2 receptor function. AT2 receptor gene disruption upregulates the number of AT1 receptors in the adrenal gland, the kidney and the brain [55–57]. Conversely, cardiac-specific AT2 receptor overexpression decreases AT1-mediated effects [58], and AT2 receptor activation negatively regulates AT1 signal transduction in bovine chromaffin cells [26]. In turn, AT2 receptor upregulation may be the consequence of the decrease in AT1 receptor activity, because AT1 receptor blockade enhances AT2 receptor expression in rat brain, adrenal zona glomerulosa and cerebral vessels [35, 36, 59, 60]. AT2 receptor upregulation may compensate for AT1 receptor downregulation, to maintain Na+,K+-ATPase and cellular sodium concentrations, since Ang II inhibits Na+,K+-ATPase through AT1 receptor stimulation [61].
Modulation of Ang II receptor types has significant effects on adrenal zona glomerulosa function. Ovariectomy does not change the adrenal aldosterone content, but estrogen treatment of OVX rats very significantly decreases aldosterone concentration, a finding consistent with the report of decreased Ang II-induced aldosterone release by estrogen replacement in OVX rats [21, 38]. That ovariectomy does not alter adrenal aldosterone levels in spite of enhanced AT1 receptor expression may be explained by the concomitant decrease in circulating Ang II [62]. The estrogen-induced reduction in adrenal aldosterone is associated with a reversal of the ovariectomyinduced increase in AT1 receptor expression. It also parallels the increase in AT2 receptor transcription and expression. Since K+ concentration in plasma is a major regulator of aldosterone release, estrogen-induced decrease in plasma K+, directly and/or through a decrease in AT1 receptor expression [46], may contribute to decrease aldosterone adrenal content. Our results support the hypothesis that AT1B receptor stimulation enhances [10, 63] and that AT2 stimulation decreases aldosterone formation and release. The role of AT2 receptors may be part of feedback mechanisms, because aldosterone infusion reduces the number of adrenal AT2 receptors [64]. There are additional reports of opposing actions of adrenal AT1 and AT2 receptors in the adrenal zona glomerulosa including AT2-mediated antagonism of the proliferative effect of AT1 receptor stimulation [65] and regulation of local blood flow through dilation of cortical arteries, antagonizing AT1-mediated vasoconstriction [66].
Estrogen modulation of adrenomedullary Ang II receptors parallels significant changes in catecholamine formation. Medullary catecholamine content decreases after ovariectomy, in agreement with previous observations [22, 67]. Ovariectomy did not reduce TH mRNA expression, but lowered the content of the essential TH cofactor tetrahydrobiopterin [68], decreasing in vivo TH activity. We showed that estrogen treatment restored adrenomedullary catecholamine content in association with enhanced TH mRNA expression in the OVX rats. Other studies demonstrated that estrogen treatment replenishes the vascular content of tetrahydrobiopterin [68] and enhances the tetrahydrobiopterin content and gene expression of GTP cyclohydrolase, the rate-limiting enzyme for tetrahydrobiopterin synthesis [69]. Our results and those of the literature [22, 23] can best be interpreted as decreased adrenomedullary catecholamine synthesis by ovariectomy, an effect reversed by estrogen treatment.
Estrogen may regulate TH transcription through activation of estrogen response elements in the tyrosine promoter [70]. In the adrenal medulla, basal and stress-induced TH transcription [7, 71] are regulated by the Fra-2, a member of the Fos family contributing to formation of the inducible activator protein-1 (AP-1) transcription factor complex. We report here that ovariectomy decreased Fra-2 expression in parallel with the decrease in catecholamine content. The decrease in Fra-2 expression was reversed by estrogen treatment, coinciding with the increase in expression of TH mRNA and the restoration of catecholamine levels. This indicates that modulation of Fra-2 expression is a likely mechanism explaining the stimulant effect of estrogen on catecholamine synthesis. Ours is the first report of a direct in vivo modulation of Fra-2 by estrogen suggesting that estrogen upregulates Fra-2 transcription. It is of interest that Fra-2 participates in the mechanisms of carcinogenesis and tumor recurrence [72], and that there is an inverse correlation between estrogen α receptor expression and Fra-2 levels in human breast cancer cells, indicating that estrogen α receptor signaling represses Fra-2 expression in these cells [73].
