Abstract
Results from birth cohort and cross-sectional studies of young children with wheezing have uncovered strong associations between both lung function and immune responses in early life and the subsequent development of persistent wheezing and chronic airway obstruction up to mid-adulthood. It is now apparent that the pattern of bronchial hyperresponsiveness, deficits in lung function, and structural airway remodeling that are characteristic of asthma may be already established during the preschool years in most patients. Interactions between acute viral infections, especially those due to rhinovirus and respiratory syncytial virus, and exposure to perennial aeroallergens may induce persistent alterations in immune responses and airway function in susceptible subjects. Similarly, deficits in airway function present shortly after birth predict airflow limitation in early adult life, which in turn is a strong predisposing factor for chronic obstructive pulmonary disease. The fact that these alterations are more likely to occur during early life and even in utero than later during childhood suggests that there a developmental window of susceptibility during which exposures can disrupt normal growth trajectories. Novel strategies for primary prevention of chronic respiratory diseases will be based on the identification of the genetic and environmental factors that interactively cause these disruptions.
Keywords: asthma, COPD, infancy, spirometry
During the last two decades, reports from several long-term cohort studies have shown that increased frequency of lower respiratory illnesses, altered immune responses by peripheral blood mononuclear cells, and changes in developmental patterns of the lungs and airways can be frequently observed in young children who will develop asthma later in life. These results provide renewed support to the hypothesis that the beginnings of many chronic diseases of adult life may be found during the growing years. The purpose of this review is to summarize emerging evidence that connects early life events with respiratory outcomes occurring years or even decades later. In addition, I will also explore the connection between these findings and the concept of “developmental windows of susceptibility” for environmentally induced changes that has recently emerged from evolutionary and developmental biology. An additional, thorough review of the early origins of chronic obstructive pulmonary disease (COPD) has been recently published (1).
DEVELOPMENTAL TRAJECTORIES AND RESPONSE MODULES: THE EXAMPLE OF CHRONIC LUNG DISEASE OF PREMATURITY
That early perturbations in the normal development of organ systems can have long-term repercussions for the health of the individual is undisputable. Environmental factors can have a major influence on lung growth, and no better example exists than chronic lung disease of prematurity (CLDP, also called bronchopulmonary dysplasia [BPD]) (2). In most patients, CLDP is a direct consequence of lung immaturity and excess oxygen and pressure needed to treat respiratory distress syndrome. Several characteristics of CLDP are illustrative of the complex interactions between the different factors that determine a disease phenotype. CLDP is heterogeneous in the scope of its clinical expression, and severity is inversely correlated with gestational age and birth weight. This pattern of expression of the CLDP suggests that there is a “developmental window of susceptibility”: the same noxious stimulus that can cause severe disease in a 24-week premature baby can have very mild or no effect in a term child. CLDP thus illustrates the fact that the development of normal lungs and airways is not the simple result of a “genetic program”: it would be as absurd to believe that CLDP is a “genetic” disease as it would be to propose “genes for head trauma.” Nevertheless, we can clearly recognize a limited set of phenotypes that can be classified under the label of CLDP, suggesting that the outcomes of the insults associated with CLDP are not random and unpredictable, but are the results of identifiable response modules. Moreover, premature infants of similar gestational age may have very different respiratory outcomes, and genetic variations can influence these outcomes. Understood in this way, there are indeed “genes for CLDP,” if by that we mean polymorphisms that modify the deleterious effects of excessive oxygen and pressure (3). There are also rare genetic mutations (for example, those causing a partial deficiency of surfactant protein B [4]) that can give rise to chronic lung syndromes in term children, the clinical manifestations of which overlap with CLDP (5). If all forms of chronic lung disease that start at birth were assigned the same disease label, then the spectrum of this “disease” would include from these single gene conditions to the severe phenotypes associated with extreme prematurity.
