Abstract
The purpose of this review is to discuss recent findings made during studies of the upper airways and sinuses of people with chronic rhinosinusitis (CRS) in the context of the literature. CRS is a chronic inflammatory disorder affecting nearly 30 million Americans and is generally resistant to therapy with antibiotics and glucocorticoids (Meltzer EO and coworkers, J Allergy Clin Immunol 2004;114:155–212). We have formed a collaboration that consists of otolaryngologists, allergists, and basic scientists to address the underlying immunologic and inflammatory processes that are occurring in, and possibly responsible for, this disease. The main emphasis of our work has been to focus on the roles that epithelium, in the sinuses and upper airways, plays as both a mediator and regulator of immune and inflammatory responses. It is not our intention here to provide a comprehensive review of the literature in this area, but we will try to put our work in the context of the findings of others (Kato A and Schleimer RP, Curr Opin Immunol 2007;19:711–720; Schleimer RP and coworkers, J Allergy Clin Immunol 2007;120:1279–1284). In particular, we discuss the evidence that epithelial cell responses are altered in CRS, including those relevant to regulation of dendritic cells, T cells, B cells, and barrier function.
Keywords: chronic rhinosinusitis, inflammation, epithelium, immunology
THE USE OF CHRONIC RHINOSINUSITIS TO STUDY EPITHELIUM IN INFLAMMATORY DISEASE
There are perhaps no mucosal surfaces in human beings that are more accessible to the study of the immune response than the upper airways and sinuses. The upper airways play an important filtering role, and as a consequence there is considerable exposure to particulates, antigens, and potential pathogens. Under optimal conditions, the upper airways and sinuses readily clear these materials and destroy or eliminate them without involvement of the adaptive immune system. When this process fails, micro-organisms may flourish on the mucosa, and acute or chronic inflammation can result. Likewise, if the barrier function of the epithelial layer fails, otherwise innocuous materials may gain access to cells of the immune response in and below the lamina propria, further stimulating the inflammatory process. Moreover, well-studied allergic nasal mucosal responses may also play a supportive role, but the degree and importance is unclear (1). Overall, the etiology and pathogenesis of CRS remains a matter of rigorous debate and while there is, in particular, a significant amount of literature supporting a role for either fungi or bacteria in chronic rhinosinusitis (CRS), this remains controversial and will not be addressed here (2–7).
The clinical presentation of CRS in individual patients covers a spectrum of severity, and CRS is probably best considered as a syndrome with persistent characteristic symptoms rather than a discrete disease entity. Until recently, research has been hampered by many factors, including most prominently the lack of a universally accepted definition. For the purposes of this review, CRS will be defined as symptomatic inflammation of the mucosa of the nose and paranasal sinuses of greater than 12 weeks duration, confirmed by computed tomography (CT) scan and nasal endoscopy (1). The most common symptoms, including nasal obstruction, smell loss, rhinorrhea, and facial pressure, have been well characterized, but confirmatory measures (CT scan and nasal endoscopy) are necessary for research purposes due to the high false-positive rate using clinical criteria alone (1). Furthermore, endoscopic analysis subdivides CRS into a form without visible nasal polyps (CRSsNP) and a form with nasal polyps (CRSwNP) (1). Although some investigators further subdivide the disease based on numerous other criteria such as the presence of fungi and fungal mucins, the presence of hyperplasia of connective tissue or mucosal glands, the presence of aspirin sensitivity or asthma, and so on, we will restrict our discussion to these two forms (1). In general, CRSwNP is associated more closely with the clinical complaints of nasal obstruction and smell loss; nasal polyp mucosa exhibits a high degree of tissue eosinophilia as well as T cells demonstrating a skewing toward Th2 cytokine expression. CRSsNP is associated more closely with facial pain and drainage, Th1 polarization, and a lesser degree of eosinophilic infiltration (8).
Regardless of the inciting antigen or the precise etiology and pathogenesis, it is clear from the cellular constituents that are elevated in CRS that a vigorous immune response is underway. We hasten to say that this immune response, though not infrequently of at least partly an allergic nature involving IgE and aeroallergens, is frequently not accompanied by demonstrable atopic sensitization; therefore, CRS should not be viewed as an allergic disease (note that there is a literature suggesting the existence of a significant number of patients with CRS with local allergen responses but not skin test sensitivity) (5, 9). Due to the value and necessity for sinus surgery to manage the disease of a significant proportion of people suffering from CRS, investigators have access to the substantial quantities of tissue that is removed during the surgical procedures. This includes sinus mucosa, turbinate mucosa, uncinate process tissue, and nasal polyps (when present) in patients with CRSsNP or CRSwNP, as well as in normal control subjects without CRS. In our studies, the primary source of normal control tissue is from patients undergoing transnasal resection of intracranial lesions.