The regulation of adrenomedullary catecholamine synthesis by estrogen probably involves modulation of AT1 and AT2 receptor function. AT1 receptor activation controls Ang II- and stress-induced enhanced TH transcription and catecholamine release in the adrenal medulla [14–16, 35]. AT1 receptor activation maintains constitutive adrenomedullary AT2 expression, and both AT1 and AT2 receptors help to maintain TH transcription, adrenomedullary catecholamine levels, and Fra-2 mRNA expression [7].
Our results may be best interpreted as follows. In the adrenal medulla, AT1, AT2 and Fra-2 expression, TH transcription and catecholamine stores are partially dependent on estrogen effects. Ovarian hormones are necessary for the full constitutive Fra-2 expression. After ovariectomy, catecholamine formation decreases probably because of reduced tetrahydrobiopterin availability [68, 69]. TH transcription is maintained by AT1 receptor activation even in the presence of reduced Fra-2 expression. Estrogen treatment enhances TH transcription, an effect associated with normalization of Fra-2 expression, restoring catecholamine synthesis. Estrogen treatment enhances AT2 receptor expression and transcription, demonstrating that AT2 receptor activity is estrogen-dependent. We hypothesize that AT2 receptor activation regulates catecholamine synthesis by modulating TH transcription through a Fra-2-dependent mechanism.
Our results may have clinical implications. We found that estrogen replacement reduced the content of aldosterone, a fluid-retentive, pro-inflammatory hormone [38], and in the adrenal medulla, reversed the AT1 receptor upregulation that results from the loss of ovarian function. Estrogen-dependent AT1 receptor downregulation, decreasing vasoconstriction, has been reported in the peripheral vasculature [74] and in the pituitary gland [75]. Downregulation of vascular and adrenal AT1 receptors, and decreased aldosterone synthesis, may be considered important mechanisms contributing to the cardiovascular protective effects associated with estrogen.
References
- 1.Corvol P, Jeunemaitre X, Charru A, Kotelevtsev Y, Soubrier F. Role of the renin-angiotensin system in blood pressure regulation and in human hypertension: new insights from molecular genetics. Recent Prog Horm Res. 1995;50:287–308. doi: 10.1016/b978-0-12-571150-0.50017-2. [DOI] [PubMed] [Google Scholar]
- 2.Castelli WP. Cardiovascular disease in women. Am J Obstet Gynecol. 1988;158:1553–1560. doi: 10.1016/0002-9378(88)90189-5. [DOI] [PubMed] [Google Scholar]
- 3.Schunkert H, Danser AH, Hense HW, Derkx FH, Kurzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation. 1997;95:39–45. doi: 10.1161/01.cir.95.1.39. [DOI] [PubMed] [Google Scholar]
- 4.Harvey PJ, Wing LM, Savage J, Molloy D. The effects of different types and doses of oestrogen replacement therapy on clinic and ambulatory blood pressure and the reninangiotensin system in normotensive postmenopausal women. J Hypertens. 1999;17:405–411. doi: 10.1097/00004872-199917030-00014. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. Randomized Aldactone Evaluation Study investigators: The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 6.Hilbers U, Peters J, Bornstein SR, Correa FM, Jöhren O, Saavedra JM, Ehrhart-Bornstein M. Local renin-angiotensin system is involved in K+-induced aldosterone secretion from human adrenocortical NCI-H295 cells. Hypertension. 1999;33:1025–1030. doi: 10.1161/01.hyp.33.4.1025. [DOI] [PubMed] [Google Scholar]
