Abstract
The application of plasmid DNA (pDNA)-based gene therapy is limited by its inefficient transgene expression. In this study, minicircle DNA was evaluated for efficient vascular endothelial growth factor (VEGF) expression in skeletal muscle cells. Production of minicircle DNA encoding VEGF was studied by a semi-quantitative electrophoresis method leading to optimized bacterial culture conditions and producing high purity (86.6 %) minicircle DNA. The VEGF minicircle DNA under control of the SV40 promoter (pMini-SV-VEGF) showed an increased amount of VEGF mRNA and up to 8 times more VEGF expression than a conventional plasmid (pSV-VEGF) in two different skeletal muscle cell lines (C2C12 and L8). Minicircle DNA with different promoters, including the SV40, CMV and chicken β-actin, was tested for VEGF expression in a C2C12 skeletal muscle cell line. The high VEGF expression generated by minicircle DNA stimulated efficient endothelial cell growth in vitro. Furthermore, minicircle DNA expressed higher VEGF compared to conventional plasmid in the tibialis anterior (TA) muscle of mice. Taken together, the results suggest that minicircle DNA is an efficacious gene vector for angiogenic VEGF expression in skeletal muscle and may be useful for treating peripheral arterial disease (PAD).
Keywords: minicircle DNA, VEGF, skeletal muscle cell
1. Introduction
pDNA has served as the simplest gene vector for in vivo gene transfection applications. Advantages of using pDNA include low toxicity, ease of large scale production, no integration into the host genome and no contamination from helper virus. As a result, pDNA-based gene transfer has been studied for the treatment of numerous diseases such as cancer [1], cardiovascular [2] and skeletal muscle related disorders [3].
The clinical significance of peripheral arterial disease (PAD) is revealed by its high occurrence rate and limited therapeutic treatments. pDNA delivery-based therapeutic angiogenesis in skeletal muscle has proven to be an alternative approach for the treatment of PAD [4, 5]. Expression of angiogenic VEGF using naked pDNA has been shown to enhance the recovery of local blood flow and improve clinical disease indexes by stimulating the proliferation of vascular endothelial cells [6]. However, clinical efficacy is limited by low transgene expression [7, 8] leading to the need for the development of more efficient gene delivery/vector systems.
Two major approaches have been proposed to improve transgene expression. The first approach is to increase the efficiency of pDNA delivery. These methods include (1) chemical methods such as polymer-based carrier mediated pDNA delivery [9, 10]; (2) physical methods such as electroporation [11, 12] or ultrasound microbubble-mediated pDNA delivery [13]. The second approach is to optimize the transgene expression cassette by testing different transcription/translation regulatory elements such as promoter [14, 15], enhancer [16, 17] and 3′ untranslated region [18, 19]. In addition to these approaches, minicircle DNA has been developed as a more efficient gene vector [20, 21].
It has been discovered that unmethylated CpG motifs in pDNA can trigger the activation of immune response which decrease gene transfection efficiency [22-25]. Bacterial-originated sequences such as the origin of replication and the antibiotic resistance gene contain the most abundant unmethylated CpG dinucleotides in pDNA. Minicircle DNA is circular double-stranded DNA which contains merely the transgene expression cassette (including promoter, enhancer, gene cDNA and poly(A) signal) without bacterial-originated sequences. Minicircle DNA is low in immunogenicity due to its lower content of unmethylated CpG dinucleotides [26, 27]. In addition, the smaller size of minicircle DNA may exhibit greater in vivo diffusivity and availability compared to the conventional plasmid with the same transgene expression cassette. CpG motif-depleted plasmids, including minicircle DNA, have been studied for production of several therapeutic proteins, for example, adiponectin [28] and human factor IX [26, 29]. The target organs for expressing these therapeutic proteins are liver and lung. To date, minicircle DNA has not been evaluated for the expression of VEGF in skeletal muscle.
In order to elucidate the limiting step for obtaining high purity minicircle DNA encoding VEGF, the production process of minicircle DNA in bacterial host cells (TOP10) was studied. In vitro transfection efficiency of minicircle DNA was compared to their respective conventional plasmids. Different promoters, including the SV40, CMV and chicken β-actin were evaluated for VEGF expression in vitro and in vivo. The enhanced proliferation of endothelial cells by higher VEGF expression from minicircle DNA was also observed in vitro.
2. Materials and Methods
2.1 Materials
Growth medium (DMEM, medium 199 and F-12k), serum (fetal bovine serum (FBS) and horse serum) and trypsin/EDTA were obtained from Invitrogen (Carlsbad, CA). Chicken embryo extract was purchase from Accurate Chemical (Westbury, NY). Branched PEI (average molecular weight of 25 kDa, average degree of polymerization 580), endothelial cell growth supplement (ECGS), heparin and L-arabinose were purchased from Sigma Aldrich (St. Louis, MO). pCMV-VEGF was constructed as previously described [9]. Luria-Bertani (LB) and Terrific broth (TB) were purchased from Fisher Scientific (Pittsburgh, PA). pGL3-basic plasmid was purchased from Promega (Madison, WI). pβ-VEGF was constructed by inserting VEGF165 cDNA (acquired from pSV-VEGF) into pβ-Null plasmid [30] using the HindIII and XbaI restriction sites.
2.2 Precursor Plasmid Construction
Precursor plasmids including p2BADφC31-SV-VEGF, p2BADφC31-CMV-VEGF and p2BADφC31-β-VEGF (Figure 1A) were constructed for producing minicircle DNA. p2BADφC31 plasmid was a generous gift from Dr. Mark A. Kay at Stanford University. To construct p2BADφC31-SV-VEGF, VEGF cDNA with the SV40 promoter was excised from pSV-VEGF by digesting at BglII and BamHI sites, then treated with Klenow fragment for blunt-end generation. p2BADφC31 was digested with SpeI and treated with Klenow fragment as well. The blunted SV40 promoter-VEGF expression cassette was ligated to blunted linear p2BADφC31 at room temperature. Transformation was performed using DH10B competent cells (Invitrogen, Carlsbad, CA) and selected on an ampicillin (100 μg/mL) containing LB agar plate. Positive clones were verified by restriction enzyme digestion and analyzed using gel electrophoresis. The same above procedure was performed to obtain p2BADφC31-CMV-VEGF and p2BADφC31-β-VEGF.
Figure 1.