What is relevant for the issues addressed in this article is that CLDP is associated with significant decreases in lung function in early life (6). Moreover, recent longitudinal studies clearly indicate that these deficits track along childhood, and persist until early adult life and perhaps beyond. Northway and coworkers (7) first reported that 68% of young adults with a history of BPD in infancy had airway obstruction, including decreases in forced expiratory volume in one second (FEV1), forced expiratory flow between 25 and 75 percent of forced vital capacity (FVC), and maximal expiratory flow velocity at 50 percent of FVC, as compared with control subjects of similar gestational age but with no BDP. More recently, Doyle and colleagues (8) reported that almost half of 18-year-olds whose birth weight was less than 1,500 g and who had BPD as infants had an FEV1/FVC ratio less than 75%, as compared with only 16% of subjects with similar birth weights and no BPD. These results clearly suggest that a specific insult occurring in the newborn period can be associated with a fixed, abnormal trajectory of airway growth that persists until the early adult years. If this abnormal trajectory is the consequence of the insult or if otherwise congenitally narrower or hyperreactive airways could predispose infants with low birth weight to require more aggressive oxygen therapy and mechanical ventilation (9) and thus, to CLDP, is currently unknown, but it is plausible to surmise that both mechanisms may be at play.
CLDP is certainly an extreme example, but it cogently illustrates that the effects of genetic predisposition, environmental factors, and their interactions during critical periods in early life can decisively influence phenotypes expressed for decades thereafter. The evidence suggesting that the same is true for asthma and also for COPD will be described below.
DEVELOPMENTAL TRAJECTORIES OF AIRWAY FUNCTION IN TERM INFANTS: ROOTS OF COPD IN INFANCY
An important first question is if the remarkable tracking of level of lung function from infancy to early adult life observed in CLDP is also present in term children who are not subject to the severe injury associated with respiratory distress syndrome. It is now well established that the maximal level of lung function reached after puberty is a crucial determinant of the risk for COPD later in life (10). Obviously, cigarette smoking is overwhelmingly the most frequent causal factor in COPD, but it is also true that only a fraction of smokers develop COPD and no less than one-fourth of cases of COPD are unrelated to smoking (11). Specifically, COPD is defined by consensus guidelines as an FEV1/FVC ratio of less than 70% (12), and thus subjects who start adult life with lower ratios will attain this threshold earlier during normal lung aging than those who start at higher levels. Moreover, the deleterious effects of smoking should also be apparent earlier in the former than in the latter.
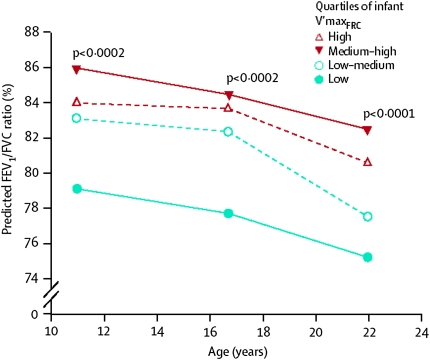
Recent results from the Tucson Children's Respiratory Study suggest that, as with abnormal airway function in CLDP, the normal spectrum of airway function is established very early in life and even in utero in term children. In that study, spirometry was performed at ages 11, 16, and 22 years in 123 unselected subjects whose maximal expiratory flows at function residual capacity (VmaxFRC) had been assessed at a mean age of 2 months by use of the chest compression technique (13). The results showed that infants in the lowest quartile for VmaxFRC had the lowest mean FEV1/FVC ratios at all ages, with a mean difference with respect to the other three quartiles of −5.2% and a 95% confidence interval of −7.4 to −3.0 (P < 0.0001); 14% of the variance for the ratio at follow-up was explained by VmaxFRC level in early life (Figure 1). Previous studies had shown that multiple measures of pulmonary function track markedly during the school years (14) and that VmaxFRC measured shortly after birth correlates strongly with school age lung function (15); however, the Tucson study was the first to track markers of airway function from birth to early adult life.
Figure 1.