- 7.Jezova M, Armando I, Bregonzio C, Yu ZX, Qian S, Ferrans VJ, Imboden H, Saavedra JM. Angiotensin II AT1 and AT2 receptors contribute to maintain basal adrenomedullary norepinephrine synthesis and tyrosine hydroxylase transcription. Endocrinology. 2003;144:2092–2101. doi: 10.1210/en.2002-0019. [DOI] [PubMed] [Google Scholar]
- 8.Chiou CY, Kifor I, Moore TJ, Williams GH. The effect of losartan on potassium-stimulated aldosterone secretion in vitro. Endocrinology. 1994;134:2371–2375. doi: 10.1210/endo.134.6.8194463. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe A, Naruse M, Arai K, Naruse K, Yoshimoto T, Seki T, Imaki T, Kobayashi M, Miyazaki H, Demura H. Angiotensin II stimulates both aldosterone secretion and DNA synthesis via type 1 but not type 2 receptors in bovine adrenocortical cells. J Endocrinol Invest. 1998;21:668–672. doi: 10.1007/BF03350796. [DOI] [PubMed] [Google Scholar]
- 10.Naruse M, Tanabe A, Sugaya T, Naruse K, Yoshimoto T, Seki T, Imaki T, Demura R, Murakami K, Demura H. Differential roles of angiotensin receptor subtypes in adrenocortical function in mice. Life Sci. 1998;63:1593–1598. doi: 10.1016/s0024-3205(98)00428-7. [DOI] [PubMed] [Google Scholar]
- 11.Volpe M, Gigante B, Enea I, Porcellini A, Russo R, Lee MA, Magri P, Condorelli G, Savoia C, Lindpaintner K, Rubattu S. Role of tissue renin in the regulation of aldosterone biosynthesis in the adrenal cortex of nephrectomized rats. Circ Res. 1997;81:857–864. doi: 10.1161/01.res.81.5.857. [DOI] [PubMed] [Google Scholar]
- 12.Kakar SS, Sellers JC, Devor DC, Musgrove LC, Neill JD. Angiotensin II type-1 receptor subtype cDNAs: differential tissue expression and hormonal regulation. Biochem Biophys Res Commun. 1992;183:1090–1096. doi: 10.1016/s0006-291x(05)80302-x. [DOI] [PubMed] [Google Scholar]
- 13.Kitami Y, Okura T, Marumoto K, Wakamiya R, Hiwada K. Differential gene expression and regulation of type-1 angiotensin II receptor subtypes in the rat. Biochem Biophys Res Commun. 1992;188:446–452. doi: 10.1016/0006-291x(92)92405-m. [DOI] [PubMed] [Google Scholar]
- 14.Hano T, Mizukoshi M, Baba A, Nakamura N, Nishio I. Angiotensin II subtype 1 receptor modulates epinephrine release from isolated rat adrenal gland. Blood Press Suppl. 1994;5:105–108. [PubMed] [Google Scholar]
- 15.Martineau D, Yamaguchi N, Briand R. Inhibition by BMS 186295, a selective non-peptide AT1 antagonist, of adrenal catecholamine release induced by angiotensin II in the dog in vivo. Can J Physiol Pharmacol. 1995;73:459–464. doi: 10.1139/y95-058. [DOI] [PubMed] [Google Scholar]
- 16.Wong PC, Hart SD, Zaspel AM, Chiu AT, Ardecky RJ, Smith RD, Timmermans PB. Functional studies of nonpeptide angiotensin II receptor subtype-specific ligands: DuP 753 (AII-1) and PD123177 (AII-2) J Pharmacol Exp Ther. 1990;255:584–592. [PubMed] [Google Scholar]
- 17.Rahmouni K, Barthelmebs M, Grima M, Imbs JL, De Jong W. Brain mineralocorticoid receptor control of blood pressure and kidney function in normotensive rats. Hypertension. 1999;33:1201–1206. doi: 10.1161/01.hyp.33.5.1201. [DOI] [PubMed] [Google Scholar]
- 18.Brownie AC. The adrenal cortex in hypertension: DOCA/salt hypertension and beyond. In: Laragh JH, editor. Hypertension: Pathophysiology, Diagnosis, and Management. Philadelphia: Lippincott Williams & Wilkins; 1995. [Google Scholar]
- 19.Joffe HV, Adler GK. Effect of aldosterone and mineralocorticoid receptor blockade on vascular inflammation. Heart Fail Rev. 2005;10:31–37. doi: 10.1007/s10741-005-2346-0. [DOI] [PubMed] [Google Scholar]
- 20.Krishnamurthi K, Verbalis JG, Zheng W, Wu Z, Clerch LB, Sandberg K. Estrogen regulates angiotensin AT1 receptor expression via cytosolic proteins that bind to the 5′ leader sequence of the receptor mRNA. Endocrinology. 1999;140:5435–5438. doi: 10.1210/endo.140.11.7242. [DOI] [PubMed] [Google Scholar]