To study the immune and inflammatory process in CRS, we routinely collect epithelial cell scrapings and nasal lavage samples in addition to the above-mentioned surgical tissue. We obtain DNA, mRNA, and protein extracts of the tissues and also retain some of the tissue for frozen sections and for fixation to perform immunohistochemistry. Protein extracts and nasal lavage are exceedingly useful to monitor production of cytokines, chemokines, host defense molecules, immunoglobulins, and so on. Messenger RNA is converted into cDNA for analysis of gene expression by real-time RT-PCR, and cells are cultured for comparative studies of responses in vitro. Data on mediators and tissue responses can be correlated with diagnosis or disease severity determined by the Lund-McKay sinus CT scoring system or by symptom-based questionnaires. It is important to note that the samples collected in our studies are from patients whose disease is of a severity sufficient to warrant surgery and is therefore not necessarily reflective of the whole CRS population.
REGULATION OF INNATE IMMUNE RESPONSES OF EPITHELIUM IN CRS AND THE ACTIONS OF GLUCOCORTICOIDS
A number of our studies have focused on the innate immune response of epithelium, both in vitro and in vivo. Studies by Sha and coworkers several years ago demonstrated that bronchial epithelial cells express mRNA for nearly all Toll-like receptors (TLR), and that stimulation resulted in a clear response especially with ligands for TLR2, TLR3, and TLR5 (10). Not only did these stimuli activate the epithelial cells to express inflammatory genes such as chemokines and cytokines, but the cells also produced a number of pathogen recognition molecules that are likely to be involved in host defense. Studies of TLR expression or responses in epithelial cells from patients and control subjects suggest that there are some alterations in expression and function of TLR2 (11). Epithelial cells from patients with CRS produce less IL-8 and respond less well to TLR2 ligands; although TLR2 mRNA appears to be elevated in nasal scrapings, flow cytometry indicates that TLR2 protein is reduced on the epithelial cells (R.P.S., unpublished observations). Other studies focusing on IL-6, the soluble IL-6 receptor, and the soluble signaling molecule gp130 that suppresses IL-6 signaling have shown that all three of these molecules are elevated in CRSwNP (A. Peters and coworkers, unpublished data). IL-6 is a STAT3 activator that is known to inhibit innate immune responses and may be important as a mediator of the shift to adaptive immunity (12). In studies by Peters and colleagues, G-CSF, another STAT3 activator, was found to be elevated in nasal lavage from patients with CRSwNP (13). Expecting to see elevated activation of STAT3 in polyp tissue as evidence of increased signaling by IL-6 and G-CSF, we were surprised to see just the opposite; Peters and coworkers found that pSTAT3 levels measured by Western blotting are decreased in nasal polyp tissue (13). Since STAT3 mediates signals for many other cytokines, including IL-10, growth hormone, and other members of the IL-6 family, this observation could be relevant to mucosal responses to numerous stimuli. In light of recent studies showing that nonfunctional mutant alleles of STAT3 lead to the hyper IgE syndrome (Job's syndrome), it is possible that the decline in activation of STAT3 that we observe bears some relevance to the pathology of polypoid CRS, which is known to include several features of hyper IgE syndrome, such as high levels of IgE, tissue structural rearrangements, eosinophilic inflammation, increased colonization with Staphylococcus aureus, and so on (14). Along these lines, it is interesting to note that loss of STAT3 function in hyper IgE syndrome leads to a deficiency in the formation of Th17 cells, which are induced in part by the presence of IL-6. T. Carr and L. Suh analyzed levels of IL-17 family members by ELISA and could not detect them in nasal lavage samples or tissue extracts from patients with CRSwNP, suggesting that a local Th17 response may not be occurring despite the inflammation and frequent colonization by S. aureus (13). A hypothesis that we are presently evaluating is that there is a diminished IL-6/STAT3 response that occurs in nasal polyp tissue, leading to a minimal Th17 response, increased IgE, and S. aureus colonization, as if there were a local equivalent of the hyper IgE syndrome.
