Abstract
Asthma currently affects the lives of more than 30 million Americans from infancy to the elderly. In many ways, pediatric asthma differs from adult asthma, including childhood-onset adult asthma. Despite many advances in our understanding of the disease, the natural history of asthma is not well defined, especially in different subsets of patients. For many with allergic asthma the disease has its origins in early childhood, associated with early sensitization to aeroallergens and exposure to repeated viral infections. These early life exposures, coupled with genetically determined susceptibility, have a major impact on the natural history of the disease. A number of risk factors during the critical early stages in the initiation of asthma have been associated with subsequent outcomes. In addition, protective factors linked to early life experiences have also been delineated which may impact the development of atopy and asthma and reduce the prevalence of these diseases. Cumulatively, the data highlight the critical nature of this early period in which immune/inflammatory responses in the lung are initiated and serve to maintain the disease in subsequent years.
Keywords: asthma, children, predictors, persistence
CHILDHOOD ASTHMA
Most cases of persistent wheezing and defined as asthma begin in early childhood and to a large extent define respiratory health throughout the individual's lifetime (1–3). Wheezing is fairly common in young children, in particular preschoolers. Because of the complexity of the initiating events (4), it is often difficult to predict whether the wheezing infant will go on to develop asthma. Cohort studies have demonstrated that roughly half of the “early” wheezing children become asymptomatic by school age. Although this group may have diminished lung function early on, by 6 years function is improved, although perhaps to levels still below normal (1). Children who had wheezing that began in infancy and continued at 6 years demonstrated normal function initially, with reduced lung function by 6 years. It thus appears that children defined as asthmatic with persistent wheezing have some ongoing chronic inflammatory process that results in airway alterations and some loss of lung function in early childhood, which extends to varying degrees into adulthood. It is of interest that adults with significant airway obstruction by age 30 to 40 already demonstrated reduced lung function by 10 years of age (5).
These findings highlight the fact that allergic asthma often begins in early childhood but the natural course of the disease can follow several pathways. In the majority of individuals with persistent wheeze, those with asthma, the disease continues through late adolescence and into adulthood, with or without a transient or permanent reprise in the second to third decade of life. In a longitudinal birth cohort study, approximately one third whose asthma appeared to be in remission at 18 years of age relapsed by 21 or 26 years of age. Relapse was not easily predicted, especially by measurements of airway responsiveness. Atopy and lower FEV1/FVC ratio at 18 years of age were significant independent prognostic factors for relapse in multiple logistic regression analyses (6). Among the factors predicting persistence or relapse were sensitization to house dust mites, airway hyperresponsiveness, female sex, smoking, and early age at onset (7).
There are many unknown features in the natural course of the disease. For example, it is not known whether patients with initially mild disease progress to experiencing moderate to severe disease or if it remains mild, and whether severe asthma begins early and remains severe. Patients with persistent disease have diminished lung function, and there is an inverse correlation between lung function and disease severity (8, 9). Outcomes in adult asthma may be determined primarily in early childhood. Although lung function abnormalities are established by the early school years, they may not progress to a large extent thereafter (7). Even in the very young, and certainly by school age, some of the characteristic inflammatory and remodeling features of chronic asthma observed in adults are found on biopsy specimens in young children (10, 11). Some of the characteristic features of asthma, including increased reticular basement membrane thickness, were seen in preschool children with confirmed wheeze between the ages of 1 and 3 years (12). Since these changes in airway remodeling are described in very young patients, well before repeated cycles of inflammation and decline in lung function, other possibilities warrant consideration. Among these potential causes, early remodeling may be the result of impaired barrier function resulting from disruption of epithelial tight junctions enabling inhaled materials to pass through to the airway wall, triggering activation of immune/inflammatory cells (13).
Although most individuals with asthma have mild to moderate disease, about 5 to 10% have severe disease that is refractory to treatment with currently available medications (14). Little is known about the age-related differences in the characteristics of asthma, especially severe asthma in children and adults. In a cross-sectional analysis of severe asthma, the clinical differences in children and adults were considerable (15). Children were more likely to be male, to be more sensitive to the suppressive effects of glucocorticoids, and to have less impaired lung function. Only 28% of the children would have been classified as having severe persistent asthma based on FEV1 data, and more than 40% would have been classified as having mild persistent disease. These findings related to FEV1 values are consistent with previous reports highlighting the inadequacy of FEV1 measures as indicators of disease severity in children (16, 17). However, despite higher mean FEV1 values in the children, they displayed a greater annual decline in FEV1 than the adults (1.8% versus 0.4%, respectively) (15). The findings illustrate the importance of monitoring lung function serially over time to better understand asthma progression in children.