- 21.Roesch DM, Tian Y, Zheng W, Shi M, Verbalis JG, Sandberg K. Estradiol attenuates angiotensin-induced aldosterone secretion in ovariectomized rats. Endocrinology. 2000;141:4629–4636. doi: 10.1210/endo.141.12.7822. [DOI] [PubMed] [Google Scholar]
- 22.Serova LI, Maharjan S, Sabban EL. Estrogen modifies stress response of catecholamine biosynthetic enzyme genes and cardiovascular system in ovariectomized female rats. Neuroscience. 2005;132:249–259. doi: 10.1016/j.neuroscience.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 23.Yanagihara N, Liu M, Toyohira Y, Tsutsui M, Ueno S, Shinohara Y, Takahashi K, Tanaka K. Stimulation of catecholamine synthesis through unique estrogen receptors in the bovine adrenomedullary plasma membrane by 17β-estradiol. Biochem Biophys Res Commun. 2006;339:548–553. doi: 10.1016/j.bbrc.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 24.Heemskerk FM, Saavedra JM. Quantitative autoradiography of angiotensin II AT2 receptors with [125I]CGP 42112. Brain Res. 1995;677:29–38. doi: 10.1016/0006-8993(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 25.Gallinat S, Busche S, Raizada MK, Sumners C. The angiotensin II type 2 receptor: an enigma with multiple variations. Am J Physiol. 2000;278:E357–E374. doi: 10.1152/ajpendo.2000.278.3.E357. [DOI] [PubMed] [Google Scholar]
- 26.Ishii K, Takekoshi K, Shibuya S, Kawakami Y, Isobe K, Nakai T. Angiotensin subtype-2 receptor (AT2) negatively regulates subtype-1 receptor (AT1) in signal transduction pathways in cultured porcine adrenal medullary chromaffin cells. J Hypertens. 2001;19:1991–1999. doi: 10.1097/00004872-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Leong DS, Terrón JA, Falcón-Neri A, Armando I, Ito T, Jöhren O, Tonelli LH, Hoe KL, Saavedra JM. Restraint stress modulates brain, pituitary and adrenal expression of angiotensin II AT1A, AT1B and AT2 receptors. Neuroendocrinology. 2002;75:227–240. doi: 10.1159/000054714. [DOI] [PubMed] [Google Scholar]
- 28.Armando I, Jezova M, Juorio AV, Terrón JA, Falcón-Neri A, Semino-Mora C, Imboden H, Saavedra JM. Estrogen upregulates renal angiotensin II AT2 receptors. Am J Physiol Renal Physiol. 2002;283:F934–F943. doi: 10.1152/ajprenal.00145.2002. [DOI] [PubMed] [Google Scholar]
- 29.Baiardi G, Macova M, Armando I, Ando H, Tyurmin D, Saavedra JM. Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regul Pept. 2005;124:7–17. doi: 10.1016/j.regpep.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Physiol. 1991;261:R209–R216. doi: 10.1152/ajpregu.1991.261.1.R209. [DOI] [PubMed] [Google Scholar]
- 31.Miller JA, Zahniser NR. The use of 14C-labeled tissue paste standards for the calibration of 125I-labeled ligands in quantitative autoradiography. Neurosci Lett. 1987;81:345–350. doi: 10.1016/0304-3940(87)90408-3. [DOI] [PubMed] [Google Scholar]
- 32.Grima B, Lamouroux A, Blanot F, Biguet NF, Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci USA. 1985;82:617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wisden W, Morris BJ. In situ hybridization with synthetic oligonucleotide probes. In: Wisden W, Morris BJ, editors. In situ Hybridization Protocols for the Brain. San Diego: Academic Press; 1994. pp. 9–34. [Google Scholar]
- 34.Jöhren O, Inagami T, Saavedra JM. AT1A, AT1B, and AT2 angiotensin II receptor subtype gene expression in rat brain. Neuroreport. 1995;6:2549–2552. doi: 10.1097/00001756-199512150-00024. [DOI] [PubMed] [Google Scholar]