During the course of our studies on TLR activation of bronchial epithelial cells, we performed some microarray analyses of the influence of glucocorticoid effects on the production of cytokines, chemokines, and host defense molecules by epithelial cells activated by the TLR3 ligand double-stranded RNA (dsRNA). As expected, we found that glucocorticoids strongly inhibited the expression of many TLR-induced inflammatory cytokines (e.g., GM-CSF, IFN-β, TNF, etc.) and chemokines (IL-8, RANTES, MCPs, MIPs, etc.). Surprisingly, we found that the steroid directly induced the expression of several host defense molecules and, when combined with dsRNA, synergistically induced these molecules. Careful review of the literature revealed that a few laboratories had observed enhancement by glucocorticoids of the expression of host defense molecules such as C3, SLPI, MBL, SpA, SpD, and so on (15, 16). We view this finding as paradoxical in the light of the widely held view that glucocorticoids are highly immunosuppressive drugs. We also note, however, that these drugs are the most effective drugs in treating several diseases in which exacerbations of disease are triggered by infections, including asthma, chronic obstructive pulmonary disease, allergic bronchopulmonary aspergillosis (ABPA), allergic fungal sinusitis, CRS, and others. It seems clear now that glucocorticoids actually enhance many aspects of innate immunity, including epithelial barrier function, mucociliary function, neutrophil survival and activity, alveolar macrophage phagocytosis, and epithelial release of a range of molecules, many of which interact directly with microorganisms (such as collectins, pentraxins, the alternate pathway of complement, etc.).
These effects in the airways are reminiscent of the ability of glucocorticoids to enhance the hepatic acute phase response, a response to TLR ligands and inflammatory cytokines that includes the release of many host defense molecules from the liver. Some of the enhancing effects of glucocorticoids on the hepatic acute phase are mediated through the transcription factor C/EBPβ. When we tested whether the epithelial response to TLR3 activation is reminiscent of a local acute phase response, we found that many, but not all, of the same proteins are produced, and found that C/EBPβ plays an important role in the response, based on siRNA knockdown, Western blot, and EMSA assays (16). We have concluded from these studies that the antiinflammatory effects of glucocorticoids, whether they are released from the adrenal gland during systemic inflammation or whether they are administered therapeutically, may be accompanied by beneficial effects that promote innate immunity at the mucosal surface, an effect that might mitigate some of their other known immunosuppressive effects on adaptive immunity. Such an innate immune-enhancing effect could be lifesaving in a situation in which a local infection drives systemic inflammation to a degree great enough to induce adrenal glucocorticoid production, by minimizing the extent to which the steroids compromise local immunity to the infection. To what extent these enhancing effects of glucocorticoids are important in the therapeutic benefit of inhaled or systemic glucocorticoids is uncertain, but is an area of active investigation for us.
REGULATION OF DENDRITIC CELLS BY EPITHELIUM
During our initial studies of TLR activation of epithelial cells, we noticed that some of the chemokines and cytokines induced were ones that would be expected to activate dendritic cells (DC), namely CCL20 (MIP-3α) and GM-CSF (10). GM-CSF is a powerful inducer of DC formation from monocytes, a cell type that can be a major component of inflammatory cell infiltrates, and CCL20 is a ligand for CCR6 and can recruit mature DC (17, 18). These studies raised the reasonable conclusion that TLR activation of epithelium could mobilize DC formation and recruitment in the airways. More recently, we have performed some studies on a recently recognized product of epithelial cells, thymic stromal lymphopoietin (TSLP), an IL-7 family member that stimulates DC to become activated and to subsequently skew T cells to become Th2 cells (19, 20). We found that TSLP is produced by epithelial cells from the airways or nasal cavity after stimulation with dsRNA, a ligand for TLR3. Interestingly, while IL-4 was not a particularly good stimulus for TSLP production in vitro, it did synergize with dsRNA to induce production of substantial quantities of TSLP (20). This led us to conclude that the simultaneous presence of TLR3 and Th2 stimulation is the most powerful inducer of TSLP, and we speculate that this could happen in vivo when RNA viruses infect airways from individuals with allergic disease. This speculation is of particular relevance to sinus disease and to asthma, since upper respiratory viruses such as rhinovirus are major activators of disease exacerbation. To test this hypothesis further, we challenged epithelial cells with rhinovirus 16 in the presence or absence of IL-4 and found the same pattern—that activation with both was clearly the strongest stimulus. TSLP has been shown to be elevated in both atopic dermatitis and in asthma (21, 22). We find TSLP mRNA to be elevated in CRSwNP, but do not find impressive changes in TSLP protein to date (A. Kato and coworkers, unpublished observations). Future studies will be required to determine whether exacerbations of asthma or CRS induced by rhinoviruses are accompanied by strong induction of TSLP in human subjects. Together, these studies suggest that epithelial activation can play an important role in determining DC maturation, recruitment, and differentiation in the airways. Considering that one subset of DC, the so-called interepithelial DC that are fractalkine receptor positive, is intimately associated with epithelial cells, some future studies will focus on the special interactions between these DC and epithelium (23).
