Abstract
Previous studies suggest that lipid peroxidation byproducts, such as 4-hydroxynonenal (HNE) and 4-oxo-2-nonenal (ONE), induces cell death in a wide variety of cell types, partly by modulating intracellular signaling pathways. However, the specific mechanisms involved, particularly for ONE, are unclear while c-Jun N-terminal kinase (JNK) has been shown to be essential in HNE-mediated cytotoxicity. In this study, we examined the role of mitogen-activated protein kinases (MAPK) signaling pathways in ONE-induced cytotoxicity in SH-SY5Y human neuroblastoma cells and found that ONE strongly induces the phosphorylation of extracellular signal-regulated kinase (ERK) and JNK, but no change in p38 MAPK. Interestingly, a transient exposure of the cells to ONE resulted in cell death, which contrasts with HNE-mediated toxicity. Importantly, blocking the ERK pathway, but not the JNK pathway, protected cells against ONE-induced cytotoxicity indicating a striking difference between the ONE-mediated cytotoxicity mechanism and that of HNE. Furthermore, inhibition of ERK reduced ONE-induced phosphorylation of p53, a key modulator of the cellular stress response, and the proteolytic cleavage of poly (ADP-ribose) polymerase (PARP), a hallmark of apoptosis. Overall, these data strongly suggest that ERK plays an essential role for ONE-mediated cytotoxicity and that ERK is an upstream component of p53-mediated apoptosis.
Keywords: cytotoxicity, lipid peroxidation, mitogen-activated protein kinase, neurodegeneration, oxidative stress, 4-oxo-2-nonenal, 4-hydroxynonenal, protein modification
Introduction
The brain is a high oxygen-consuming organ and constantly exposed to oxidative stress. In addition, since the brain contains high concentrations of polyunsaturated fatty acids and has a relatively weak antioxidative capacity, it is particularly vulnerable to oxidative stress (Halliwell et al. 1992, Facchinetti et al. 1998). Polyunsaturated lipid in lipoproteins and membranes are particularly susceptible to oxidative stress damage, leading to the production of several reactive aldehydes such as 4-hydroxynonenal (HNE) and 4-oxo-2-nonenal (ONE) (Esterbauer et al. 1991, Lee & Blair 2000, Lee et al. 2001), which are capable of modifying protein (Sayre et al. 2001) and DNA (Marnett et al. 2003). Indeed, there is substantial evidence for increased lipoperoxidation in neurodegenerative disease associated with oxidative stress, such as Alzheimer disease and Parkinson disease (Yoritaka et al. 1996, Sayre et al. 1997, Takeda et al. 2000).
It is relatively well recognized that HNE-mediated cytotoxicity involves the mitogen-activated protein kinases (MAPK) pathway (Tamagno et al. 2005, Song et al. 2001). MAPK includes extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase/stress-regulated protein kinase (JNK/SAPK), and p38 MAPK, which are involved in many cellular functions, ranging from proliferation to differentiation and apoptosis (Chang & Karin 2001). In various cell types, JNK and p38 MAPK are frequently associated with the induction of apoptosis while the ERK pathway is mainly induced in response to mitogens and growth factors and plays a major role in regulating cell growth, survival, and differentiation (Xia et al. 1995, Cobb 1999). However, ERK activation can also contribute to apoptosis induced by H2O2 in oligodendrocytes (Bhat & Zhang 1999, Brand et al. 2001), mesanglial cells (Ishikawa & Kitamura 2000), and SH-SY5Y neuroblastoma cells (Kelicen et al. 2002).
In previous studies, HNE appears to markedly upregulate the activity of JNK and p38 kinase and, importantly, blocking of these pathways prevents cell death (Tamagno et al. 2005). HNE has also been shown to upregulate the transcription factor activator protein 1 followed by the activation of JNK rather than ERK (Uchida et al. 1999, Parola et al. 1998) in all cell types tested, including myofibroblasts, hepatocytes, neuronal cells (Camandola et al. 2000, Tamagno et al. 2005), and cells of the macrophage lineage (Poli et al. 2004).
While the majority of the previous studies focus on HNE, ONE has recently been reported to be more reactive with protein (Sayre et al. 2006) and DNA (Lee & Blair 2001, Pollack et al. 2006) and is more cytotoxic than HNE (Lin et al. 2005, Shibata et al. 2006), emphasizing the importance of ONE in pathogenic mechanisms. ONE is causally involved in broad pathophysiological effects associated with oxidative stress in cells and tissues in vivo (West et al. 2004, Shibata et al. 2006). Furthermore, ONE adducts have been shown to accumulate in the spinal cord motor neurons of patients with amyotrophic lateral sclerosis (Shibata et al. 2006). However, the mechanisms of ONE-mediated cytotoxicity remain to be elucidated. Specifically, while JNK is a known key player in HNE-mediated toxicity, it is unclear whether the same mechanism(s) are involved in ONE-mediated cytotoxicity. In this regard, it is very interesting to note that Shibata and colleagues (Shibata et al. 2006) recently identified ONE, but not HNE, as a selective inducer of the activation of p53 in SH-SY5Y neuroblastoma cells. These data strongly suggest that ONE, despite its structural similarity to HNE, may exert its cytotoxicity through a different apoptotic mechanism than that used by HNE (i.e., JNK signal pathway). Therefore, in the present study, to gain insights into the mechanisms of cytotoxic response by ONE, we investigated the involvement of MAPK pathways involved in ONE-mediated cytotoxicity in SH-SY5Y human neuroblastoma cells and their relationship with p53 signaling pathway.