- 35.Armando I, Carranza A, Nishimura Y, Hoe K-L, Barontini M, Terrón JA, Falcón-Neri A, Ito T, Juorio AV, Saavedra JM. Peripheral administration of an angiotensin II AT1 receptor antagonist decreases the hypothalamic-pituitary-adrenal response to isolation stress. Endocrinology. 2001;142:3880–3889. doi: 10.1210/endo.142.9.8366. [DOI] [PubMed] [Google Scholar]
- 36.Gigante B, Rubattu S, Russo R, Porcellini A, Enea I, De Paolis P, Savoia C, Natale A, Piras O, Volpe M. Opposite feedback control of rennin and aldosterone biosynthesis in the adrenal cortex by angiotensin II AT1-subtype receptors. Hypertension. 1997;30:563–568. doi: 10.1161/01.hyp.30.3.563. [DOI] [PubMed] [Google Scholar]
- 37.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17βthroughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 38.Wu Z, Maric C, Roesch DM, Zheng W, Verbalis JG, Sandberg K. Estrogen regulates adrenal angiotensin AT1 receptors by modulating AT1 receptor translation. Endocrinology. 2003;144:3251–3261. doi: 10.1210/en.2003-0015. [DOI] [PubMed] [Google Scholar]
- 39.Owonikoko TK, Fabucci ME, Brown PR, Nisar N, Hilton J, Mathews WB, Ravert HT, Rauseo P, Sandberg K, Dannals RF, Szabo Z. In vivo investigation of estrogen regulation of adrenal and renal angiotensin (AT1) receptor expression by PET. J Nucl Med. 2004;45:94–100. [PMC free article] [PubMed] [Google Scholar]
- 40.Suarez C, Díaz-Torga G, González-Iglesias A, Cristina C, Becu-Villalobos D. Upregulation of angiotensin II type 2 receptor expression in estrogen-induced pituitary hyperplasia. Am J Physiol. 2004;286:E786–E794. doi: 10.1152/ajpendo.00477.2003. [DOI] [PubMed] [Google Scholar]
- 41.Mancina R, Susini T, Renzetti A, Forti G, Razzoli E, Serio M, Maggi M. Sex steroid modulation of AT2 receptors in human myometrium. J Clin Endocrinol Metab. 1996;81:1753–1757. doi: 10.1210/jcem.81.5.8626829. [DOI] [PubMed] [Google Scholar]
- 42.Brosnihan KB, Hodgin JB, Smithies O, Maeda N, Gallagher P. Tissue-specific regulation of ACE/ACE2 and AT1/AT2 receptor gene expression by estrogen in ApoE/ERa knockout mice. Exp Physiol. 2008;93:658–664. doi: 10.1113/expphysiol.2007.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res. 2004;62:587–593. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 44.Jöhren O, Sanvitto GL, Egidy G, Saavedra JM. Angiotensin II AT1A receptor mRNA expression is induced by estrogen-progesterone in dopaminergic neurons of the female rat arcuate nucleus. J Neurosci. 1997;17:8283–8292. doi: 10.1523/JNEUROSCI.17-21-08283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeuchi K, Alexander RW, Nakamura Y, Tsujino T, Murphy TJ. Molecular structure and transcriptional function of the rat vascular AT1A angiotensin receptor gene. Circ Res. 1993;73:612–621. doi: 10.1161/01.res.73.4.612. [DOI] [PubMed] [Google Scholar]
- 46.Zheng W, Shi M, Yoo S-E, Ji H, Roesch DM. Estrogens contribute to a sex difference in plasma potassium concentration: a mechanism for regulation of adrenal angiotensin receptors. Gender Med. 2006;3:43–53. doi: 10.1016/s1550-8579(06)80193-2. [DOI] [PubMed] [Google Scholar]
- 47.Ray PE, Ruley EJ, Saavedra JM. Different effects of chronic K+ depletion on forebrain and peripheral angiotensin II receptors in young rats. Brain Res. 1991;556:240–246. doi: 10.1016/0006-8993(91)90311-i. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi S, Ohnishi J, Nibu Y, Nishimatsu S, Umemura S, Ishii M, Murakami K, Miyazaki H. Cloning of the rat angiotensin II type 2 receptor gene and identification of its functional promoter region. Biochim Biophys Acta. 1995;1262:155–158. doi: 10.1016/0167-4781(95)00076-s. [DOI] [PubMed] [Google Scholar]