REGULATION OF T CELLS BY EPITHELIUM
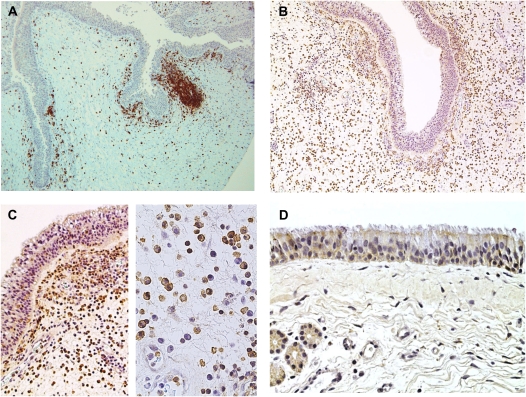
Increased numbers of T cells have been reported in CRS, and increased activation of the T cells that are present in nasal polyps has been reported (see Figure 1) (24). We have pursued several lines of investigation that relate to the T cell participation in sinus disease and the role that epithelial cells play in regulating these T cell responses. One line of investigation has focused on the roles that Staphylococcus aureus and the superantigens that it produces play in CRS. These studies followed up the earlier studies of and Bernstein and colleagues (25) and Van Zele and coworkers (26) providing evidence for increased presence of S. aureus organisms and providing evidence for activation of CRS tissues by superantigens. Using flow cytometric analysis of tissue from patients with CRSwNP, we found evidence for a superantigen effect on T cells based on clear evidence for skewing of the T cell receptor Vβ expressed on cells in the tissue (27–30). This agreed with evidence from L. Suh (unpublished observations) showing the presence of SEB and SEA in many of the CRS nasal samples and the presence of IgE that is specific for the superantigens in the serum. At this point, it is our impression that S. aureus colonization and a superantigen effect are common but not consistent features of CRS, especially in CRSwNP, and it is not clear whether these are primary drivers of disease or secondary manifestations resulting from increased susceptibility to colonization.
Figure 1.
(A) T cells in chronic rhinosinusitis (CRS). (B) BAFF staining in CRS with nasal polyp (CRSwNP). (C) BAFF in nasal polyp tissue. (D) SPINK5 in sinus mucosa.
Another line of investigation has focused on the role of epithelial cells in the functional regulation of the T cells that are in the airways and sinuses. Like DC, there are special subsets of interepithelial T cells that interact intimately with the epithelium and express adhesion molecules, such as the αEβ7 integrin, that maintain their proximity to the mucosa (31). This raises the possibility that physical interactions between epithelium and T cells can regulate the function of both cell types. Several years ago, we found that epithelial cells express members of the B7 family of costimulatory molecules, notably B7-H1 (PD-L1), B7-H2 (ICOS-L), and B7-DC (PD-L2). B7-H1 and B7-DC are of interest because they are able to reduce T cell activation via binding to PD-1, an ITIM-containing regulatory molecule (32). B7-H1 and B7-DC are both inducible on epithelial cells by stimulation with either TLR3 ligand or rhinovirus (33, 34). Interestingly, we found clear up-regulation of these two B7 homologs in nasal scrapings after rhinovirus challenge of normal volunteer subjects (34). In CRS, we found elevation of these same homologs by analysis of mRNA and found elevations of B7-H1 by immunohistochemistry, especially in Samter's triad, a particularly severe form of CRSwNP that is characterized by nasal polyps, asthma, and aspirin sensitivity (33). At present, it is not clear whether increased levels of B7-H1 and B7-DC are reflective of a feedback inhibitory mechanism that is activated during disease or whether they are involved in the pathology of CRS, since these molecules can also play a proinflammatory role via reverse signaling (35). Regardless of the precise role, if any, played in pathogenesis, increased expression of B7-H1 and B7-DC is likely to be valuable as a marker of epithelial activation in diseases of the upper airways and sinuses. Since PD-1 is expressed and functionally active on B lymphocytes as well as T lymphocytes, the epithelial expression of B7-H1 and B7-DC may reflect epithelial regulation of the B cell responses discussed below (35).