Materials and Methods
Chemicals and reagents
U0126 was obtained from Cell Signaling Technology (Beverly, MA). SB203580 and SP600125 were obtained from Calbiochem (San Diego, CA). Antibodies against phosphorylated ERK1/2 (Thr202/Tyr204), p38 MAPK (Thr180/Tyr182), JNK1/2 (Thr183/Tyr185), p53 (Ser15), total ERK1/2 and cleaved poly (ADP-ribose) polymerase (PARP) were purchased from Cell Signaling Technology (Beverly, MA). The anti-plain p38 MAPK and JNK1/2 were obtained from the StressGen Biotechnology (Victoria, BC). The anti-Actin antibody was obtained from Sigma (Saint Louis, MO). ONE and HNE were prepared as described in our previous studies (Nadkarni & Sayre 1995, Zhang et al. 2003).
Cell culture and cytotoxicity assays
SH-SY5Y human neuroblastoma cells were maintained in Opti-MEM media (Life Technologies, Gaithersberg, MD) with 5% fetal bovine serum and 1% penicillin/streptomycin with fungizone (Life Technologies). For cytotoxicity in SH-SY5Y, the cells were seeded in a density of 2×104 cells/well on to 96 well plates and cultured overnight for lactate dehydrogenase (LDH) assay. For ONE treatment, the cell culture medium was switched to serum-free Opti-MEM containing different concentrations of ONE or HNE. The cytotoxicity of ONE and HNE was evaluated by the LDH assay kit (Roche Diagnostic, Indianapolis, IN), according to the manufacturer’s instructions. Briefly, cell media were collected after each treatment and the collected media were mixed with LDH substrate in a 96 well plate. After incubation for 30 min at room temperature, the optical density was measured at 490 nm using a microplate reader (Molecular Devices, Sunnyvale, CA). The measured optical density was converted after standardization with low (no treatment; 0% toxicity) and high controls (1% Triton X-100; 100% toxicity) by the following equation: cytotoxicity (%) = [(experimental value-low control)/(high control-low control)]×100.
Western blot analysis
Cells were washed twice with ice cold phosphate-buffered saline without Ca2+ and Mg2+ and then suspended in a lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM sodium chloride, 1% Triton, 1 mM EGTA, 1 mM EDTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 μg/ml leupeptin, 1 mM PMSF). After centrifugation at 10,000 ×g for 10 min, equal amounts of cellular protein lysates were separated by SDS-PAGE and electrophoretically transferred to PVDF membranes (Millipore, Billerica, MA). Following treatment with 10% nonfat milk at room temperature for 1 hour, the membranes were probed with each antibody at 4°C for overnight followed by horseradish peroxidase-conjugated anti-rabbit or mouse IgG secondary antibodies (Cell Signaling Technology, Beverly, MA). Bound antibodies were visualized by chemiluminescence detection on autoradiographic film. For quantitative analysis of the immunoblot bands, the densities of the bands were measured by scanning densitometry (BioRad, Hercules, CA). The densitometric data were presented as mean ± SD of values obtained for four controls versus four experimental samples.
JNK kinase assay
For the JNK kinase assay, cell lysates were prepared as described previously, and JNK activity was determined using a JNK assay kit according to the manufacturer’s instruction (Cell Signaling Technology, Beverly, MA). Briefly, GST-c-Jun (amino acids 1-89) fusion protein bound to glutathione-sepharose beads was incubated with cell lysates and allowed to react on a rotating rocker for 2 hours at 4°C. The reactants were centrifugated at 10,000 g for 15 min to pull down JNK. After washing with 1× kinase assay buffer (kit component), the samples were resuspended in 50 μL 1× kinase assay buffer containing 200 μM ATP for 30 min at 30°C, subjected to SDS-PAGE and transferred to a PVDF membrane. Kinase activity was analyzed by western blotting using rabbit anti-phospho-c-Jun antibody.
Statistical analysis
The significance of difference between experimental values was determined by Student’s t-test (two tailed, paired t). Values were considered significantly different at p < 0.05.