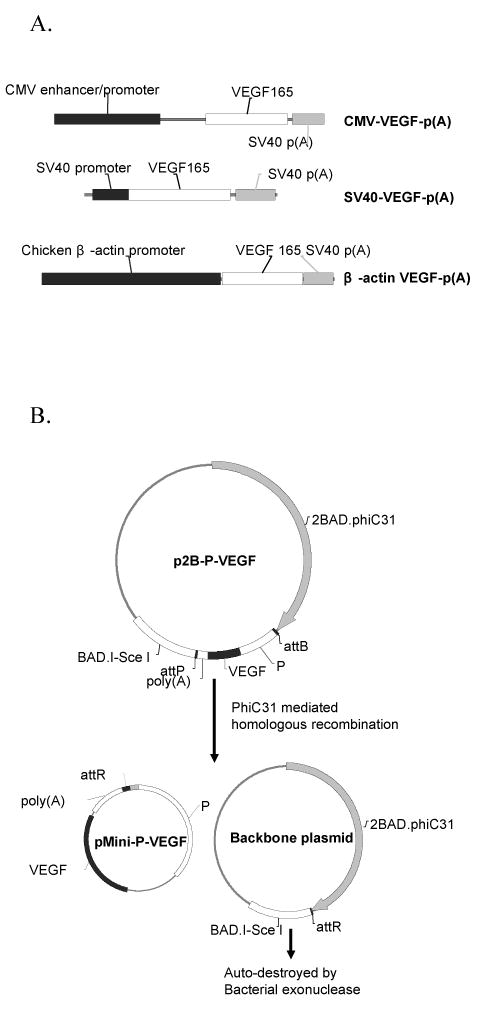
(A) Structure of VEGF expression cassettes containing three different promoters including the CMV, SV40 and chicken β-actin promoters. (B) Schematic representation of the production process of minicircle DNA encoding a VEGF expression cassette. P: promoter; p2B-P-VEGF: precursor plasmid; pMini-P-VEGF: minicircle DNA.
2.3 Induction and Purification of Minicircle DNA
The procedure for the homologous recombination reaction and the purification of minicircle DNA developed by Z.Y. Chen et al. [29] was followed with minor modifications. Five different protocols were designed to optimize the incubation conditions for producing minicircle DNA encoding VEGF. Protocol I, III & V were performed in duplicate. Step 1: a fresh single colony of E.coli TOP10 (Invitrogen, Carlsbad, CA) transformed with precursor plasmid was inoculated in designated medium (LB for Protocol I & II; TB for Protocol III, IV & V) and incubated at 37°C/250 rpm overnight (16∼18 hours). Cell pellets were obtained by centrifuging at 2800 rpm for 20 minutes at 20 °C. Step 2: fresh LB medium containing 1% (w/v) L-arabinose was added at a designated volume ratio to overnight culture medium (1:1 for Protocol I & III; 1:4 for Protocol II & IV; 1:8 for Protocol V) to re-suspend the cell pellet. The intra-bacterial φC31 integrase-mediated recombination was promoted at 32 °C for 2 hours at 250 rpm. SceI endonuclease activity was then optimized by increasing the culture temperature to 37 °C for an additional 2 hours. Plasmid purification was performed using Qiagen Maxiprep (Qiagen, Valencia, CA). As a result of recombination, the precursor plasmid splits into two smaller plasmids: backbone plasmid and minicircle DNA (Figure 1B). The SceI site containing backbone plasmid was linearized by the subsequent expressed SceI endonuclease and finally cleaned up by intracellular exonuclease. The size of minicircle DNA is reduced to at least half of its original size compared to the conventional counterpart. For example, the size of pMini-CMV-VEGF (2 kb) is decreased from a larger size of pCMV-VEGF (4.6 kb).
2.4 Calculation of Recombination and Digestion Efficiency
Upon induction with 1% arabinose, a precursor plasmid splits into two smaller plasmids including minicircle DNA and backbone plasmid. The amount of precursor plasmid or backbone plasmid depends on the activities of φC31 recombinase and SceI endonuclease. Assuming minicircle DNA was only produced from the designated recombination reaction and not subjected to SceI digestion, the efficiency for each reaction can be calculated semi-quantitatively based on the amount of minicircle DNA using the following equations:
Where,
TBM: Theoretical weight ratio of backbone plasmid /minicircle DNA
ABM: Actual weight ratio of backbone plasmid /minicircle DNA
WPb: Amount of precursor plasmid before recombination and SceI digestion
WPa: Amount of precursor plasmid after recombination and SceI digestion
The amount of each plasmid population can be quantified from gel electrophoresis analysis (Quanti-One, Bio-Rad, Hercules, CA). The molecular weight (MW) of minicircle DNA and backbone plasmid are required to calculate TBM. For example, MW of pBADφC31-CMV-VEGF is 12 kb; backbone plasmid is 10 kb, and MW of pMini-CMV-VEGF is 2 kb. Assuming the amount of minicircle DNA is X, then WPb is 6 X. WPa can be calculated by dividing the amount of precursor plasmid by the amount of minicircle DNA.
2.5 Cell Culture and In Vitro Transfection
Primary skeletal muscle (L8), mouse skeletal muscle (C2C12) and human umbilical vein endothelial cells (HUVEC) were purchased from ATCC (Rockville, MD). L8 cells were grown in culture flasks containing 10% horse serum, 5% chicken embryo extract and 20% medium 199 in DMEM medium. C2C12 cells were grown in culture flasks containing 10% fetal bovine serum (FBS) in DMEM medium. HUVEC cells were grown in culture flasks containing 10% FBS, 2 mM L-glutamine, 1.5 mg/mL sodium bicarbonate, 0.1 mg/mL heparin, and 0.04 mg/mL endothelial cell growth supplement (ECGS) in F-12K medium. Every other day, the medium was replaced until cells became 80% confluent and then subcultured using 0.25% trypsin/EDTA treatment.
For the transfection assay, conventional plasmid or minicircle DNA was complexed with branched polyethylenimine (bPEI, 25k) at a nitrogen/phosphate (N/P) ratio of 20:1. In order to maintain the same pDNA copy number for comparison, the amount of minicircle DNA was compensated by its molecular weight. For example, 0.43 μg pMini-CMV-VEGF (2 kb) was compared with 1 μg pCMV-VEGF (4.6kb). pGL3-basic plasmid was used as an empty plasmid when complexing minicircle DNA with bPEI(25k). Thirty minutes after complexion, bPEI(25k)/pDNA complex was added to transfect cells seeded at a density of 3∼5×104 cells/well in 24-wells plates. Prior to transfection, medium was replaced with serum free medium. Transfections for each group was performed in triplicate and allowed to incubate subsequently at 37 °C/5% CO2 for 4 hours. After this time, medium was replaced with 1 mL of serum containing medium.