FEV1/FVC ratio at ages 11, 16, and 22 years by quartiles of airway function measured at a mean age of 2 months. Reprinted by permission from Reference 13.
Biologically, and taken together with those described for CLDP, these results suggest that a growth module is established very early in life that controls the proportional (also called “allometric” [16]) changes taking place in the many determinants of airway function, as assessed by use of maximal expiratory flows. This proportional growth, however, maintains the relative position (or rank) of each individual with respect to her/his peers. This relative position is not irreversible and fixed; environmental stimuli can alter the ranked course of airway growth, and these stimuli have clear windows of opportunity during which they can induce meaningful changes.
ASTHMA AND AIRWAY FUNCTION: TRACKING AND DIVERGING
Adults with asthma are at increased risk of developing COPD (17), and many studies have shown that, as a group, patients with asthma have reduced airway function, and more specifically, reduced FEV1/FVC ratio, compared with subjects without asthma (18). The two longest, ongoing cohort studies of asthma in which lung function was measured repeatedly, starting during the school years, have shed considerable light on the origins of these alterations. In the Melbourne Asthma Study, a group of children with a past history of wheezing and a control group without wheezing were randomly selected at the age of 7 years in 1964, and a further group of children with severe wheezing was selected from the same birth cohort at the age of 10 years (19). These subjects have been followed prospectively at 7-year intervals, with the last published review in 1999, when their average age was 42 years. Throughout adolescence and adult life, children who were originally classified as having either mild or severe asthma had consistently lower FEV1/FVC ratios than those without a diagnosis of asthma. Most notably, these lower levels were already established by the age of 7 to 10 years, with no further deficits occurring up to the age of 42 years, in spite of the fact that most patients, and especially those with severe asthma, had continued asthma symptoms throughout the follow-up (19). In the Dunedin Multidisciplinary Health and Development Study, over 1,100 children were enrolled at birth in 1972 to 1973 and followed prospectively (20). Lung function and history of asthma-like symptoms were assessed at 2- to 5-year intervals between ages 9 and 26 years. Subjects who were classified as “persistent wheezers” (that is, who reported wheeze at all surveys between ages 9 and 26 years or at all surveys after inception of wheezing), and those who were classified as “relapsers” (that is, those for whom wheezing was reported at two or more successive surveys, then was absent at one or more successive assessments, and then was reported at all subsequent assessments), had lower FEV1/FVC ratios, starting at age 9 years, than children with no wheezing. Similarly to what was found in the Melbourne cohort, the lung function deficits of these two groups of children, relative to those with milder wheezing or without wheezing, remained unchanged between 9 and 26 years (20).
These results suggest that in patients with asthma considered as a class, very little deterioration in FEV1/FVC ratio, relative to subjects without asthma, is observed from school age and up to mid-adult life. Two scenarios are thus possible: that children with asthma are born with “small airways” and this congenital abnormality increases their risk for subsequent asthma; or that deficits in lung function are acquired, perhaps as a consequence of acute or persistent airway inflammation occurring during a critical period for the development of lung and airways. A birth cohort study in Norway has yielded evidence in support of the first scenario. Håland and coworkers (21) found that children in whom an index derived from tidal breathing curves (tPTEF/tE) measured shortly after birth was below the median were more likely to have a history of asthma at 10 years of age than those whose index was above the median (24.3% versus 16.2%, P = 0.01). Children with low tPTEF/tE were also more likely to have current asthma (14.6% versus 7.5%, P = 0.005), and to have severe bronchial hyperresponsiveness, defined as a methacholine dose of less than 1.0 μmol causing a 20% fall in the FEV1 at age 10 (9.1% versus 4.9%, P = 0.05). As compared with children whose respiratory system compliance was above the median, children with respiratory compliance at or below the median more often had a history of asthma (27.4% versus 14.8%; P = 0.001) and current asthma (15.0% versus 7.7%, P = 0.009) at age 10.