Results
ONE activates ERK1/2, JNK but not p38 MAPK
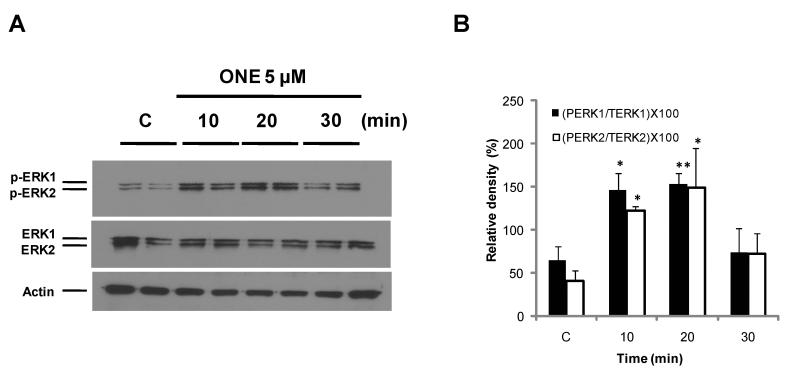
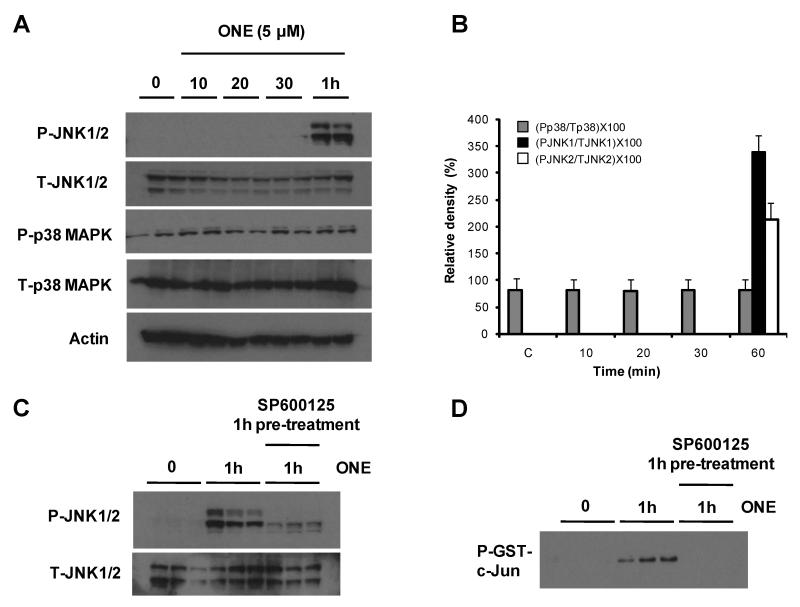
To investigate whether ONE can activate MAPK, cells were treated with 5 μM ONE. Under these conditions, 5 μM ONE induced about 50% cytotoxicity in 24 hours (Lin et al. 2005). The activation of each kinase was analyzed by phospho-specific antibodies and, as shown in Figure 1, ERK phosphorylation significantly increased within 10-20 min by ONE treatment and then gradually decreased to basal levels after 30 min (Fig. 1). No significant changes in the amounts of total ERK1/2 were observed in all of the samples indicating that ONE modulates the posttranslational regulation of ERK rather than transcriptional activation. In contrast to ERK1/2, the activation of JNK required 1 hour treatment of ONE while there was no significant change in phospho-p38 (Fig. 2A and B).
Figure 1. The activation of ERK.
SH-SY5Y cells were treated with 5 μM ONE for 30 min and phosphorylation of ERK was monitored by Western blotting with highly specific anti-phospho-ERK antibody (A). ERK phosphorylation increased within 10 min by ONE treatment and then gradually decreased to baseline over 30 minutes. The densities of phospho-ERK1 and ERK2 were normalized with those of total-ERK1, ERK2 and actin, respectively (B). * p < 0.05 and ** p < 0.01 compared with the control group as assessed by Student’s t-test (n=4).
Figure 2. ONE activates JNK, but not p38 MAPK.
SH-SY5Y cells were treated with 5 μM ONE for different time intervals as indicated. JNK, and p38 MAPK was monitored by Western blotting 15 μg of whole cell lysate with highly specific anti-phospho-JNK, and phospho-p38 MAPK antibodies (A). The densities of phospho-JNK and phospho-p38 MAPK were normalized with total level of each protein detected by anti-JNK and p38 MAPK antibodies, and actin, respectively (n=4) (B). While there was no significant change in the level of phospho-p38, the level of phospho-JNK increased 1 h after ONE treatment and this increase was prevented by JNK specific inhibitor (SP600125) treatment (C). The significant increase of JNK activity by ONE and the specific inhibitory effect of SP600125 were also confirmed by the kinase assay using GST-c-Jun as a substrate (D).
The early activation of ERK1/2 plays a role in ONE-induced neurotoxicity
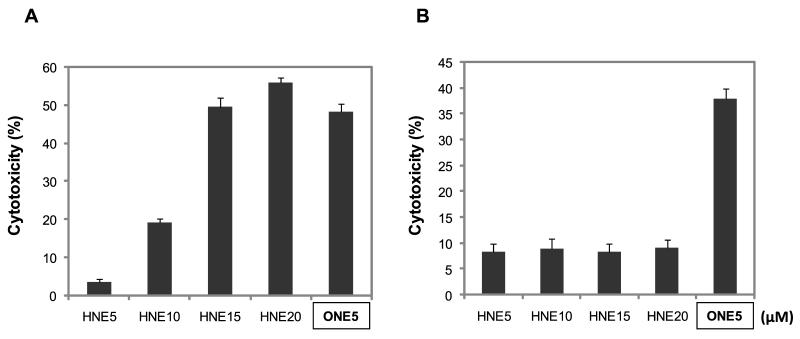
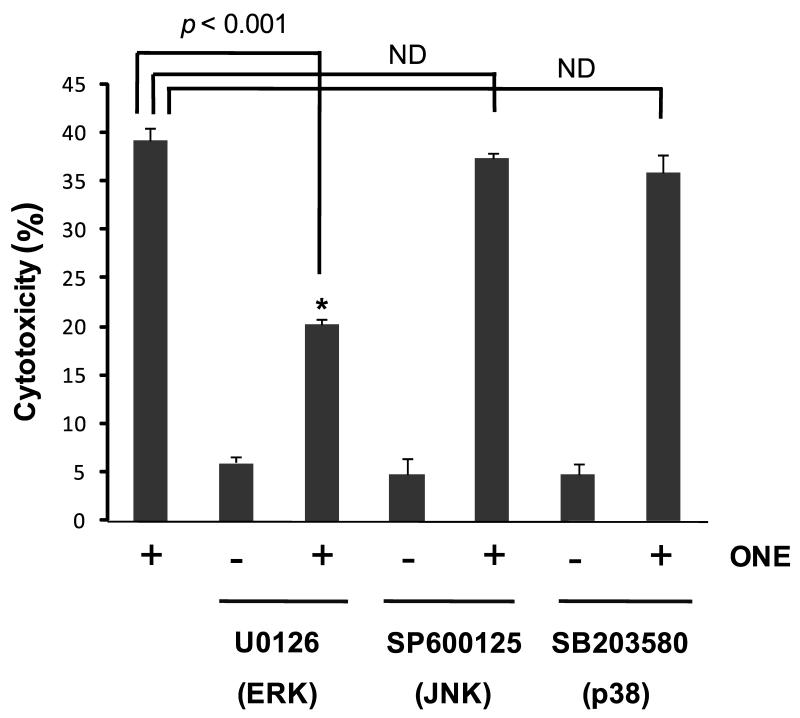
Since JNK has been reported to be an essential factor in HNE-mediated cytotoxicity (Tamagno et al. 2005), we were interested in the early signal pathway by ONE or HNE treatment and whether it is significant to induce cell death because of time-gap between the activation of ERK and JNK. To address this, one group of cells was treated with ONE or HNE for 30 min and the media was replaced with ONE- or HNE-free media (Fig. 3B), and the other group of cells was continuously treated with ONE or HNE (Fig. 3A). In this experiment, ONE was sufficient to induce the cell death even at a low concentration (5 μM) after 30 min treatment but HNE treatment for 30 min did not induce cell death at 20 μM (Fig. 3). These results are consistent with our previous report showing higher toxicity of ONE than HNE (Lin et al. 2005) and, furthermore, suggest that the signal pathway mechanisms involved in ONE-mediated cytotoxicity are different than the pathways involved in HNE-mediated cytotoxicity. Since both pro- and anti-apoptotic roles for ERK have been reported following oxidative stress mediated injury (Arany et al. 2004, Zhuang et al. 2007), we further evaluated the role of ERK activation in ONE-induced cell death using U0126, a specific inhibitor for ERK upstream kinase MEK1/2 (Favata et al. 1998). Inhibition of ERK activation using U0126 significantly reduced ONE-induced cytotoxicity (Fig. 4) although the exposure of SH-SY5Y cells to U0126 alone for 1 hour had no effect on apoptosis and selectively inhibited ERK1/2 phosphorylation (data not shown). We also examined the role of p38 and JNK pathways in ONE-induced cytotoxicity using specific inhibitors for each kinase. In contrast to inhibition of ERK, the inhibition of p38 MAPK by SB203580, or JNK by SP600125, had no effect on ONE-induced cell death (Fig. 4). Since it has been suggested that JNK is a major player in HNE-mediated toxicity (Tamagno et al. 2005), we further confirmed the effect of SP600125 on JNK activation to confirm. Specifically, we examined both the level of phospho-JNK with Western blot (Fig. 2C) and the activity of JNK with kinase assay using GST-c-Jun protein as a substrate (Fig. 2D). A significant increase of JNK activity was observed in the cell lysate treated with ONE for 1 hour and co-treatment of SP600125 completely inhibited JNK activity (Fig. 2C,D). These data strongly suggest that the early activation of ERK, but not JNK or p38, mediates neurotoxicity in SH-SY5Y cells following ONE treatment.
Figure 3. The transient short-term treatment of ONE triggers cell death.
One group of cells was treated with various concentrations of ONE and HNE for 30 minutes and the media was changed (B). The other group of cells was continuously treated with the ONE and HNE (A). After overnight culture, we assessed the cytotoxicity using LDH assay. As a result, the cytotoxicity of 30 minutes-treated group was similar with those of continuous-treated group in ONE-treated cells (A and B). This result showed that ONE (5 μM) for 30 minutes treatment is sufficient to induce the cell death, whereas HNE treatment for 30 minutes did not induce cell death (B). Each column is the mean ± SD from five independent experiments.
Figure 4. The early activation of ERK plays a role in ONE-induced neurotoxicity.
The cells were pre-incubated with each MAPK inhibitor (U0126, SP600125, and SB203580) for 1 h and followed by treatment with ONE. Pre-incubation of cells with p38 MAPK inhibitor or JNK inhibitor had no effect on ONE-induced cell death, but ERK inhibitor decreased ONE-induced cytotoxicity. These data showed that the early activation of ERK plays a role in ONE-induced neurotoxicity. Each column is the mean ± SD from five independent experiments. Data are expressed as the percentage of maximum LDH release (defined as 100% cell death) induced by incubating cells for 45 min with 1% Triton X-100 as detained in the manufacturer’s protocol. * Significantly different (p < 0.001) from the control cells treated with 5 μM ONE. ND: No difference.