2.6 RT-PCR
RT-PCR was performed as described previously [2]. Total RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA). The concentration of total RNA was measured at 260 nm. Reverse transcription was performed using AMV transcriptase (Promega, Madison, WI) to amplify the first cDNA strand from 2 μg of RNA. cDNA strands were further amplified with platinum pfx polymerase (Invitrogen, Carlsbad, CA). The RT-PCR of β-actin was also performed as an internal control. The sequences of primers were as follows: VEGF forward primer, 5′-CCC AAG CTT GAA ACC ATG AAC TTG CT-3′; VEGF backward primer, GCT CTA GAT CAT TCA TTC ACC GCC T-3′; β-actin forward primer, 5′-TGG AAT CCT GTG GCA TCC ATG AAA-3′; β-actin backward primer, 5′-TAA AAC GCA GCT CAG TAA CAG TCC G-3′. PCR reaction was: 45 °C for 45 minutes, 94 °C for 3 minutes, 20 cycles at 94 °C for 30 seconds, 58 °C for 30 seconds, and 72 °C for 1 minute. The PCR products were separated by electrophoresis on a 0.8 % agarose gel at 30 minutes.
2.7 In Vitro Endothelial Cell Proliferation Assay
An in vitro endothelial cell proliferation assay was performed as described previously [2]. Medium (250 μl) was collected from C2C12 cells transfected with minicircle DNA or conventional plasmid, then added to ECGS-starved HUVECs (750 μl 10% FBS/DMEM) plated at a density of 2.5 × 104 cells/well in 24-well plates. After 5 days, proliferation of HUVECs was measured using a MTT assay. Briefly, 50 μl of 5 mg/mL MTT solution was added into each well and incubated for an additional 3 hours. The medium was replaced by adding 100 μl of DMSO and the absorbance at a wavelength of 570 nm (OD570) was measured using a microplate reader (Bio-Rad, Hercules, CA). HUVEC cells without any treatment were used as the relative control. The effect of VEGF on HUVEC proliferation is calculated by dividing the corrected absorbance of sample (OD570(sample) – OD570(control)) by the absorbance of control (OD570(control)):
2.8 In Vivo Transfection
Male Balb/c mice (approximate 15∼20 g) were maintained in accordance with the guidance of the University of Utah Animal Care and Use Committee. Animals were anesthetized with 90 mg/kg ketamine and 10 mg/kg xylazine through intraperitoneal injection. An incision was made on the skin accessing the tiabialis anterior (TA) muscle. Minicircle DNA or conventional plasmid prepared in 30 μl of saline was injected into the TA muscle horizontally. After injection, the incision was sutured, and antibiotic cream was applied to prevent infection. Mice were euthanized by CO2 inhalation two days after injection. TA muscles were completely excised, weighed and homogenized with an appropriate amount (2.5 μl/mg tissue) of reporter lysis buffer (Promega, Madison, WI) containing 2 % protease inhibitor cocktail (Sigma, St. Louis, MO) in liquid nitrogen. The lysed mixtures were vortexed at room temperature for 30 ∼ 40 minutes then centrifuged at 6000 g for 15 minutes. Supernatants were collected and preserved at -70°C before further treatment.
2.9 VEGF ELISA
ELISA was performed using the Quantikine Human VEGF ELISA kit (R&D Systems, MN). Each sample (200 μl) was added into the designated wells. Similarly, VEGF standards ranging from 0.03 to 2 ng/ml were added to microwell plates. Calibrator diluent alone was added in blank wells and incubated at room temperature for 2 hours. After washing three times with wash buffer, 200 μl polyclonal antibody against VEGF conjugated to horseradish peroxidase was added and incubated at room temperature for 2 hours. After washing wells again three times, tetramethylbenzidine substrate solution was added and incubated at room temperature for 20 minutes. The enzymatic reaction was stopped by adding 50 μl of stop solution to each well, and absorbance was determined by spectrophotometric reading at 450 nm subtracting 540 nm. The VEGF concentration in samples was calculated based on a standard curve.
3. Results
3.1 Study of Minicircle DNA Production
pMini-CMV-VEGF was used for studying the process of minicircle DNA production. Minicircle DNA produced by using designated protocols was purified using Qiagen Maxiprep and analyzed by gel electrophoresis (Figure 2). Weight purity of each plasmid population was analyzed and calculated using Quanti-One program (Bio-Rad, Hercules, CA). The initial use of LB broth (Protocol I) as overnight culture medium was found to give a moderate weight purity (53.8 %) and molar purity (88.0 %). To further improve this process, it is necessary to elucidate the production process of each plasmid population. To analyze the production process of minicircle DNA, equations were derived for calculating overall efficiency, φC31-mediated recombination efficiency and SceI-mediated digestion as described in the Materials and Methods section. According to these equations, the φC31-mediated recombination efficiency was 90.7 % and SceI-mediated digestion efficiency was 97.1 % from using Protocol I (Table 1). By using TB (Protocol III), the purity of minicircle DNA was increased from 53.8 % to 72.1 % (Table 1). Since TB is an enriched culture medium and allows up to three times higher density of host cell growth, we tested three different volume ratios of 1% arabinose containing LB (1:1, 1:4 and 1:8) for re-suspending cell pellets in step 2. The purity of produced minicircle DNA was further improved from 72.1 % to 86.6 % by using a lower volume ratios (1:4 and 1:8) of re-suspended medium (Table 1).
Figure 2.
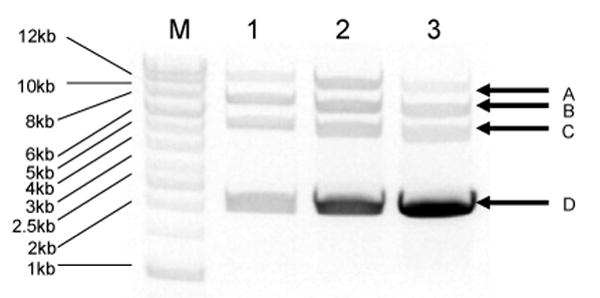
Analysis of the production of minicircle DNA (pMini-CMV-VEGF) under different culture conditions (Protocol I, III & V). Minicircle DNA was purified as described in the Materials and Methods. Purified DNA was digested with EcoRV and Spe1. After digestion, backbone plasmid was linearized (10 kb, arrow A), p2B-CMV-VEGF was cut into two linear fragments: (6.5 kb, arrow B) and (5.1 kb, arrow C), and mini-CMV-VEGF was linearized (2.1 kb, arrow D). M: DNA ladder (1kb); 1: Protocol I; 2: Protocol III; 3: Protocol V.