ASTHMA, AIRWAY FUNCTION, AND AIRWAY REMODELING: A DEVELOPMENTAL WINDOW
The observation that lung function at birth may predispose to the subsequent development of asthma is not incompatible with the existence of acquired mechanisms for the deficits in lung function and increased bronchial responsiveness observed in asthma. Indeed, both epidemiologic studies and carefully performed studies of biopsies in wheezing young children suggest that the preschool years are a developmental window of opportunity for the establishment of deficits in lung function and airway remodeling in asthma. Using data from the Tucson study, Morgan and colleagues (22) showed that preschool persistent wheezers (e.g., children who had wheezing lower respiratory illnesses in the first 3 years of life and were still wheezing at age 6) were as likely as late-onset wheezers (e.g., children who wheezed at age 6 but who had no reports of wheezing before age 3) to have frequent wheezing episodes during the school years. However, although these two groups had similar VmaxFRC levels measured shortly after birth, persistent wheezers had significantly lower maximal flows by age 6 and up to age 16 and significantly lower FEV1/FVC ratio at ages 11 and 16 years than late-onset wheezers. Interestingly, as in the Melbourne and Dunedin cohorts, maximal flows showed no further deterioration after age 6 in either group, in spite of the fact that both showed significant respiratory morbidity between ages 6 and 16 years.
These results are only in apparent contradiction with recent reports that used data from the Childhood Asthma Management Program (CAMP). In children with mild to moderate asthma enrolled in CAMP, Strunk and coworkers showed a significant pattern of airway obstruction that increased between ages 5 and 18, when compared with a reference population (23). However, for the most reliable index of airway obstruction, the FEV1/FVC ratio, the authors showed that at ages 6 and 18, the deficit in boys with asthma was −7.3% and −9.8%, respectively, while in girls with asthma the deficits were −7.1% and −9.9%, respectively. The data thus suggest that almost three-fourths of the mean deficits in lung function growth observed in these patients were already present at age 6. Given the large numbers of children with asthma enrolled in CAMP, this study had much greater power to show the smaller, additional increases in airway obstruction observed between ages 6 and 18 years.
These results thus suggest that children who will go on to develop asthma are more likely to show acquired, persistent deficits in lung function if the first symptoms of the disease occur during the first 3 years of life. Structural and functional studies of children with asthma symptoms at different ages have provided strong support for the existence of such a “window of opportunity” before age 3. Saglani and colleagues (24) obtained endobronchial biopsies from wheezy preschool children and controls with stridor. Reticular basement membrane thickness, a measure of airway remodeling, was significantly higher in subjects with confirmed wheeze than in control subjects, as was eosinophilic inflammation. In contrast, no increased reticular basement membrane thickness was observed in wheezy infants aged less than 1 year (25). The authors thus suggested that remodeling in asthma occurs very early in life and may track into adult life in patients with persistent disease (26).
Further support for the presence of a developmental “window of opportunity” for the development of asthma in early life comes from studies of patterns of asthma prevalence in immigrants. Recently, Kuehni and coworkers (27) administered a questionnaire to several thousand South Asian and White young mothers randomly sampled in Leicestershire, United Kingdom. The reported prevalence of asthma was 10.9% in South Asian and 21.8% in White women. South Asian women who migrated to the United Kingdom aged 5 years or older reported less asthma (6.5%) than those born in the United Kingdom or who migrated before age 5 (16.0%). For those who migrated aged over 5 years, the prevalence did not alter with the duration of residence in the United Kingdom. These data clearly suggest that events occurring in the first years of life have decisive influence on developmental trajectories that influence the subsequent development of asthma.
Together, these data thus suggest that most of the acquired structural and functional changes that are often observed in asthma occur in the first years of life, at a time when the main triggers of wheezing are viral infections (28). It is thus plausible to surmise that abnormal inflammatory responses to viruses in these first years of life could predispose to the structural (“airway remodeling”) and functional changes in the lungs and airways that are characteristic of asthma.