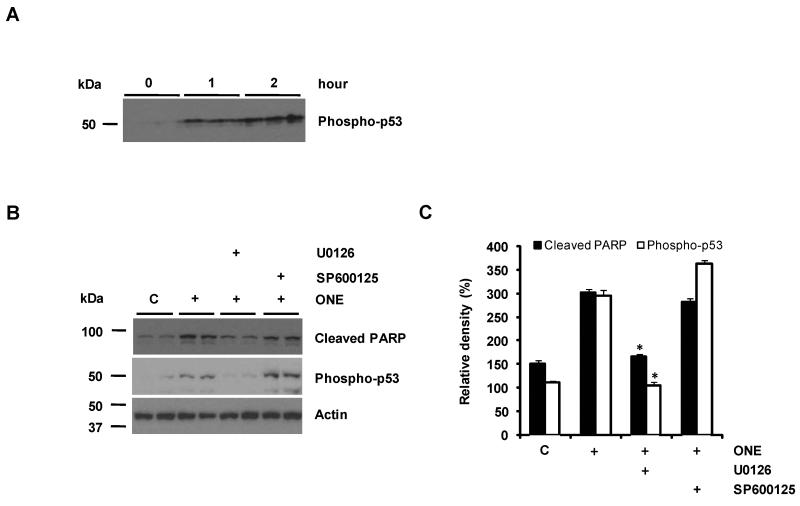
ERK acts as an upstream factor for p53 activation in ONE-treated cells
Recently, Shibata et al. (Shibata et al. 2006) reported that ONE induces apoptosis in SH-SY5Y neuroblastoma cells by the p53-dependent apoptotic pathway. This prompted us to determine whether the ERK activity is involved in the p53-dependent apoptotic pathway. To confirm p53 activation in response to treatment with ONE, we examined the activation of p53 by measuring the phosphorylation of p53 (Ser15). Consistent with a previous report (Shibata et al. 2006), ONE treatment enhanced p53 phosphorylation in a time-dependent manner (Fig. 5A). Next, we treated cells with U0126 to prevent ERK activation for determining whether ONE-induced p53 response is mediated via the ERK pathway. As shown in Figure 5B, pretreatment with U0126 significantly reduced ONE-induced phosphorylation of p53, suggesting ERK is an upstream pathway for p53 activation by ONE. The tumor suppressor gene product, p53, has been reported to mediate apoptosis in many experimental systems and ONE-induced cell death has been shown to be an apoptotic cell death (Shibata et al. 2006, West et al. 2004). Consistently, the exposure of SH-SY5Y cells to ONE led to a proteolytic cleavage of PARP, one of the molecular hallmarks for apoptosis, resulting in the accumulation of the 85 kDa fragment and ERK inhibition significantly reduced the level of this PARP fragment (Fig. 5B,C) in line with its neuroprotective actions. However, JNK inhibition failed to reduce the level of phospho-p53 and PARP fragment (Fig. 5B,C). These data indicate that p53-activation and its apoptotic activity are mediated by ERK activation in ONE-treated cells. Therefore, ERK may be a fundamental early signal pathway involved in ONE-induced cytotoxicity.
Figure 5. p53 activation is mediated by ERK activity in ONE-treated cells.
ONE treatment enhanced the p53 phosphorylation in a time-dependent manner (A). The cells was pretreated with ERK or JNK inhibitor and then treated with ONE for 1 h. p53-activation was prevented by ERK inhibitor but not by JNK inhibitor. In addition, cleaved PARP was also reduced by ERK inhibitor (B). The densities of cleaved PARP and phospho-p53 were normalized with actin (n=4) (C). * Significantly different (p<0.001) from the cells treated with 5 μM ONE.
Discussion
Lipid peroxidation-derived aldehydes generated during the process of lipid peroxidation can diffuse within or even escape from the cell and attack targets are far from the original initiation site. Therefore, it has been suggested that they are not only end products of lipid peroxidation but also act as a mediator of oxidative stress (Uchida et al. 1999). The present study supports this hypothesis by demonstrating the activation of MAPK by ONE. ONE activates both ERK and JNK, but not p38 MAPK. Additionally we show that ERK is an upstream regulator of p53-mediated apoptosis by ONE in SH-SY5Y human neuroblastoma cells. Treatment of SH-SY5Y cells with ONE induced the early activation of ERK followed by the late activation of JNK. While inhibition of ERK significantly reduced toxicity, JNK inhibition did not affect cytotoxicity induced by ONE. This is particularly interesting since JNK plays an important role in HNE induced cytotoxicity (Uchida et al. 1999, Tamagno et al. 2005).
Compared to HNE, ONE is structurally analogous but contains a different functional group at the C4 position that is predicted to make the compound more reactive toward nucleophiles than HNE. It has been shown that both HNE and ONE react with amino acid nucleophiles via Michael addition, however the reactivity of these lipid aldehydes toward amino acid nucleophiles differs qualitatively, with Arg being a target for ONE but not HNE, and quantitatively, by a remarkable > 100-fold difference in the rate of Cys modification between HNE and ONE (Doorn & Petersen 2002). Moreover, ONE has some unique adducts from HNE. Even after Michael addition, ONE adduct, a 4-ketoaldehyde, cannot rearrange to a cyclic hemiacetal like HNE and can further condense with Lys side chain to form a stable pyrrole cross-linking (Zhang et al. 2003, Liu et al. 2003, Zhu & Sayre 2007). In addition, ONE, but not HNE, forms 4-ketoamide with Lys (Zhu & Sayre 2007). Therefore, a possible explanation for the difference between ONE and HNE cytotoxicity found in this study may stem from the difference in the reactions between ONE and HNE. This finding is consistent with our previous findings that ONE is more neurotoxic to neuroblastoma cells as well as more reactive in modifying and cross-linking proteins than HNE (Lin et al. 2005, Zhang et al. 2003).