Table 1.
Summary of reaction efficiency for minicircle DNA production. The amount of each plasmid population can be quantified from gel electrophoresis analysis (Quanti-One, Bio-Rad, Hercules, CA). The reaction efficiency was calculated using the equations in the Materials and Methods.
| Batch number | Precursor plasmid (%) | Backbone plasmid (%) | Minicircle DNA (%) | PhiC31-mediated recombination efficiency (%) | SceI-mediated digestion efficiency (%) | Overall efficiency (%) | ||
|---|---|---|---|---|---|---|---|---|
| Protocol I | LB 1:1 | 0116 | 39.9 | 7.6 | 52.5 | 89.9 | 97.1 | 87.3 |
| 0503 | 36.9 | 8.0 | 55.1 | 91.5 | 97.1 | 88.8 | ||
| Protocol II | LB 1:4 | 0503 | 29.9 | 7.8 | 62.3 | 94.3 | 97.5 | 92.0 |
| Protocol III | TB 1:1 | 0116 | 21.1 | 8.4 | 70.5 | 97.3 | 97.6 | 95.0 |
| 0503 | 17.4 | 8.8 | 73.7 | 98.4 | 97.6 | 96.1 | ||
| Protocol IV | TB 1:4 | 0503 | 13.2 | 2.6 | 84.1 | 98.0 | 99.4 | 97.4 |
| Protocol V | TB 1:8 | 0116 | 9.5 | 1.7 | 89.0 | 98.5 | 99.7 | 98.2 |
| 0503 | 11.5 | 4.3 | 84.2 | 98.7 | 99.0 | 97.7 | ||
3.2 Expression of VEGF Protein by pMini-SV-VEGF in Mouse and Rat Skeletal Muscle Cell Line (C2C12 and L8 Respectively)
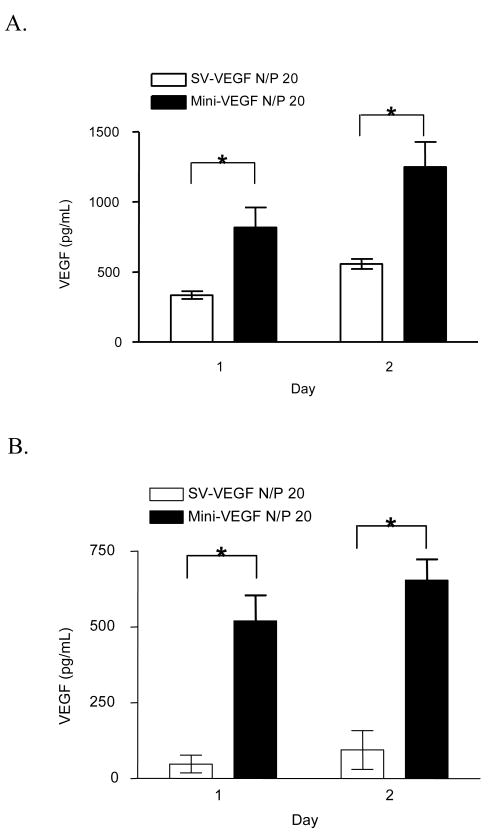
The SV40 promoter has been used for transgene expression in skeletal muscle [31] and myocardial muscle [2]. bPEI (25k) was used to deliver pMini-SV-VEGF and pSV-VEGF into skeletal muscle cell culture. The same copy number of each plasmid type was compared for VEGF production. The results (Figure 3) show that VEGF expression from minicircle DNA was 2 to 8 times higher than conventional plasmid in mouse or rat skeletal muscle cell cultures, respectively.
Figure 3.
VEGF gene expression after transfecting skeletal muscle cells with pMini-SV-VEGF or pSV-VEGF. The comparison was performed by using 1 μg pSV-VEGF or 0.25 μg pMini-SV-VEGF combining with 0.75 μg pGL3-basic (empty plasmid) on 4 × 104 cells per well. (A) L8 rat skeletal muscle cell. (B) C2C12 mouse skeletal muscle cell. Data represents the mean ± SEM (n = 3). Statistical analysis was done using Student's t-test. * p<0.05 compared to pSV-VEGF.
3.3 Expression of VEGF mRNA by Different Plasmid Constructs in Mouse Skeletal Muscle Cell Line (C2C12)
The higher transgene expression by minicircle DNA was also confirmed by comparing VEGF mRNA expression from transfected cells. RT-PCR was used as a specific and semi-quantitative method to evaluate the amount of VEGF mRNA expression. From the results in Figure 4, amplified VEGF cDNA (∼600 bp) from pMini-SV-VEGF transfected cells (lane 1 & 2) was higher than pSV-VEGF transfected cells (lane 3 & 4) and no VEGF cDNA could be detected from the control group (lane 5 & 6).
Figure 4.
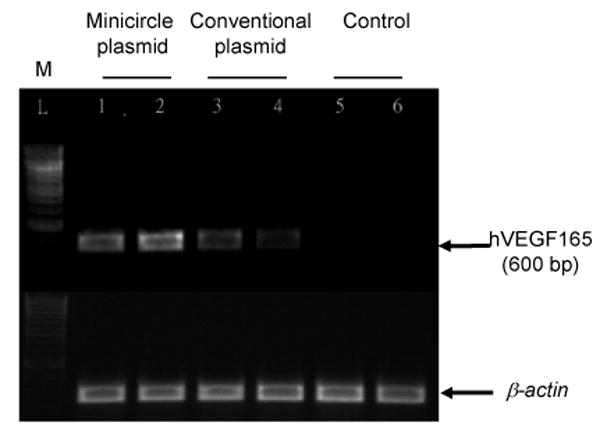
Detection of VEGF mRNA expression after transfecting C2C12 cells with pMini-SV-VEGF or pSV-VEGF. Forty eight hours after transfection, total RNA was extracted and VEGF mRNA level was measured by RT-PCR. Detection of β-actin was used as the control.