VIRAL INFECTION IN EARLY LIFE AND SUBSEQUENT ASTHMA AND AIRWAY OBSTRUCTION
The association between viral infection in early life and the subsequent development of asthma and asthma-related phenotypes has been extensively scrutinized, and the most widely studied organism has been the respiratory syncytial virus (RSV) (29). There is now strong evidence suggesting that lower respiratory illnesses due to RSV are associated with increased likelihood of subsequent wheezing, at least until the pre-pubertal years (30), and deficits in lung function (31). If this association is causal or if otherwise it is due to common risk factor for the illness itself and the outcomes it is associated with is currently unknown, but most likely, the mechanisms involved are heterogeneous (29).
More recently, the development of new molecular tools has enhanced viral diagnostics during acute respiratory illnesses, and these advances have uncovered infection due to rhinoviruses (RV) as an important determinant of acute episodes of airway obstruction in infancy. In a longitudinal study in Madison, Wisconsin, RV and RSV were isolated during a very high proportion of wheezing episodes occurring during the first years of life in newborns at high risk for allergies (32). Infection with either virus in early life was a strong risk factor for asthma at age 6, but the association was much stronger for RV (relative risks of almost 10) than for RSV (33). Although longer follow-up is needed to determine if the risk for subsequent wheezing decreases with age as it does for RSV (30), these results indicate that susceptibility to RV infection may be a common thread linking wheezing in early life and asthma later during childhood. This conclusion is strongly supported by the finding that the most common factor associated with exacerbations of asthma in older children and adults is RV infection (34).
DETERMINANTS OF SUSCEPTIBILITY TO RHINOVIRUS INFECTION
The above findings suggest that one potential explanation for the strong connection between early life wheezing and subsequent asthma could be a common susceptibility to rhinovirus infection. There are very few studies of risk factors for wheezing during RV infection in children aged less than 3 years. Kusel and colleagues (35) reported no association between allergic sensitization and wheezing lower respiratory illnesses in the first or second year of life in a Perth (Australia) cohort of high risk children, but the authors did not divide the illnesses according to the virus that caused them (RV or RSV). Jackson and coworkers (33) showed that children enrolled in the Wisconsin cohort who became sensitized to local aeroallergens by age 1 were not more likely to have wheezing RV illnesses by that age. However, children who became sensitized by age 3 were significantly more likely to have RV wheezing illnesses when compared with those who were not sensitized by that age. No information was provided for the relation between RSV illnesses and allergic sensitization at either age. These results could thus suggest that the stronger association between asthma at age 6 and RV wheezing in the first 3 years of life could be due to a common predisposition for atopy among children who have airway obstruction during these two age periods. Although it is not possible to determine from the available data the direction of the association between allergic sensitization and RV illness in early life, it is reasonable to postulate that sensitization to aeroallergens may provide an airway milieu that increases susceptibility to RV infection. However, there is surprisingly little evidence supporting this contention. Parry and colleagues showed that in subjects with allergies, IFN-γ responses by peripheral blood mononuclear cells were inversely correlated with viral shedding during experimental infection with rhinovirus, but not with severity of respiratory symptoms (36). Moreover, in the study by Jackson and coworkers, both allergic sensitization and RV infection by age 3 contributed independently to the risk of having asthma at age 6 (33).
What determines this persistent susceptibility to rhinovirus in young children who will go on to develop asthma is not known. Macaubas and colleagues (37) showed that children who would go on to develop asthma by age 6 had significantly lower cord serum IL-4 and IFN-γ levels than children who did not develop asthma. Similarly, Stern and coworkers (38) showed diminished IFN-γ responses by peripheral blood mononuclear cells during the first year of life in children who subsequently developed chronic wheezing. Recently, Wark and colleagues (39) showed that primary bronchial epithelial cells from subjects with asthma had significantly impaired IFN-β response when infected with rhinovirus compared with responses of healthy control subjects. In a similar experiment, bronchial epithelial cells from patients with asthma showed marked impairment in IFN-λ responses to rhinovirus infection (40). Whether these abnormalities preceded the development of asthma or were a consequence of the chronic inflammatory responses present in the asthmatic airway could not be determined from these experiments. However, the longitudinal studies linking rhinovirus infection in early life to subsequent asthma risk suggest that congenital, perhaps genetically mediated, impairment of responses to rhinovirus could explain the increase susceptibility to airway obstruction in these subjects. It is possible that this increased susceptibility to airway obstruction may be due to altered inflammatory responses during viral infections, and these altered responses may, in turn, predispose to chronic deficits in airway function.