Childhood asthma, at least initially, is more likely to be episodic. In addition to differences in FEV1, children tend to hyperinflate, to increase total lung capacity and residual volume, and to have less airway resistance (Raw) and better airway conductance to airflow (18). Thus, FEF25–75 may be a more sensitive measure of airflow obstruction than FEV1 in children with asthma (16), although the test is very effort dependent and results potentially inconsistent.
NATURAL HISTORY OF CHILDHOOD-ONSET ASTHMA
Asthma in early life may also differ from asthma in childhood. Asthma in early life is characterized by parental asthma, a history of eczema, and early allergic sensitization, as discussed further below. The infants appear less responsive to inhaled glucocorticoids, and the inflammatory response, if present, may be neutrophilic in nature (19). Roughly 80 to 90% of children with asthma will have allergic asthma, eosinophilic inflammation, some changes consistent with airway remodeling, and a generally good response to inhaled glucocorticoids (20).
Lung development may be affected by the asthmatic processes, an important and not well-defined aspect of asthma in children. Significant impairment of lung function may occur early, even prenatally (1, 21). Reduced lung function in early infancy has been associated with later obstructive airway disease. Lung function was measured in a prospective birth cohort study of healthy infants shortly after birth, using tidal breathing flow–volume loops. Compared with infants with lung function above the median, children at or below the median were more likely at 10 years of age to have a history of asthma, to have current asthma, and to have severe bronchial hyperresponsiveness (22).
Another risk factor for persistent wheezing in later childhood is transient tachypnea of the newborn. Maternal asthma, birthweight over 4,500 g, male sex, and urban location were risk factors for development of transient tachypnea of the newborn period, and these infants were at significant risk for persistent wheezing later in life (23).
This could lead to different models of disease progression, as illustrated in Figure 1, with one group beginning normally, but rapidly deteriorating with progressive loss of lung function. Another group, after an initial insult, more slowly lose lung function. Another group may begin with low function, and only lose further function slowly over time. It is unclear at present whether this apparent heterogeneity in natural history reflects the activation of different pathophysiologic pathways resulting from different environmental exposures and/or genetic susceptibilities, or are the consequences of abnormalities in lung development.
Figure 1.
Models of disease progression from childhood to adulthood. (A) Normal: remains normal, (B) begins normal: steep slope resulting in severe obstruction over time, (C) begins normal: rapid decline early on resulting in severe obstruction, and (D) Begins with low values and continues with low lung function. Adapted by permission from Reference 47.
This modeling of early asthma heterogeneity becomes important after considering the needs for specific pharmacologic interventions early in the onset of disease. Based on several studies, it appears that inhaled corticosteroids (ICS) do not interfere with the progressive loss of lung function in children with asthma. In the first well-controlled longitudinal study, the Children's Asthma Management Program (CAMP), ICS were no better than placebo or nedocromil in maintaining lung function (postbronchodilator FEV1) over the 4 years of the study (24). At least three other studies have reached similar conclusions, showing that ICS have little benefit on the natural course of the disease or maintenance of lung function at end-point (25–27). This is despite the obvious benefits of ICS on symptoms, use of rescue medications, and urgent care visits while on the medications. Somewhat more revealing in the CAMP cohort was a group of decliners, children who had a significant decline in lung function over the duration of the study (28). These patients were equally distributed over the steroid, nedocromil, and placebo arms, further highlighting the inability of corticosteroids to prevent the progressive loss of lung function.
EARLY ORIGINS OF ASTHMA
Much of the data point to the initiation or inception of asthma in infancy and early childhood. It is thus important to consider the factors that may influence this critical period. Currently, the development of allergic asthma is proposed to be the result of gene–environment interactions. Atopy and asthma show a strong genetic susceptibility with parental asthma associating with asthma prevalence in the offspring. How other atopic diseases, eczema, food allergy, or rhinitis influence the likelihood of developing asthma is unclear. In infants with atopic dermatitis, mutations in the filaggrin gene in the skin or other keratinizing epithelia were associated with an increased risk for eczema by more than threefold, a substantial risk for allergic rhinitis, as well as asthma occurring in the context of eczema (29). Surprisingly, eczema in the first 2 years of life was associated with an increased risk of asthma in boys but not girls (30).