- 49.Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 50.Ichiki T, Kambayashi Y, Inagami T. Transcription of the rat angiotensin II type 2 receptor gene. Biochem Biophys Res Commun. 1996;222:566–571. doi: 10.1006/bbrc.1996.0784. [DOI] [PubMed] [Google Scholar]
- 51.Tsai ML, Chang CC, Lee CL, Huang BY. The differential effects of tamoxifen and ICI 182,780 on the reduction of Na+,K+-ATPase activity and spontaneous oscillations by 17β-estradiol. Chin J Physiol. 2003;46:55–62. [PubMed] [Google Scholar]
- 52.Foster RH, Prat H, Rothman I. Is ouabain produced by the adrenal gland? Gen Pharm. 1998;31:499–501. doi: 10.1016/s0306-3623(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 53.Tamura M, Wanaka Y, Landon EJ, Inagami T. Intracellular sodium modulates the expression of angiotensin II subtype 2 receptor in PC12W cells. Hypertension. 1999;33:626–632. doi: 10.1161/01.hyp.33.2.626. [DOI] [PubMed] [Google Scholar]
- 54.Laredo J, Shah JR, Lu ZR, Hamilton BP, Hamlyn JM. Angiotensin II stimulates secretion of endogenous ouabain from bovine adrenocortical cells via angiotensin type 2 receptors. AT2 receptors stimulate endogenous ouabain from adrenal glands. Hypertension. 1997;29:401–407. doi: 10.1161/01.hyp.29.1.401. [DOI] [PubMed] [Google Scholar]
- 55.Saavedra JM, Armando I, Terrón JA, Falcón-Neri A, Jöhren O, Häuser W, Inagami T. Increased AT1 receptors in adrenal gland of AT2 receptor gene-disrupted mice. Regul Pept. 2001;102:41–47. doi: 10.1016/s0167-0115(01)00303-2. [DOI] [PubMed] [Google Scholar]
- 56.Saavedra JM, Häuser W, Ciuffo G, Egidy G, Hoe KL, Jöhren O, Sembonmatsu T, Inagami T, Armando I. Increased AT1 receptor expression and mRNA in kidney glomeruli of AT2 receptor gene-disrupted mice. Am J Physiol Renal Physiol. 2001;280:F71–F78. doi: 10.1152/ajprenal.2001.280.1.F71. [DOI] [PubMed] [Google Scholar]
- 57.Armando I, Terrón JA, Falcón-Neri A, Ito T, Häuser W, Inagami T, Saavedra JM. Increased angiotensin II AT1 receptor expression in paraventricular nucleus and hypothalamic-pituitary-adrenal axis stimulation in AT2 receptor gene disrupted mice. Neuroendocrinology. 2002;76:137–147. doi: 10.1159/000064525. [DOI] [PubMed] [Google Scholar]
- 58.Masaki H, Kurihara T, Yamaki A, Inomata N, Nozawa Y, Mori Y, Murasawa S, Kizima K, Maruyama K, Horiuchi M, Dzau VJ, Takahashi H, Iwasaka T, Inada M, Matsubara H. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J Clin Invest. 1998;101:527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seltzer A, Bregonzio C, Armando I, Baiardi G, Saavedra JM. Oral administration of an AT1 receptor antagonist prevents the central effects of angiotensin II in spontaneously hypertensive rats. Brain Res. 2004;1028:9–18. doi: 10.1016/j.brainres.2004.06.079. [DOI] [PubMed] [Google Scholar]
- 60.Zhou J, Pavel J, Macova M, Yu ZX, Imboden H, Ge L, Nishioku T, Dou J, Delgiacco E, Saavedra JM. AT1 receptor blockade regulates the local angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke. 2006;37:1271–1276. doi: 10.1161/01.STR.0000217404.64352.d7. [DOI] [PubMed] [Google Scholar]
- 61.Hajnóczky G, Csordás G, Hunyady L, Kalapos MP, Balla T, Enyedi P, Spät A. Angiotensin-II inhibits Na+/K+ pump in rat adrenal glomerulosa cells: possible contribution to stimulation of aldosterone production. Endocrinology. 1992;130:1637–1644. doi: 10.1210/endo.130.3.1311245. [DOI] [PubMed] [Google Scholar]