DEFICITS IN LUNG FUNCTION AND EARLY ALLERGIC SENSITIZATION
In both the Perth and the Wisconsin cohort studies, allergic sensitization and viral infection in early life were independent determinants of asthma risk by the early school years. Other newborn cohorts have explored the mechanisms by which early allergic sensitization can influence asthma susceptibility. Lombardi and coworkers showed that early (by age 6 years) allergic sensitization to Alternaria, the most common asthma-related allergen in the Tucson area, was strongly associated with hyperresponsiveness to cold, dry air measured concomitantly, and to both persistent and incident asthma up to age 11 years (41). In the Manchester (UK) asthma birth cohort, Lowe and colleagues (42) measured specific airway resistance using a plethysmograph and both sensitization and exposure to aeroallergens (mite, dog, and cat) in almost 500 unselected, 3-year-old children. They found that neither sensitization nor exposure to the allergens, when present separately, had any significant influence on lung function. However, children who were exposed and sensitized had significantly higher specific airway resistance than those who only had one or neither of these two risk factors. Very similar findings were reported by Illi and coworkers (43) in the German Multicenter Allergy birth cohort. They observed that children who were sensitized to perennial allergens (mite, dog, and cat) by age 3 had significantly lower levels of lung function during the school years than those who were not sensitized. Moreover, exposure to high levels of perennial allergens aggravated these effects, and thus exposure was also associated with increased bronchial responsiveness, especially among children who wheezed. In contrast, sensitization and exposure later in life had only marginal effects on lung function and bronchial responsiveness. The German study also confirmed that most children who wheezed during the preschool years but were not sensitized to perennial allergens in early life lost their symptoms by school age. The data thus suggest that the airways of young children may be particularly susceptible to presumably persistent airway inflammatory responses triggered by exposure to perennial allergens in sensitized subjects.
CONCLUSIONS
The results of several newborn cohort studies have markedly increased our understanding of the natural history of asthma and of the factors that determine its inception. The roots of several asthma-related phenotypes can be found in immune and airway characteristics that are either present at birth or that can be detected shortly thereafter. This novel finding is consistent with the observation that most somatic phenotypes show marked plasticity (i.e., susceptibility to change under the influence of environmental exposures) early during development, and this plasticity tends to decrease with aging (16). Developmental plasticity has profound evolutionary consequences, because individuals in whom phenotypic changes induced by novel exposures are associated with increased fitness are more likely to transmit the hereditary traits that predisposed them to those adaptive changes to their offspring. However, developmental plasticity can also be associated with harmful phenotypic changes that track with age in susceptible individuals and that predispose them to chronic diseases such as asthma and COPD. Elucidating these complex gene by environment by development interactions is a necessary step for the development of successful strategies for the primary prevention of these debilitating diseases.
Funded by grants HL056177, HL080083, and HL064307 from the National Heart, Lung, and Blood Institute.
Conflict of Interest Statement: F.D.M. has served on the Merck Advisory Board and participated in one MedImmune Advisory Board meeting. He also served as a consultant for GlaxoSmithKline, Pfizer, Genentech, and MedImmune. In the last 3 years he has also received lecture fees for events sponsored by Merck and Genentech. In each of the last 3 years he was selected as a Pfizer Visiting Scholar, a program meant to increase opportunities for scientific exchange and education at medical schools, teaching hospitals and other organizations. No additional relationships exist between Dr. Martinez and these (or any other) commercial entities.