Epidemiologically, asthma prevalence appears to be increasing more in younger than older children. Based on all of the epidemiologic information gathered, a critical area requiring investigation is the nature and consequences of early life experiences on the natural history of asthma and their potential impact on asthma heterogeneity. Early exposures to environmental factors are thought to play important roles in concert with genetic susceptibility. These environmental factors include exposures to microbial products, indoor and outdoor allergens, poor air quality, and environmental tobacco smoke. Economic status may also play an important role in this susceptibility (31). In addition to the risk factors, there are a number of potential protective factors that have been identified.
A balance between these different exposures ultimately determines outcome. This balance is perhaps best illustrated from numerous epidemiologic studies comparing antenatal and perinatal exposure to farms and livestock, endotoxin, and consumption of unpasteurized farm milk, and their preventative influences on development of atopy and asthma (32). Moreover, it may be that it is antenatal maternal exposure to diverse mircrobial compounds that is required for protection against the development of atopic sensitization in the offspring (33). Although not entirely defined, these studies collected under the umbrella of the “hygiene hypothesis” emphasize the importance of early life experiences in dictating whether a susceptible infant will develop atopy and asthma or not.
FACTORS IMPACTING THE INDUCTION AND PROGESSION OF ASTHMA
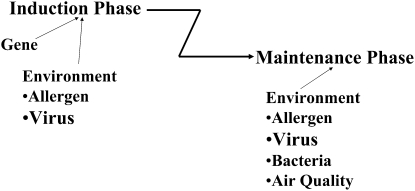
Taking advantage of these findings, a model can be developed that distinguishes (at least) two stages of asthma: an induction (inception) or sensitization phase and a maintenance or progression phase. Conceptually, the pathway(s) and triggers leading to the first phase, which represents the “origins of asthma,” differs from the pathways contributing to the later, maintenance phase. In animal models, these two phases are easily distinguished; for example, IL-4 is essential in the initial phase, whereas IL-13 becomes critical in the second phase. Importantly, this distinction may also explain the failure of clinical trials with certain drugs, such as those targeting IL-4 in asthmatics who are clearly beyond the induction phase and where preventing the effects of IL-4 have limited benefit in the maintenance phase.
Among the factors that may impact the induction phase in infancy, early allergen sensitization and respiratory syncytial virus (RSV) infection appear to be important (Figure 2). The role of early sensitization to allergen on the natural history of wheezing was studied in a large multicenter allergy study group (34). The study followed 1,300 children from birth to 13 years of age who had early wheezing in the first 3 years of life. Allergen exposure was assessed at 6 and 18 months, and 3, 4, and 5 years of age. Lung function was assessed at 7, 10, and 13 years of age. Of the children who wheezed by 3 years of age, 90% who were not atopic lost their symptoms by school age and had normal lung function at puberty. In contrast, early sensitization in the first 3 years of life (positive skin test responses to house dust mite, dog, or cat) was associated with a loss of lung function at school age. Exposure to high levels of perennial allergens early in life further aggravated disease. Sensitization and exposure later in life or sensitization to food allergens had little impact on asthma development. These findings suggest that the likelihood of developing a chronic course of asthma, bronchial hyperresponsiveness, presumably continuing airway inflammation, and loss of lung function at school age and puberty were influenced by early aeroallergen sensitization in the first 3 years of life. This study, if validated, offers a means of predicting outcomes early in the course of the disease and confirms the importance of age at initial sensitization in defining later outcomes. The findings indicate that the processes initiating chronic airway inflammation, altered airway function, even remodeling, begin in early life. These results have important therapeutic implications; the eradication of culprit allergens may not be achievable and could accelerate rather than decrease the risk of atopy (35). Reducing house dust mite exposure by encasing mattresses and pillows has failed to impact development of asthma (36). As reported recently, exposure thresholds to different allergens for sensitization or asthma appear to be sufficiently low, making it unlikely that interventional measures to reduce domestic allergen levels alone will impact the incidence of sensitization or asthma in childhood (37).