3.4 VEGF Expression from Minicircle DNA with Different Promoters in Skeletal Muscle Cell Line
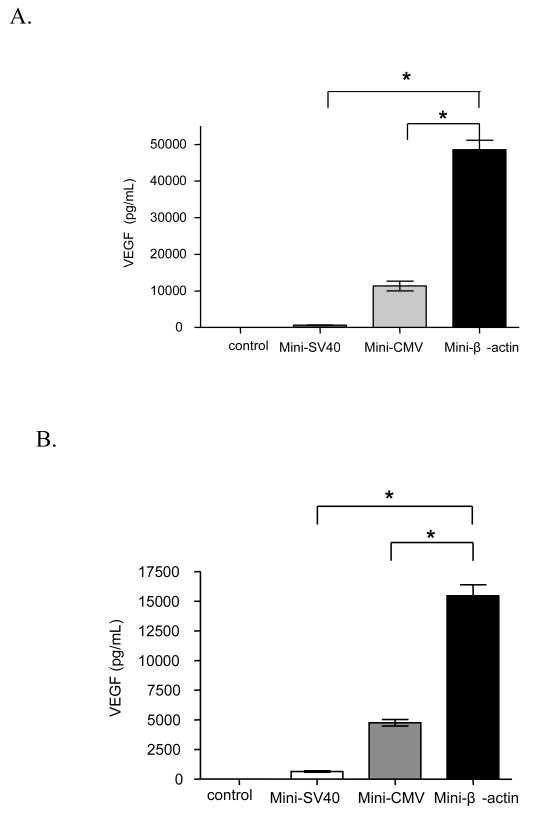
In this study, we compared VEGF expression by the SV40 promoter with two other commonly used promoters to obtain efficient VEGF expression from minicircle DNA in skeletal muscle cell line. The chicken β-actin promoter has been used to efficiently express GLP-1 [30] and adiponectin [28] in vivo. From the results (Figure 5A, B), VEGF protein expression driven by the chicken β-actin promoter was higher than the CMV promoter and the SV40 promoter in C2C12 skeletal muscle cells 48 or 72 hours after transfection. In addition, enhanced VEGF expression was observed from cells transfected by minicircle DNA with the chicken β-actin promoter or CMV promoter compared to their respective conventional plasmid (Figure 5C).
Figure 5.
VEGF protein expression after transfecting C2C12 cells with pMini-SV-VEGF, pMini-CMV-VEGF or pMini-β-VEGF. C2C12 cells without transfection were used as control group. (A) Forty eight hours after transfection. (B) Seventy two hours after transfection. (C) Comparison of VEGF protein expression between minicircle DNA and conventional plasmid under the CMV or chicken β-actin promoter. Data represents mean ± SEM (n = 3). Statistical analysis was done using one-way ANOVA and a Tukey's multiple comparison post-test. * p<0.05.
3.5 In Vitro Endothelial Cell Proliferation Assay
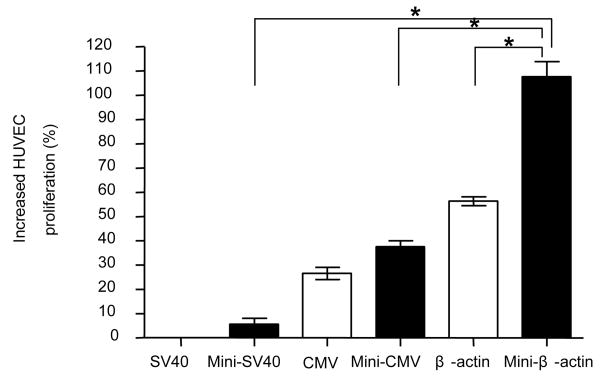
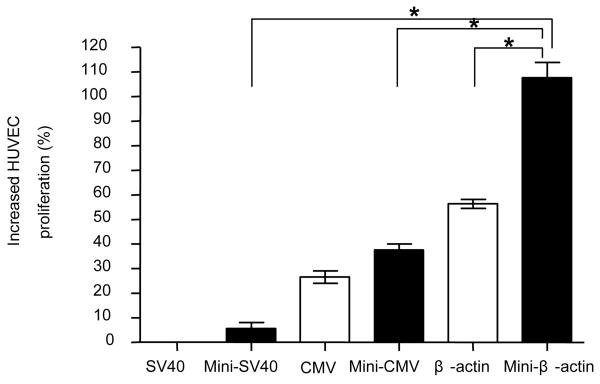
The advantage of achieving higher expression of VEGF by minicircle DNA was further investigated using an endothelial cell proliferation assay (Figure 6). Two days after muscle cells were transfected with VEGF pDNA, VEGF protein was secreted into 10% FBS/DMEM medium. The VEGF protein containing medium was collected and added to ECGS starving endothelial cells. According to our preliminary study, 10 % FBS/DMEM does not enhance the proliferation of endothelial cells (data not shown). Therefore, the observed proliferation will be proportional to the amount of added VEGF protein. Five days after adding the VEGF protein containing medium from pMini-β-VEGF transfected cells, the number of endothelial cells was greater than cells receiving medium from pβ-VEGF transfected cells (Figure 6).
Figure 6.
In vitro endothelial cell proliferation assay. Forty eight hours after transfecting VEGF plasmid into C2C12 cells, the VEGF containing medium was added for HUVEC cell incubation. Five days after incubation, the cell population was measured by a MTT assay. Data represents mean ± SEM (n = 3). Statistical analysis was done using one-way ANOVA and a Tukey's multiple comparison post-test. * p<0.05 is considered as statistical difference.
3.6 In Vivo Transfection
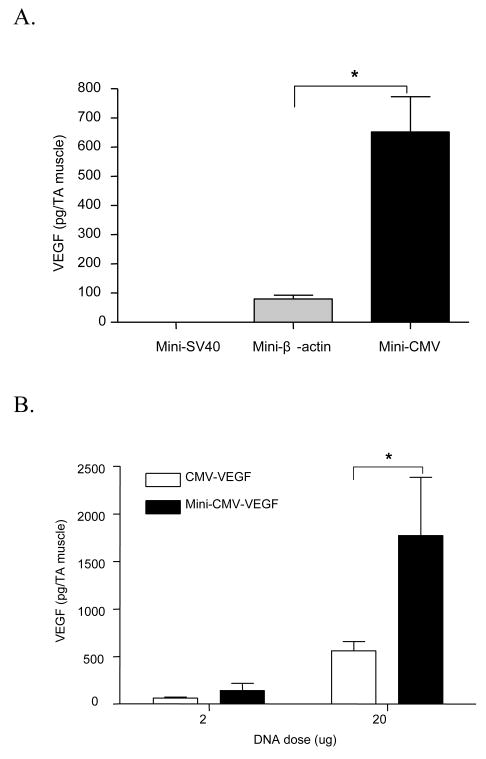
Minicircle DNA or conventional plasmid was injected into TA muscle of mice. It has been shown previously that the peak time for VEGF expression is two days after injection [9]. In order to make an in vivo comparison, the expressed VEGF protein was extracted from muscle tissue two days after injection and quantified using ELISA. The order of VEGF expression by different promoters in minicircle DNA was: CMV> chicken β-actin > SV40 (Figure 7A). Subsequently, minicircle DNA or conventional plasmid with the CMV promoter was compared for VEGF expression at two different doses (2 and 20 μg). Results (Figure 7B) show that VEGF expression was three times higher with pMini-CMV-VEGF compared to conventional plasmid.