In recent study, a series of toxic α, β-unsaturated aldehydes, including ONE, have shown to induce apoptosis involves the activation of caspases, proteolysis of downstream caspase targets, and nucleosomal DNA fragmentation (West et al. 2004). More recently, ONE, but not HNE, was identified as a potential trigger of p53, a key modulator of cellular stress reponses in human neuroblastoma cell lines (Shibata et al. 2006).
In the present study, pre-treatment with a specific inhibitor of MEK, the upstream kinase of ERK, markedly suppressed p53 phosphorylation at Ser 15 at 1 h after ONE treatment (Fig. 5), suggesting that ERK is an upstream regulator of p53-mediated cell death by ONE. Since mutation at Ser 15 impairs the functional activation of p53 in apoptosis (Unger et al. 1999), phosphorylation of p53 at Ser15 appears to play a critical role in the stabilization, upregulation and functional activation of p53 during cellular stress (Lambert et al. 1998, Shieh et al. 1997, Siliciano et al. 1997), suggesting an essential role for Ser15 phosphorylation in the induction of cell death. In this regard, it is interesting to note that ERK regulates p53 phosphorylation at Ser15 in response to resveratrol (Alkhalaf & Jaffal 2006), or nitric oxide (Ridnour et al. 2005). While the mechanism(s) underlying ERK activation by ONE remain to be determined, we hypothesize that oxidative stress associated with ONE may be an important participant in the mechanism (Fig. 6). Indeed, since ONE is a potent electrophile like HNE, ONE treatment can trigger a change in the cellular redox status such that it may exert oxidative stress and ERK activation (Wang et al. 1998, Chu et al. 2004). In support of this idea, oxidative stress has been known to induce ERK activation as shown in hydrogen peroxide- or glutamate-mediated oxidative stress in neurons (Zhuang et al. 2007, Stanciu et al. 2000) and antioxidants or ROS scavengers prevent the activation of ERK in these conditions. Furthermore, the inhibition of ERK by U0126, a specific inhibitor of MEK, protects the cells from oxidative stress mediated cytotoxicity (Zhuang et al. 2007). Based on these previous findings and the fact that ONE is intimately linked with oxidative stress, our data showing the protective effect of ERK inhibition strongly suggest the interaction of oxidative stress and ERK pathway in ONE-induced cytotoxicity. Alternatively, ERK could be directly regulated by modification with ONE as shown in rat hepatocytes treated with HNE (Parola et al. 1998, Sampey et al. 2007). Further study is required to explore this possibility.
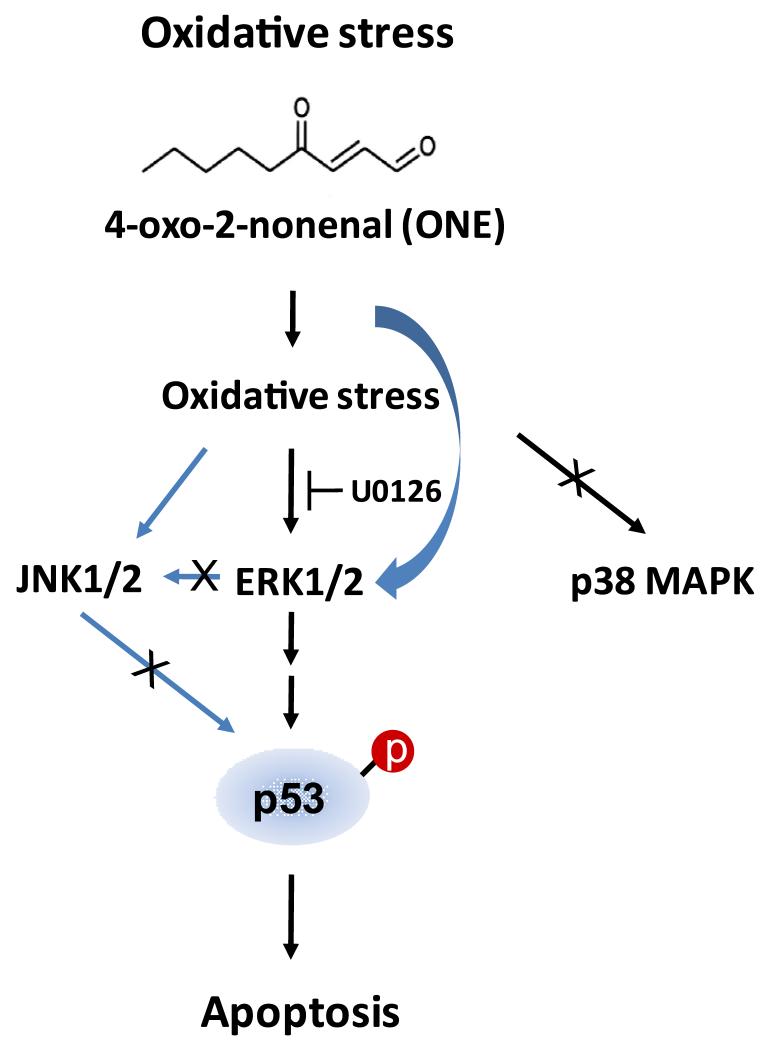
Figure 6. A schematic model of ONE-induced signaling pathway.