References
- 1.Bush A. COPD: a pediatric disease. COPD 2008;5:53–67. [DOI] [PubMed] [Google Scholar]
- 2.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007;357:1946–1955. [DOI] [PubMed] [Google Scholar]
- 3.Hilgendorff A, Heidinger K, Pfeiffer A, Bohnert A, Konig IR, Ziegler A, Merz C, Frey G, Chakraborty T, Gortner L, et al. Association of polymorphisms in the mannose-binding lectin gene and pulmonary morbidity in preterm infants. Genes Immun 2007;8:671–677. [DOI] [PubMed] [Google Scholar]
- 4.Hamvas A, Cole FS, Nogee LM. Genetic disorders of surfactant proteins. Neonatology 2007;91:311–317. [DOI] [PubMed] [Google Scholar]
- 5.Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med 2002;347:2141–2148. [DOI] [PubMed] [Google Scholar]
- 6.Stocks J, Coates A, Bush A. Lung function in infants and young children with chronic lung disease of infancy: the next steps? Pediatr Pulmonol 2007;42:3–9. [DOI] [PubMed] [Google Scholar]
- 7.Northway WH Jr, Moss RB, Carlisle KB, Parker BR, Popp RL, Pitlick PT, Eichler I, Lamm RL, Brown BW Jr. Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med 1990;323:1793–1799. [DOI] [PubMed] [Google Scholar]
- 8.Doyle LW, Faber B, Callanan C, Freezer N, Ford GW, Davis NM. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics 2006;118:108–113. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand JM, Riley SP, Popkin J, Coates AL. The long-term pulmonary sequelae of prematurity: the role of familial airway hyperreactivity and the respiratory distress syndrome. N Engl J Med 1985;312:742–745. [DOI] [PubMed] [Google Scholar]
- 10.Weiss ST, Ware JH. Overview of issues in the longitudinal analysis of respiratory data. Am J Respir Crit Care Med 1996;154:S208–S211. [DOI] [PubMed] [Google Scholar]
- 11.King ME, Mannino DM, Holguin F. Risk factors for asthma incidence: a review of recent prospective evidence. Panminerva Med 2004;46:97–110. [PubMed] [Google Scholar]
- 12.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance–United States, 1971–2000. MMWR Surveill Summ 2002;51:1–16. [PubMed] [Google Scholar]
- 13.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet 2007;370:758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG Jr. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol 1993;15:75–88. [DOI] [PubMed] [Google Scholar]
- 15.Turner SW, Palmer LJ, Rye PJ, Gibson NA, Judge PK, Cox M, Young S, Goldblatt J, Landau LI, Le Souef PN. The relationship between infant airway function, childhood airway responsiveness, and asthma. Am J Respir Crit Care Med 2004;169:921–927. [DOI] [PubMed] [Google Scholar]
- 16.Pigliucci M, Schlichting C. Phenotypic evolution: a reaction norm perspective. Sunderland, MA: Sinauer Associates, Inc.; 1996.