Figure 7.
In vivo transfection of minicircle DNA. Minicircle DNA or conventional plasmid was injected into TA muscle of mice. Two days after injection, the amount of VEGF in muscle was quantitated by ELISA. (A) Comparison of transcriptional activity of three different promoters: the SV40, chicken β-actin and CMV promoters in minicircle DNA. Ten μg of each minicircle DNA was injected into TA muscle for comparison. (B) Comparison of VEGF expression between minicircle DNA and conventional plasmid. Data represents mean ± SEM (n ≥ 6). Statistical analysis was done using Student t-test. * p<0.05 is considered as statistical difference.
4. Discussion
Skeletal muscle is an immunogenic tissue [32] and is currently being studied as a gene vaccination target [25, 33]. After injecting into skeletal muscle, unmethylated CpG motifs containing oligonucleotides or pDNA induces both immune [34] and inflammatory responses [35] accompanied with the secretion of IFN-γ which has been shown to strongly attenuate transgene expression from pDNA [36]. The use of minicircle DNA may reduce transgene silencing by eliciting lower immune responses compared to conventional plasmid. Also, the smaller size of minicircle DNA may contribute to a better extracellular and intracellular diffusivity [37] thus increasing its availability for transcription and translation processes.
The reaction efficiency for producing minicircle DNA from precursor plasmid depends on at least three factors including (1) size of expression cassette in minicircle DNA, (2) viability or activity of transformed host cells and (3) type of recombinant protein overexpressed in the host cells. First, the small size of VEGF expression cassette presents a significant problem for obtaining high weight purity of minicircle DNA. The short length of the VEGF expression cassette (1.1∼2.2 kb in this study) between two recombination sites (attP and attB) may also affect the recombination efficiency. Second, assuming neither φC31 mediated recombination nor SceI mediated digestion in dead host cells, a small decrease (i.e. 10 %) of host cell viability or activity will result in a significant decrease on weight purity (40 %). This is due to the low molecular weight of minicircle DNA (2 kb for pMini-CMV-VGF) compared to the larger molecular weight precursor (12 kb) or backbone plasmid (10 kb). For the effect of transgene product from pDNA in host cells, it has been shown that overexpression of recombinant proteins often results in serious metabolic changes and growth retardation [38]. All these factors discussed above may attribute to the production of minicircle DNA with low weight purity using Protocol I.
By using Protocol I, minicircle DNA with low weight purity (53.8 %) and moderate molar purity (88.0 %) was obtained. Assuming the precursor and backbone plasmid are equally susceptible to the degradation by SceI, a higher weight ratio (3:1) of precursor plasmid to backbone plasmid indicated that a significant portion of host cells in which precursor plasmids never initiated the recombination process. This speculation is also supported by obtaining minicircle DNA with higher SceI digestion efficiency (97.1 %) and lower φC31 recombination efficiency (90.7 %). We hypothesize that the decrease of host cell viability or activity is responsible for this observation. To increase the cell viability or activity, TB was used for overnight culture. TB has been discovered as an enriched medium for host cell (E.coli) growth to achieve better cell growth with higher plasmid yield. By using TB as the overnight culture medium, the recombination efficiency was increased from 90.7 % to 98.6 %. In TB, a favorable culture environment was provided for host cell growth and enzyme expression and activity. As a result, the weight purity of minicircle DNA was increased from 53.8 % (Protocol I) to 86.6 % (Protocol V).
For the in vitro transfection studies, bPEI(25k) was used as a cationic carrier to enhance gene transfection efficiency in C2C12 cell culture. Higher expression of VEGF on both protein and mRNA levels were observed from minicircle DNA compared to conventional plasmids under different circumstances including different skeletal muscle cell lines and different days after transfection. The chicken β-actin promoter in minicircle DNA was found to show the highest VEGF expression among the promoters we tested in vitro. However, in skeletal muscle tissue, the CMV promoter showed higher VEGF expression compared to the chicken β-actin promoter. We speculate that the discrepancy of promoter comparison in vitro and in vivo might be due to the different genetic environment between immortal C2C12 myoblast culture and differentiated myotubes (muscle tissue). It has been reported that transcriptional activity of a promoter can be regulated by the differentiation status of muscle cells [39]. Our results support the concept that in vivo evaluation of promoter activity is crucial and may not be substituted by in vitro assay as suggested by other individuals [40].
VEGF-mediated therapeutic angiogenesis provides an effective alternative compared to conventional approaches such as surgical bypass and angioplasty. VEGF has been discovered as a survival and growth promotion factor for vascular endothelial cell proliferation under hypoxic conditions [41]. Histological chemistry studies have shown a correlation between increases of capillary density and recovery of blood perfusion [42, 43]. In this study, HUVEC cells were used due to their resemblance to vascular endothelial cells [2]. By stimulation with higher VEGF expressed from minicircle DNA, we observed a dramatic increase of HUVEC cell proliferation, which is an essential step for new capillary growth. It has been reported that VEGF expression in skeletal muscle can significantly stimulate the local blood perfusion. In this study, three times higher VEGF expression with minicircle DNA compared to conventional plasmid was observed in mouse skeletal muscle. The efficient in vivo VEGF expression from minicircle DNA holds great potential for the treatment of ischemia.
5. Conclusion
We have successfully cloned and purified minicircle DNA encoding the VEGF gene. Equations were derived to analyze the limiting step(s) for minicircle DNA production to optimize the production of minicircle DNA encoding VEGF expression cassettes specifically. The superior VEGF expression from minicircle DNA was demonstrated at the level of both transcription and translation levels in skeletal muscle cells. The potential of using minicircle DNA to stimulate vascular growth was evaluated on an in vitro endothelial cell proliferation model. The greater expression of VEGF from minicircle DNA resulted in a greater proliferation of endothelial cells. Furthermore, higher VEGF expression with minicircle DNA compared to conventional plasmid was observed in mouse skeletal muscle. To conclude, minicircle DNA is a potentially useful gene vector for therapeutic angiogenesis-based gene therapy.