Figure 2.
Stages of asthma. Asthma may be divided into two main stages: an initiation or inception phase, and a maintenance or progression phase. The triggers and pathways activated may differ in the different stages. In the initiation phase, early allergen exposure and repeated viral infections coupled with genetic susceptibility may be important in setting the outcomes in the second stage.
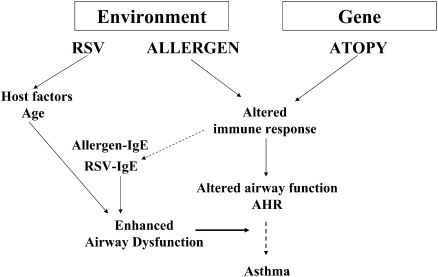
In infancy, exposure to many viruses, even repeatedly, is common. During early life, exposure to RSV has been linked to asthma. Whereas RSV bronchiolitis in infancy was not associated with persistent wheezing, in infants hospitalized for severe RSV bronchiolitis, the link to persistent wheezing and asthma may be stronger (reviewed in Reference 38). Peculiar to RSV is the failure to develop protective immunity, so reinfection with RSV is not uncommon. Using an animal model, we examined the consequences of age at initial infection on the response to reinfection 5 weeks later (39). In mice initially infected with RSV at weaning (3 wk of age), reinfection 5 weeks later elicited a significant airway and tissue lymphocytosis, but the animals were protected against development of airway hyperresponsiveness (AHR) to inhaled methacholine. In contrast, after initial infection with RSV of newborn mice (< 1 wk of age), reinfection 5 weeks later resulted in a marked airway eosinophilic inflammatory response, enhanced goblet cell metaplasia and mucus hyperproduction, and heightened AHR to inhaled methacholine (increased lung resistance). Infected initially as newborns, these mice also developed increases in RSV-specific IgE (a risk factor in young infants as well), and this was shown to augment AHR on reinfection (40). Thus, similar to early allergen exposure, early infection with RSV can result in altered airway function. Further, the combination of RSV infection and allergen sensitization enhances development of airway inflammation and altered airway function (41–44). The potential interactions of allergen and virus in potentiating asthma is illustrated in Figure 3, where environmental exposure, genetic susceptibility, and age-dependent factors intersect to increase immune/inflammatory responses in the lung, resulting in increased airway dysfunction. The propensity of the young to develop allergen-specific and virus-specific IgE further enhances lung allergic responses, contributing to greater airway inflammation and altered airway function.
Figure 3.
Importance of gene–environment interaction on the origins of asthma. Gene–environment interactions contribute to the development of altered airway function and airway inflammation. Host factors and the age at initial exposure to allergen and/or virus are important determinants of the specific immune responses to the exposure. The development of allergen-specific and virus-specific IgE enhances the immune/inflammatory responses in the lung.
Although much of the work associating virus infection and later risks for developing asthma has focused on RSV, recent studies have emphasized potential associations with other viruses in infancy. Among viral illnesses developing in infancy and early childhood in an outpatient setting, rhinovirus infections may be among the predictors of the subsequent development of asthma at age 6 in a high-risk cohort (45). The risk factors may not be restricted to early virus infections. Neonates colonized in the hypopharynx with bacteria such as Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis may also be at risk for recurrent wheeze and asthma early in life (46).
Support for this notion of a critical susceptibility in early life to the initiation of disease is that neonates are skewed to developing a more pronounced Th2, proallergic cytokine response, which in turn contributes to the development of atopy. Although the concept of a Th1/Th2 imbalance is attractive for its simplicity, further study of events in the early sensitization period are required to define specific predisposing factors and what truly renders the genetically at-risk infant susceptible to developing an atopic disease such as asthma.
INTERVENTION AND PREVENTION OF ASTHMA
What then are the opportunities in at-risk infants and toddlers to intervene in this critical initiation or inception phase? Can we take advantage of the epidemiologic studies on farms to understand how tolerance to specific allergens may be induced at a critical stage of exposure? The timing for intervention is particularly critical, as tolerance induction is more easily achieved early in the developing immune response as opposed to reversing an established immune response. Corticosteroids are not the answer, as they impart little to no disease-modifying benefits. All of the data point to a critical window of opportunity to induce tolerance to specific allergens. A number of strategies have been proposed, even initiated, in manipulating at-risk infants. These strategies have included allergen avoidance and purposeful allergen exposure, and vaccination with different microbial products or the (killed) microbes themselves. Immunotherapy in infancy is another avenue currently under investigation. Given the ethical issues of researching infants, it will take considerable time to demonstrate the efficacy of any one of these strategies. Nonetheless, as Sears and coworkers have noted, even delaying the onset of asthma by 10 years may reduce the risk of relapse by almost 70% (7).