ONE induces strong ERK activation at an early time point, which is subsequently followed by p53-mediated apoptosis. In this model, ERK, but not JNK or p38, plays as an upstream regulator of ONE-induced cytotoxicity. While the mechanisms for ERK activation by ONE are unclear and require further study, we hypothesize that either direct interaction of ONE and ERK or ONE-mediated generation of reactive oxygen species might be an important factor in this regulatory mechanism.
In conclusion, in ONE treated SHSY5Y human neuroblastoma cells, we demonstrate that ERK is an early and important signal pathway associated with subsequent apoptosis and that an essential role for ERK signaling pathway in p53-dependent apoptosis. Studies focusing on these biochemical steps would extend our understanding of the regulation of stress signaling cascades stimulated by various reactive products generated by lipid peroxidation.
Acknowledgments
Work in the authors’ laboratories is partly supported by the National Institutes of Health and Alzheimer Association (IIRG-06-25798).
Abbreviations
- (ERK)
extracellular signal-regulated kinase
- (HNE)
4-hydroxynonenal
- (JNK)
c-Jun N-terminal kinase
- (LDH)
lactate dehydrogenase
- (MAPK)
mitogen-activated protein kinases
- (ONE)
4-oxo-2-nonenal
- (PARP)
poly (ADP-ribose) polymerase
- (SAPK)
stress-regulated protein kinase
References
- Alkhalaf M, Jaffal S. Potent antiproliferative effects of resveratrol on human osteosarcoma SJSA1 cells: Novel cellular mechanisms involving the ERKs/p53 cascade. Free Radic. Biol. Med. 2006;41:318–325. doi: 10.1016/j.freeradbiomed.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Arany I, Megyesi JK, Kaneto H, Tanaka S, Safirstein RL. Activation of ERK or inhibition of JNK ameliorates H(2)O(2) cytotoxicity in mouse renal proximal tubule cells. Kidney Int. 2004;65:1231–1239. doi: 10.1111/j.1523-1755.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- Bhat NR, Zhang P. Hydrogen peroxide activation of multiple mitogen-activated protein kinases in an oligodendrocyte cell line: role of extracellular signal-regulated kinase in hydrogen peroxide-induced cell death. J. Neurochem. 1999;72:112–119. doi: 10.1046/j.1471-4159.1999.0720112.x. [DOI] [PubMed] [Google Scholar]
- Brand A, Gil S, Seger R, Yavin E. Lipid constituents in oligodendroglial cells alter susceptibility to H2O2-induced apoptotic cell death via ERK activation. J. Neurochem. 2001;76:910–918. doi: 10.1046/j.1471-4159.2001.00085.x. [DOI] [PubMed] [Google Scholar]
- Camandola S, Poli G, Mattson MP. The lipid peroxidation product 4-hydroxy-2,3-nonenal increases AP-1-binding activity through caspase activation in neurons. J. Neurochem. 2000;74:159–168. doi: 10.1046/j.1471-4159.2000.0740159.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury. The dark side of ERK1/2. Eur. J. Biochem. 2004;271:2060–2066. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb MH. MAP kinase pathways. Prog. Biophys. Mol. Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- Doorn JA, Petersen DR. Covalent modification of amino acid nucleophiles by the lipid peroxidation products 4-hydroxy-2-nonenal and 4-oxo-2-nonenal. Chem. Res. Toxicol. 2002;15:1445–1450. doi: 10.1021/tx025590o. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Dawson VL, Dawson TM. Free radicals as mediators of neuronal injury. Cell. Mol. Neurobiol. 1998;18:667–682. doi: 10.1023/A:1020685903186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J. Lab. Clin. Med. 1992;119:598–620. [PubMed] [Google Scholar]
- Ishikawa Y, Kitamura M. Anti-apoptotic effect of quercetin: intervention in the JNK-and ERK-mediated apoptotic pathways. Kidney Int. 2000;58:1078–1087. doi: 10.1046/j.1523-1755.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- Kelicen P, Cantuti-Castelvetri I, Pekiner C, Paulson KE. The spin trapping agent PBN stimulates H2 O2 -induced Erk and Src kinase activity in human neuroblastoma cells. Neuroreport. 2002;13:1057–1061. doi: 10.1097/00001756-200206120-00016. [DOI] [PubMed] [Google Scholar]
- Lambert PF, Kashanchi F, Radonovich MF, Shiekhattar R, Brady JN. Phosphorylation of p53 serine 15 increases interaction with CBP. J. Biol. Chem. 1998;273:33048–33053. doi: 10.1074/jbc.273.49.33048. [DOI] [PubMed] [Google Scholar]
- Lee SH, Blair IA. Characterization of 4-oxo-2-nonenal as a novel product of lipid peroxidation. Chem. Res. Toxicol. 2000;13:698–702. doi: 10.1021/tx000101a. [DOI] [PubMed] [Google Scholar]
- Lee SH, Blair IA. Oxidative DNA damage and cardiovascular disease. Trends Cardiovasc. Med. 2001;11:148–155. doi: 10.1016/s1050-1738(01)00094-9. [DOI] [PubMed] [Google Scholar]
- Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- Lin D, Lee HG, Liu Q, Perry G, Smith MA, Sayre LM. 4-Oxo-2-nonenal is both more neurotoxic and more protein reactive than 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2005;18:1219–1231. doi: 10.1021/tx050080q. [DOI] [PubMed] [Google Scholar]