- 17.Burrows B, Knudson RJ, Cline MG, Lebowitz MD. A reexamination of risk factors for ventilatory impairment. Am Rev Respir Dis 1988;138:829–836. [DOI] [PubMed] [Google Scholar]
- 18.Weiss ST, Tosteson TD, Segal MR, Tager IB, Redline S, Speizer FE. Effects of asthma on pulmonary function in children: a longitudinal population-based study. Am Rev Respir Dis 1992;145:58–64. [DOI] [PubMed] [Google Scholar]
- 19.Phelan PD, Robertson CF, Olinsky A. The Melbourne Asthma Study: 1964–1999. J Allergy Clin Immunol 2002;109:189–194. [DOI] [PubMed] [Google Scholar]
- 20.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med 2003;349:1414–1422. [DOI] [PubMed] [Google Scholar]
- 21.Håland G, Carlsen KC, Sandvik L, Devulapalli CS, Munthe-Kaas MC, Pettersen M, Carlsen KH. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med 2006;355:1682–1689. [DOI] [PubMed] [Google Scholar]
- 22.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med 2005;172:1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strunk RC, Weiss ST, Yates KP, Tonascia J, Zeiger RS, Szefler SJ. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol 2006;118:1040–1047. [DOI] [PubMed] [Google Scholar]
- 24.Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, Jeffery PK. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med 2007;176:858–864. [DOI] [PubMed] [Google Scholar]
- 25.Saglani S, Malmstrom K, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, Turpeinen M, Rogers AV, Payne DN, Bush A, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med 2005;171:722–727. [DOI] [PubMed] [Google Scholar]
- 26.Bush A. How early do airway inflammation and remodeling occur? Allergol Int 2008;57:11–19. [DOI] [PubMed] [Google Scholar]
- 27.Kuehni CE, Strippoli MP, Low N, Silverman M. Asthma in young south Asian women living in the UK: the importance of early life. Clin Exp Allergy 2007;37:47–53. [DOI] [PubMed] [Google Scholar]
- 28.Lemanske RF Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, Kirk CJ, Reisdorf E, Roberg KA, Anderson EL, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol 2005;116:571–577. [DOI] [PubMed] [Google Scholar]
- 29.Martinez FD. Heterogeneity of the association between lower respiratory illness in infancy and subsequent asthma. Proc Am Thorac Soc 2005;2:157–161. [DOI] [PubMed] [Google Scholar]
- 30.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;353:541–545. [DOI] [PubMed] [Google Scholar]
- 31.Openshaw PJ, Dean GS, Culley FJ. Links between respiratory syncytial virus bronchiolitis and childhood asthma: clinical and research approaches. Pediatr Infect Dis J 2003;22(2, Suppl)S58–S64. (discussion S64–55). [DOI] [PubMed] [Google Scholar]
- 32.Gern JE, Martin MS, Anklam KA, Shen K, Roberg KA, Carlson-Dakes KT, Adler K, Gilbertson-White S, Hamilton R, Shult PA, et al. Relationships among specific viral pathogens, virus-induced interleukin-8, and respiratory symptoms in infancy. Pediatr Allergy Immunol 2002;13:386–393. [DOI] [PubMed] [Google Scholar]
- 33.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, Dasilva DF, Tisler CJ, Gern JE, Lemanske Jr RF. Wheezing rhinovirus illnesses in early life predict asthma development in high risk children. Am J Respir Crit Care Med 2008;178:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Message SD, Johnston SL. Viruses in asthma. Br Med Bull 2002;61:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol 2007;119:1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parry DE, Busse WW, Sukow KA, Dick CR, Swenson C, Gern JE. Rhinovirus-induced PBMC responses and outcome of experimental infection in allergic subjects. J Allergy Clin Immunol 2000;105:692–698. [DOI] [PubMed] [Google Scholar]
- 37.Macaubas C, de Klerk NH, Holt BJ, Wee C, Kendall G, Firth M, Sly PD, Holt PG. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet 2003;362:1192–1197. [DOI] [PubMed] [Google Scholar]
- 38.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol 2007;120:835–841. [DOI] [PubMed] [Google Scholar]
- 39.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005;201:937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med 2006;12:1023–1026. [DOI] [PubMed] [Google Scholar]
- 41.Lombardi E, Morgan WJ, Wright AL, Stein RT, Holberg CJ, Martinez FD. Cold air challenge at age 6 and subsequent incidence of asthma. a longitudinal study. Am J Respir Crit Care Med 1997;156:1863–1869. [DOI] [PubMed] [Google Scholar]
- 42.Lowe LA, Woodcock A, Murray CS, Morris J, Simpson A, Custovic A. Lung function at age 3 years: effect of pet ownership and exposure to indoor allergens. Arch Pediatr Adolesc Med 2004;158:996–1001. [DOI] [PubMed] [Google Scholar]
- 43.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet 2006;368:763–770. [DOI] [PubMed] [Google Scholar]