CONCLUSIONS
Children with asthma are different than adults with asthma. Asthma may also have very different phenotypes at various stages of the disease or at different ages, from infancy to adolescence. In terms of lung function, lung physiology, and immunopathology, these differences are now being defined and likely contribute to the significant heterogeneity of the disease in children. The inception phase of the disease occurs at a critical time-point with early allergen exposure and viral infections intersecting with genetic susceptibility, setting the stage for future outcomes. Little is known about whether the natural history of the disease or the specific pathophysiologic pathways, once initiated, set the stage for long-standing disease. Importantly, there is a critical absence of validated predictive markers, especially noninvasive markers, to monitor disease progression in children. Currently available medications have either not been tested as part of an early intervention strategy for their influence on the natural history of childhood asthma or, as in the case of corticosteroids, have shown no disease-modifying effects. The ability to influence development of early immune tolerance is one of the important challenges today. “Asthma is like a tin of sardines—we are all looking for the key.”
Supported by NIH grants HL-36577, HL-61005, and AI-77609, and by EPA grant R825702. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH.
Conflict of Interest Statement: E.W.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. N Engl J Med 1995;332:133–138. [DOI] [PubMed] [Google Scholar]
- 2.Stick S. The contribution of airway development to paediatric and adult lung disease. Thorax 2000;55:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dezateux C, Stocks J. Lung development and early origins of childhood respiratory illness. Br Med Bull 1997;53:40–57. [DOI] [PubMed] [Google Scholar]
- 4.Martinez FD. The epidemiology of wheezing in infants and preschool children. In: Martinez FD, Godfrey S, Editors. Wheezing disorders in the preschool child: pathophysiology and management. Abingdon, UK: Taylor & Francis; 2003. pp. 1–19.
- 5.Oswald H, Phelan PD, Lanigan A, Hibbert M, Carlin JB, Bowes G, Olinsky A. Childhood asthma and lung function in mid-adult life. Pediatr Pulmonol 1997;23:14–20. [DOI] [PubMed] [Google Scholar]
- 6.Taylor DR, Cowan JO, Greene JM, Willan AR, Sears MR. Asthma in remission: can relapse in early adulthood be predicted at 18 years of age? Chest 2005;127:845–850. [DOI] [PubMed] [Google Scholar]
- 7.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med 2003;349:1414–1422. [DOI] [PubMed] [Google Scholar]
- 8.Weiss ST, Tosteson TD, Segal MR, Tager IB, Redline S, Speizer FE. Effects of asthma on pulmonary function in children: a longitudinal population-based study. Am Rev Respir Dis 1992;145:58–64. [DOI] [PubMed] [Google Scholar]
- 9.Cline MG, Dodge R, Lebowitz MD, Burrows B. Determinants of percent predicted FEV1 in current asthmatic subjects. Chest 1994;106:1089–1093. [DOI] [PubMed] [Google Scholar]
- 10.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med 2005;172:1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins HA, Cool C, Szefler SJ, Covar R, Brugman S, Gelfand EW, Spahn JD. Histopathology of severe childhood asthma: a case series. Chest 2003;124:32–41. [DOI] [PubMed] [Google Scholar]
- 12.Saglani S, Payne DN, Jin J, Wang Z, Nicholson AG, Bush A, Jeffery PK. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med 2007;176:858–864. [DOI] [PubMed] [Google Scholar]
- 13.Holgate ST, Leung DYM, Ledford DK. Epithelium dysfunction in asthma. J Allergy Clin Immunol 2007;120:1233–1244. [DOI] [PubMed] [Google Scholar]