- Liu Z, Minkler PE, Sayre LM. Mass spectroscopic characterization of protein modification by 4-hydroxy-2-(E)-nonenal and 4-oxo-2-(E)-nonenal. Chem. Res. Toxicol. 2003;16:901–911. doi: 10.1021/tx0300030. [DOI] [PubMed] [Google Scholar]
- Marnett LJ, Riggins JN, West JD. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Invest. 2003;111:583–593. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni DV, Sayre LM. Structural definition of early lysine and histidine adduction chemistry of 4-hydroxynonenal. Chem. Res. Toxicol. 1995;8:284–291. doi: 10.1021/tx00044a014. [DOI] [PubMed] [Google Scholar]
- Parola M, Robino G, Marra F, et al. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J. Clin. Invest. 1998;102:1942–1950. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Leonarduzzi G, Biasi F, Chiarpotto E. Oxidative stress and cell signalling. Curr. Med. Chem. 2004;11:1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- Pollack M, Yang IY, Kim HY, Blair IA, Moriya M. Translesion DNA Synthesis across the heptanone--etheno-2′-deoxycytidine adduct in cells. Chem. Res. Toxicol. 2006;19:1074–1079. doi: 10.1021/tx0600503. [DOI] [PubMed] [Google Scholar]
- Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13147–13152. doi: 10.1073/pnas.0502979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampey BP, Carbone DL, Doorn JA, Drechsel DA, Petersen DR. 4-Hydroxy-2-nonenal adduction of extracellular signal-regulated kinase (Erk) and the inhibition of hepatocyte Erk-Est-like protein-1-activating protein-1 signal transduction. Mol. Pharmacol. 2007;71:871–883. doi: 10.1124/mol.106.029686. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Lin D, Yuan Q, Zhu X, Tang X. Protein adducts generated from products of lipid oxidation: focus on HNE and one. Drug Metab. Rev. 2006;38:651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Smith MA, Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr. Med. Chem. 2001;8:721–738. doi: 10.2174/0929867013372922. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J. Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- Shibata T, Iio K, Kawai Y, Shibata N, Kawaguchi M, Toi S, Kobayashi M, Yamamoto K, Uchida K. Identification of a lipid peroxidation product as a potential trigger of the p53 pathway. J. Biol. Chem. 2006;281:1196–1204. doi: 10.1074/jbc.M509065200. [DOI] [PubMed] [Google Scholar]
- Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Canman CE, Taya Y, Sakaguchi K, Appella E, Kastan MB. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Soh Y, Bae M, Pie J, Wan J, Jeong K. Apoptosis of PC12 cells by 4-hydroxy-2-nonenal is mediated through selective activation of the c-Jun N-terminal protein kinase pathway. Chem. Biol. Interact. 2001;130-132:943–954. doi: 10.1016/s0009-2797(00)00247-7. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Wang Y, Kentor R, et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J. Biol. Chem. 2000;275:12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- Takeda A, Smith MA, Avila J, Nunomura A, Siedlak SL, Zhu X, Perry G, Sayre LM. In Alzheimer’s disease, heme oxygenase is coincident with Alz50, an epitope of tau induced by 4-hydroxy-2-nonenal modification. J. Neurochem. 2000;75:1234–1241. doi: 10.1046/j.1471-4159.2000.0751234.x. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Parola M, Bardini P, et al. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J. Neurochem. 2005;92:628–636. doi: 10.1111/j.1471-4159.2004.02895.x. [DOI] [PubMed] [Google Scholar]
- Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J. Biol. Chem. 1999;274:2234–2242. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- Unger T, Sionov RV, Moallem E, Yee CL, Howley PM, Oren M, Haupt Y. Mutations in serines 15 and 20 of human p53 impair its apoptotic activity. Oncogene. 1999;18:3205–3212. doi: 10.1038/sj.onc.1202656. [DOI] [PubMed] [Google Scholar]
- Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem. J. 1998;333(Pt 2):291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JD, Ji C, Duncan ST, Amarnath V, Schneider C, Rizzo CJ, Brash AR, Marnett LJ. Induction of apoptosis in colorectal carcinoma cells treated with 4-hydroxy-2-nonenal and structurally related aldehydic products of lipid peroxidation. Chem. Res. Toxicol. 2004;17:453–462. doi: 10.1021/tx034248o. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WH, Liu J, Xu G, Yuan Q, Sayre LM. Model studies on protein side chain modification by 4-oxo-2-nonenal. Chem. Res. Toxicol. 2003;16:512–523. doi: 10.1021/tx020105a. [DOI] [PubMed] [Google Scholar]
- Zhu X, Sayre LM. Mass spectrometric evidence for long-lived protein adducts of 4-oxo-2-nonenal. Redox Rep. 2007;12:45–49. doi: 10.1179/135100007X162176. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol Renal Physiol. 2007;292:F440–447. doi: 10.1152/ajprenal.00170.2006. [DOI] [PubMed] [Google Scholar]