- 62.Xu X, Xiao JC, Luo LF, Wang S, Zhang JP, Huang JJ, Liu ML, Liu CG, Xu KQ, Li YJ, Song HP. Effects of ovariectomy and 17β-estradiol treatment on the renin-angiotensin system, blood pressure, and endothelial structure. Int J Cardiol. 2007 doi: 10.1016/j.ijcard.2007.08.041. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 63.Chen X, Li W, Yoshida H, Tsuchida S, Nishimura H, Takemoto F, Okubo S, Fogo A, Matsusaka T, Ichikawa I. Targeting deletion of angiotensin type 1B receptor gene in the mouse. Am J Physiol. 1997;272:F299–F304. doi: 10.1152/ajprenal.1997.272.3.F299. [DOI] [PubMed] [Google Scholar]
- 64.Wang DH, Qiu J, Hu Z. Differential regulation of angiotensin II receptor subtypes in the adrenal gland: role of aldosterone. Hypertension. 1998;32:65–70. doi: 10.1161/01.hyp.32.1.65. [DOI] [PubMed] [Google Scholar]
- 65.Mazzocchi G, Malendowicz LK, Gottardo G, Rebuffat P, Nussdorfer GG. Angiotensin-II stimulates DNA synthesis in rat adrenal zona glomerulosa cells: receptor subtypes involved and possible signal transduction mechanism. Endocr Res. 1997;23:191–203. doi: 10.3109/07435809709031853. [DOI] [PubMed] [Google Scholar]
- 66.Gauthier KM, Zhang DX, Edwards EM, Holmes B, Campbell WB. Angiotensin II dilates bovine adrenal cortical arterioles: role of endothelial nitric oxide. Endocrinology. 2005;146:3319–3324. doi: 10.1210/en.2005-0129. [DOI] [PubMed] [Google Scholar]
- 67.Kholer C, Berkowitz BA, Spector S. Sex hormones and tyrosine hydroxylase activity in vascular and adrenal tissue. Endocrinology. 1975;97:1316–1320. doi: 10.1210/endo-97-5-1316. [DOI] [PubMed] [Google Scholar]
- 68.Lam K-K, Lee Y-M, Hsiao G, Chen S-Y, Yen M-H. Estrogen therapy replenishes vascular tetrahydrobiopterin and reduces oxidative stress in ovariectomized rats. Menopause. 2006;13:294–302. doi: 10.1097/01.gme.0000182806.99137.5e. [DOI] [PubMed] [Google Scholar]
- 69.Miyazaki-Akita A, Hayashi T, Ding QF, Shiraishi H, Nomura T, Hattori Y, Iguchi A. 17β-Estradiol antagonizes the down-regulation of endothelial nitric-oxide synthase and GTP cyclohydrolase I by high glucose: relevance to postmenopausal diabetic cardiovascular disease. J Pharmacol Exp Ther. 2007;320:591–598. doi: 10.1124/jpet.106.111641. [DOI] [PubMed] [Google Scholar]
- 70.Maharjan S, Serova L, Sabban EL. Transcriptional regulation of tyrosine hydroxylase by estrogen: opposite effects with estrogen receptors α and β and interactions with cyclic AMP. J Neurochem. 2005;93:1502–1514. doi: 10.1111/j.1471-4159.2005.03142.x. [DOI] [PubMed] [Google Scholar]
- 71.Nankova BB, Rivkin M, Kelz M, Nestler EJ, Sabban EL. Fos-related antigen 2: potential mediator of the transcriptional activation in rat adrenal medulla evoked by repeated immobilization stress. J Neurosci. 2000;20:5647–5653. doi: 10.1523/JNEUROSCI.20-15-05647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Milde-Langosch K. The Fos family of transcription factors and their role in tumorigenesis. Eur J Cancer. 2005;41:2449–2461. doi: 10.1016/j.ejca.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Wang X, Belguise K, Kersual N, Kirsch KH, Mineva ND, Galtier F, Chalbos D, Sonenshein GE. Oestrogen signaling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat Cell Biol. 2007;9:470–478. doi: 10.1038/ncb1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nickenig G, Baumer AT, Grohe C, Kahlert S, Strehlow K, Rosenkranz S, Stablein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Bohm M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation. 1998;97:2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 75.Seltzer A, Pinto JE, Viglione PN, Correa FM, Libertun C, Tsutsumi K, Steele MK, Saavedra JM. Estrogens regulate angiotensin-converting enzyme and angiotensin receptors in female rat anterior pituitary. Neuroendocrinology. 1992;55:460–467. doi: 10.1159/000126157. [DOI] [PubMed] [Google Scholar]