- 14.Busse WW, Banks-Schlegal S, Wenzel SE. Pathophysiology of severe asthma. J Clin Immunol 2000;106:1033–1042. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins HA, Cherniack R, Szefler SJ, Covar R, Gelfand EW, Spahn JD. A comparison of the clinical characteristics of children and adults with severe asthma. Chest 2003;24:1318–1324. [DOI] [PubMed] [Google Scholar]
- 16.Bacharier LB, Mauger DT, Lemanske RF, Schend V, Sorkness C, Strunk RC. Classifying asthma severity in children: is measuring lung function helpful? J Allergy Clin Immunol 2002;109:S266. [Google Scholar]
- 17.Fuhlbrigge AL, Kitch BT, Paltiel AD, Kuntz KM, Neumann PJ, Dockery DW, Weiss S. FEV1 is associated with risk of asthma attacks in a pediatric population. J Allergy Clin Immunol 2004;107:61–67. [DOI] [PubMed] [Google Scholar]
- 18.Paull K, Covar R, Jain N, Gelfand EW, Spahn JD. Do NHLBI lung function criteria apply to children? A cross-sectional evaluation of childhood asthma at National Jewish Medical and Research Center, 1999–2002. Pediatr Pulmonol 2005;39:311–317. [DOI] [PubMed] [Google Scholar]
- 19.Marguet C, Jouen-Boedes F, Dean TP, Warner JO. Bronchoalveolar cell profiles in children with asthma, infantile wheeze, chronic cough, or cystic fibrosis. Am J Respir Crit Care Med 1999;159:1533–1540. [DOI] [PubMed] [Google Scholar]
- 20.Covar RA, Szefler SJ, Zeiger RS, Sorkness CA, Moss M, Mauger DT, Boehmer SJ, Strunk RC, Martinez FD, Taussig LM; Childhood Asthma Research and Education Network. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J Allergy Clin Immunol 2008;122:741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children's Respiratory Study: 1980 to present. J Allergy Clin Immunol 2003;111:661–675. [DOI] [PubMed] [Google Scholar]
- 22.Haland G, Carlsen KCL, Sandvik L, Devulapalli CS, Munthe-Kaas MC, Pettersen M, Carlsen K-H; ORAACLE. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med 2006;355:1682–1689. [DOI] [PubMed] [Google Scholar]
- 23.Liem JJ, Huq SI, Ekuma O, Becker AB, Kozyrskyj AL. Transient tachypnea of the newborn may be an early clinical manifestation of wheezing symptoms. J Pediatr 2007;151:29–33. [DOI] [PubMed] [Google Scholar]
- 24.The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054–1063. [DOI] [PubMed] [Google Scholar]
- 25.Murray CS, Woodcock A, Langley SJ; the IFWIN study team. Secondary prevention of asthma by the use of inhaled fluticasone propionate in wheezy infants (IFWIN): double-blind, randomized, controlled study. Lancet 2006;368:754–762. [DOI] [PubMed] [Google Scholar]
- 26.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, Bacharier LB, Lemanske RF Jr, Strunk RC, Allen DB, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med 2006;354:1985–1997. [DOI] [PubMed] [Google Scholar]
- 27.Bisgaard H, Hermansen MN, Loland L, Halkjaer LB, Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med 2006;354:1998–2005. [DOI] [PubMed] [Google Scholar]
- 28.Covar RA, Spahn JD, Murphy JR, Szefler SJ. Progression of asthma measured by lung function in the Childhood Asthma Management Program. Am J Respir Crit Care Med 2004;170:234–241. [DOI] [PubMed] [Google Scholar]
- 29.Weidinger S, O'Sullivan M, Illig T, Baurecht H, Depner M, Rodriguez E, Ruether A, Klopp N, Vogelberg C, Weiland SK, et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J Allergy Clin Immunol 2008;121:1203–1209. [DOI] [PubMed] [Google Scholar]
- 30.Lowe AJ, Carlin JB, Bennett CM, Hosking CS, Abramson MJ, Hill DJ, Dharmage SC. Do boys do the atopic march while girls dawdle? J Allergy Clin Immunol 2008;121:1190–1195. [DOI] [PubMed] [Google Scholar]
- 31.Weinmayr G, Weiland SK, Björkstén B, Brunekreef B, Büchele G, Cookson WOC, Garcia-Marcos L, Gotua M, Gratziou C, van Hage M, et al. Atopic sensitization and the international variation of asthma symptom prevalence in children. Am J Respir Crit Care Med 2007;176:565–574. [DOI] [PubMed] [Google Scholar]