Acknowledgments
This work was supported by NIH grant HL 65477. We thank Dr. Mark A. Kay for providing p2BADφC31 plasmid. We thank Dr. Zhi-Ying Chen, Dr. James W. Yockman and Dr. Jeong Hyun Park for their valuable suggestions on minicircle DNA purification.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim WJ, Yockman JW, Lee M, Jeong JH, Kim YH, Kim SW. Soluble Flt-1 gene delivery using PEI-g-PEG-RGD conjugate for anti-angiogenesis. J Control Release. 2005;106(12):224–234. doi: 10.1016/j.jconrel.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Lee M, Rentz J, Bikram M, Han S, Bull DA, Kim SW. Hypoxia-inducible VEGF gene delivery to ischemic myocardium using water-soluble lipopolymer. Gene Ther. 2003;10(18):1535–1542. doi: 10.1038/sj.gt.3302034. [DOI] [PubMed] [Google Scholar]
- 3.Foster K, Foster H, Dickson JG. Gene therapy progress and prospects: Duchenne muscular dystrophy. Gene Ther. 2006;13(24):1677–1685. doi: 10.1038/sj.gt.3302877. [DOI] [PubMed] [Google Scholar]
- 4.Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow ST, Passeri J, Horowitz JR, Symes JF, Isner JM. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation. 1996;94(12):3281–3290. doi: 10.1161/01.cir.94.12.3281. [DOI] [PubMed] [Google Scholar]
- 5.Tsurumi Y, Kearney M, Chen D, Silver M, Takeshita S, Yang J, Symes JF, Isner JM. Treatment of acute limb ischemia by intramuscular injection of vascular endothelial growth factor gene. Circulation. 1997;96 9:II-382–388. [PubMed] [Google Scholar]
- 6.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation. 2004;109(21):2487–2491. doi: 10.1161/01.CIR.0000128595.79378.FA. [DOI] [PubMed] [Google Scholar]
- 7.Collinson DJ, Donnelly R. Therapeutic angiogenesis in peripheral arterial disease: can biotechnology produce an effective collateral circulation? Eur J Vasc Endovasc Surg. 2004;28(1):9–23. doi: 10.1016/j.ejvs.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Rissanen TT, Vajanto I, Yla-Herttuala S. Gene therapy for therapeutic angiogenesis in critically ischaemic lower limb - on the way to the clinic. Eur J Clin Invest. 2001;31(8):651–666. doi: 10.1046/j.1365-2362.2001.00864.x. [DOI] [PubMed] [Google Scholar]
- 9.Chang CW, Choi D, Kim WJ, Yockman JW, Christensen LV, Kim YH, Kim SW. Non-ionic amphiphilic biodegradable PEG-PLGA-PEG copolymer enhances gene delivery efficiency in rat skeletal muscle. J Control Release. 2007;118(2):245–253. doi: 10.1016/j.jconrel.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Zhu J, Sriadibhatla S, Gebhart C, Alakhov V, Kabanov A. Promoter- and strain-selective enhancement of gene expression in a mouse skeletal muscle by a polymer excipient Pluronic P85. J Control Release. 2005;108(23):496–512. doi: 10.1016/j.jconrel.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Trollet C, Bloquel C, Scherman D, Bigey P. Electrotransfer into skeletal muscle for protein expression. Curr Gene Ther. 2006;6(5):561–578. doi: 10.2174/156652306778520656. [DOI] [PubMed] [Google Scholar]
- 12.Schertzer JD, Plant DR, Lynch GS. Optimizing plasmid-based gene transfer for investigating skeletal muscle structure and function. Mol Ther. 2006;13(4):795–803. doi: 10.1016/j.ymthe.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Miyoshi H, Nakamura M. Encapsulated ultrasound microbubbles: therapeutic application in drug/gene delivery. J Control Release. 2006;114(1):89–99. doi: 10.1016/j.jconrel.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 14.van Gaal EV, Hennink WE, Crommelin DJ, Mastrobattista E. Plasmid engineering for controlled and sustained gene expression for nonviral gene therapy. Pharm Res. 2006;23(6):1053–1074. doi: 10.1007/s11095-006-0164-2. [DOI] [PubMed] [Google Scholar]
- 15.Xu ZL, Mizuguchi H, Ishii-Watabe A, Uchida E, Mayumi T, Hayakawa T. Optimization of transcriptional regulatory elements for constructing plasmid vectors. Gene. 2001;272(12):149–156. doi: 10.1016/s0378-1119(01)00550-9. [DOI] [PubMed] [Google Scholar]
- 16.Dai Y, Roman M, Naviaux RK, Verma IM. Gene therapy via primary myoblasts: long-term expression of factor IX protein following transplantation in vivo. Proc Natl Acad Sci U S A. 1992;89(22):10892–10895. doi: 10.1073/pnas.89.22.10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, MacLaughlin FC, Fewell JG, Gondo M, Wang J, Nicol F, Dean DA, Smith LC. Muscle-specific enhancement of gene expression by incorporation of SV40 enhancer in the expression plasmid. Gene Ther. 2001;8(6):494–497. doi: 10.1038/sj.gt.3301419. [DOI] [PubMed] [Google Scholar]
- 18.Choi BH, Ha Y, Ahn CH, Huang X, Kim JM, Park SR, Park H, Park HC, Kim SW, Lee M. A hypoxia-inducible gene expression system using erythropoietin 3′ untranslated region for the gene therapy of rat spinal cord injury. Neurosci Lett. 2006 doi: 10.1016/j.neulet.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Lee M, Choi D, Choi MJ, Jeong JH, Kim WJ, Oh S, Kim YH, Bull DA, Kim SW. Hypoxia-inducible gene expression system using the erythropoietin enhancer and 3′-untranslated region for the VEGF gene therapy. J Control Release. 2006;115(1):113–119. doi: 10.1016/j.jconrel.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Darquet AM, Rangara R, Kreiss P, Schwartz B, Naimi S, Delaere P, Crouzet J, Scherman D. Minicircle: an improved DNA molecule for in vitro and in vivo gene transfer. Gene Ther. 1999;6(2):209–218. doi: 10.1038/sj.gt.3300816. [DOI] [PubMed] [Google Scholar]