- 32.von Mutius E. Asthma and allergies in rural areas of Europe. Proc Am Thorac Soc 2007;4:212–216. [DOI] [PubMed] [Google Scholar]
- 33.Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Üblagger E, Schram-Bijkerk D, Brunekreef B, van Hage M, Scheynius A, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol 2006;117:817–823. [DOI] [PubMed] [Google Scholar]
- 34.Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U. Perennial allergen sensitization early in life and chronic asthma in children: a birth cohort study. Lancet 2006;368:763–770. [DOI] [PubMed] [Google Scholar]
- 35.Woodcock A, Lowe LA, Murray CS, Simpson BM, Pipis SD, Kissen P, Simpson A, Custovic A. Early life environmental control: effect on symptoms, sensitization, and lung function at age 3 years. Am J Respir Crit Care Med 2004;170:433–439. [DOI] [PubMed] [Google Scholar]
- 36.Koopman LP, van Strien RT, Kerkhof M, Wijga A, Smit HA, de Jongste JC, Gerritsen J, Aalberse RC, Brunekreef B, Neijens HJ. Placebo-controlled trial of house dust mite-impermeable mattress covers: effect on symptoms in early childhood. Am J Respir Crit Care Med 2002;166:307–313. [DOI] [PubMed] [Google Scholar]
- 37.Torrent M, Sunyer J, Garcia R, Harris J, Iturriaga MV, Puig C, Vall O, Anto JM, Newman Taylor AJ, Cullinan P. Early-life allergen exposure and atopy, asthma, and wheeze up to 6 years of age. Am J Respir Crit Care Med 2007;176:446–453. [DOI] [PubMed] [Google Scholar]
- 38.Singh AM, Moore PE, Gern JE, Lemanske RF Jr, Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. Am J Respir Crit Care Med 2007;175:108–119. [DOI] [PubMed] [Google Scholar]
- 39.Dakhama A, Park JW, Taube C, Joetham A, Balhorn A, Miyahara N, Takeda K, Gelfand EW. The enhancement or prevention of airway hyperresponsiveness during re-infection with respiratory syncytial virus is critically dependent on the age at first infection and interleukin-13 production. J Immunol 2005;175:1876–1883. [DOI] [PubMed] [Google Scholar]
- 40.Dakhama A, Park JW, Taube C, Chayama K, Balhorn A, Joehtam A, Wei X, Fan RH, Swasey C, Miyahara N, et al. The role of virus-specific immunoglobulin E in airway hyperresponsiveness. Am J Respir Crit Care Med 2004;170:952–959. [DOI] [PubMed] [Google Scholar]
- 41.Schwarze J, Hamelmann E, Bradley KL, Takeda K, Gelfand EW. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest 1997;100:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwarze J, Makela M, Cieslewicz G, Dakhama A, Lahn M, Ikemura T, Joetham A, Gelfand EW. Transfer of the enhancing effect of respiratory syncytial virus infection on subsequent allergic airway sensitization by T lymphocytes. J Immunol 1999;163:5729–5734. [PubMed] [Google Scholar]
- 43.Schwarze J, Gelfand EW. Respiratory viral infections as promoters of allergic sensitization and asthma development. Eur Respir J 2002;19:341–349. [DOI] [PubMed] [Google Scholar]
- 44.Makela MJ, Kanehiro A, Dakhama A, Borish L, Joetham A, Tripp R, Anderson L, Gelfand EW. The failure of IL-10-deficient mice to develop AHR is overcome by RSV infection in allergen sensitized/challenged mice. Am J Respir Crit Care Med 2002;165:824–831. [DOI] [PubMed] [Google Scholar]
- 45.Jackson DJ, Gangnon RE, Evans MD, Robert KA, Anderson EL, Pappas TE, Printz MC, Lee W-M, Shult PA, Reisdorf E, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 2008;178:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, Brashold M, Heltberg A, Vissing NH, Thorsen SV, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 2007;357:1487–1495. [DOI] [PubMed] [Google Scholar]
- 47.Szefler SJ. Facing the challenges of childhood asthma: what changes are necessary? J Allergy Clin Immunol 2005;115:685–688. [DOI] [PubMed] [Google Scholar]