- 21.Schakowski F, Gorschluter M, Junghans C, Schroff M, Buttgereit P, Ziske C, Schottker B, Konig-Merediz SA, Sauerbruch T, Wittig B, Schmidt-Wolf IG. A novel minimal-size vector (MIDGE) improves transgene expression in colon carcinoma cells and avoids transfection of undesired DNA. Mol Ther. 2001;3(5 Pt 1):793–800. doi: 10.1006/mthe.2001.0322. [DOI] [PubMed] [Google Scholar]
- 22.Tan Y, Li S, Pitt BR, Huang L. The inhibitory role of CpG immunostimulatory motifs in cationic lipid vector-mediated transgene expression in vivo. Hum Gene Ther. 1999;10(13):2153–2161. doi: 10.1089/10430349950017149. [DOI] [PubMed] [Google Scholar]
- 23.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 24.Krieg AM. Direct immunologic activities of CpG DNA and implications for gene therapy. J Gene Med. 1999;1(1):56–63. doi: 10.1002/(sici)1521-2254(199901/02)1:1<56::aid-jgm5>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Coban C, Ishii KJ, Gursel M, Klinman DM, Kumar N. Effect of plasmid backbone modification by different human CpG motifs on the immunogenicity of DNA vaccine vectors. J Leukoc Biol. 2005;78(3):647–655. doi: 10.1189/jlb.1104627. [DOI] [PubMed] [Google Scholar]
- 26.Yew NS, Zhao H, Przybylska M, Wu IH, Tousignant JD, Scheule RK, Cheng SH. CpG-depleted plasmid DNA vectors with enhanced safety and long-term gene expression in vivo. Mol Ther. 2002;5(6):731–738. doi: 10.1006/mthe.2002.0598. [DOI] [PubMed] [Google Scholar]
- 27.Reyes-Sandoval A, Ertl HC. CpG methylation of a plasmid vector results in extended transgene product expression by circumventing induction of immune responses. Mol Ther. 2004;9(2):249–261. doi: 10.1016/j.ymthe.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Park JH, Lee M, Kim SW. Non-viral adiponectin gene therapy into obese type 2 diabetic mice ameliorates insulin resistance. J Control Release. 2006;114(1):118–125. doi: 10.1016/j.jconrel.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Chen ZY, He CY, Kay MA. Improved production and purification of minicircle DNA vector free of plasmid bacterial sequences and capable of persistent transgene expression in vivo. Hum Gene Ther. 2005;16(1):126–131. doi: 10.1089/hum.2005.16.126. [DOI] [PubMed] [Google Scholar]
- 30.Oh S, Lee M, Ko KS, Choi S, Kim SW. GLP-1 gene delivery for the treatment of type 2 diabetes. Mol Ther. 2003;7(4):478–483. doi: 10.1016/s1525-0016(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 31.Wells DJ, Goldspink G. Age and sex influence expression of plasmid DNA directly injected into mouse skeletal muscle. FEBS Lett. 1992;306(23):203–205. doi: 10.1016/0014-5793(92)81000-c. [DOI] [PubMed] [Google Scholar]
- 32.Wiendl H, Hohlfeld R, Kieseier BC. Immunobiology of muscle: advances in understanding an immunological microenvironment. Trends Immunol. 2005;26(7):373–380. doi: 10.1016/j.it.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Corr M, Lee DJ, Carson DA, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184(4):1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells KE, Maule J, Kingston R, Foster K, McMahon J, Damien E, Poole A, Wells DJ. Immune responses, not promoter inactivation, are responsible for decreased long-term expression following plasmid gene transfer into skeletal muscle. FEBS Lett. 1997;407(2):164–168. doi: 10.1016/s0014-5793(97)00329-3. [DOI] [PubMed] [Google Scholar]
- 35.McMahon JM, Wells KE, Bamfo JE, Cartwright MA, Wells DJ. Inflammatory responses following direct injection of plasmid DNA into skeletal muscle. Gene Ther. 1998;5(9):1283–1290. doi: 10.1038/sj.gt.3300718. [DOI] [PubMed] [Google Scholar]
- 36.Qin L, Ding Y, Pahud DR, Chang E, Imperiale MJ, Bromberg JS. Promoter attenuation in gene therapy: interferon-gamma and tumor necrosis factor-alpha inhibit transgene expression. Hum Gene Ther. 1997;8(17):2019–2029. doi: 10.1089/hum.1997.8.17-2019. [DOI] [PubMed] [Google Scholar]
- 37.Lukacs GL, Haggie P, Seksek O, Lechardeur D, Freedman N, Verkman AS. Size-dependent DNA mobility in cytoplasm and nucleus. J Biol Chem. 2000;275(3):1625–1629. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]
- 38.Soriano E, Borth N, Katinger H, Mattanovich D. Optimization of recombinant protein expression level in Escherichia coli by flow cytometry and cell sorting. Biotechnol Bioeng. 2002;80(1):93–99. doi: 10.1002/bit.10353. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Capetanaki Y. Regulation of the mouse desmin gene: transactivated by MyoD, myogenin, MRF4 and Myf5. Nucleic Acids Res. 1993;21(2):335–343. doi: 10.1093/nar/21.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnhart KM, Hartikka J, Manthorpe M, Norman J, Hobart P. Enhancer and promoter chimeras in plasmids designed for intramuscular injection: a comparative in vivo and in vitro study. Hum Gene Ther. 1998;9(17):2545–2553. doi: 10.1089/hum.1998.9.17-2545. [DOI] [PubMed] [Google Scholar]
- 41.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 42.Taniyama Y, Morishita R, Aoki M, Nakagami H, Yamamoto K, Yamazaki K, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat and rabbit hindlimb ischemia models: preclinical study for treatment of peripheral arterial disease. Gene Ther. 2001;8(3):181–189. doi: 10.1038/sj.gt.3301379. [DOI] [PubMed] [Google Scholar]
- 43.Namba T, Koike H, Murakami K, Aoki M, Makino H, Hashiya N, Ogihara T, Kaneda Y, Kohno M, Morishita R. Angiogenesis induced by endothelial nitric oxide synthase gene through vascular endothelial growth factor expression in a rat hindlimb ischemia model. Circulation. 2003;108(18):2250–2257. doi: 10.1161/01.CIR.0000093190.53478.78. [DOI] [PubMed] [Google Scholar]