REGULATION OF B CELLS BY EPITHELIUM
It has become increasingly clear that B lymphocytes undergo local recruitment, activation, class switch recombination, and differentiation to plasma cells in the lamina propria and mucosa of the airways and sinuses, and that these processes play a role in asthma and hay fever (36, 37). Class switch recombination to produce IgE and IgA is of particular relevance to both allergic diseases and immunity in the airways and sinuses, and has been one area of investigation by our laboratory and others. Both of these immunoglobulin isotypes are highly elevated in CRS, especially the polypoid form (38, 39). Like T cells, B cells can be found in isolated lymphoid follicles in CRSwNP and there are numerous plasma cells in nasal polyp tissue. We have recently demonstrated that BAFF (also known as BLyS and TNFSF13B), a member of the TNF family of molecules, is produced by airway epithelial cells in the lungs and upper airways (40). This molecule is induced by stimulation with several cytokines and TLR3 activation. Studies in mice and humans indicate that BAFF and one of its receptors, BAFFR, are essential for B cell development and that another receptor, TACI, is essential in class switch recombination for the formation of IgA (41, 42).
Analysis of tissues from the upper airways and sinuses from control subjects and patients with CRS showed that BAFF mRNA and protein are highly elevated in nasal polyp tissue but not in unaffected tissue in CRSwNP or in tissue from control subjects or patients with CRSsNP. BAFF was also elevated in nasal lavage samples from patients with CRSwNP. Levels of BAFF mRNA in tissues correlated with levels of mRNA for CD20 and TACI, suggesting that BAFF may be involved in the increase in B cells in CRS (39). Along with elevated levels of IL-6 (discussed above), BAFF may help to provide a milieu for the expansion of B cells in sinus disease. We have recently confirmed this finding in the lower airways in a segmental bronchial challenge model in humans, showing that BAFF was increased dramatically after antigen challenge and that the BAFF that was detected was probably produced locally (43). As to the increased levels of IgA found in nasal polyps, recent studies have given insight into the potential role of an IL-13–induced enzyme, 15-lipoxygenase-1, in regulating both IgA production in the airway mucosa and in regulating the expression, and possibly function, of the IgA transport molecule polymeric immunoglobulin receptor (pIgR). Mice deficient in the murine equivalent of this enzyme (12/15LO) have been found to have dramatic elevations of IgA in the airway lumen in conjunction with increased pIgR expression (44). Together, these results suggest that induction of 15-LO-1 by Th2 responses may decrease IgA transport and lead to accumulation of IgA in tissue. Recent studies in CRSwNP suggest that IgA is elevated in the tissue but not in the nasal lavage (39). On the other hand, secretory IgA does appear to be increased in the lavage fluid of patients with CRSwNP. We are presently uncertain what the role of IgA is in CRS pathogenesis. As to the mechanisms of B cell recruitment, recently M. Patadia has detected elevated levels of BLC (CXCL13) and SDF-1α (CXCL12) in extracts from nasal polyps, suggesting that increased recruitment of B cells may be another mechanism by which they accumulate in the upper airways (45). Following along with this line of investigation, Julian Dixon has recently found elevated levels of mRNA encoding receptors for these chemokines, namely, CXCR4 and CXCR7, in nasal polyp tissue extracts (J. Dixon and colleagues, unpublished observations). Together, these findings suggest the hypothesis that during the formation of nasal polyps there are processes that induce the recruitment, activation, class switch recombination, immunoglobulin production, and differentiation of B cells to plasma cells (see Figure 2). Two important questions that we are presently attempting to evaluate are: (1) Is local production of immunoglobulins pathogenic? and (2) What is the antigen specificity of the immunoglobulins produced?
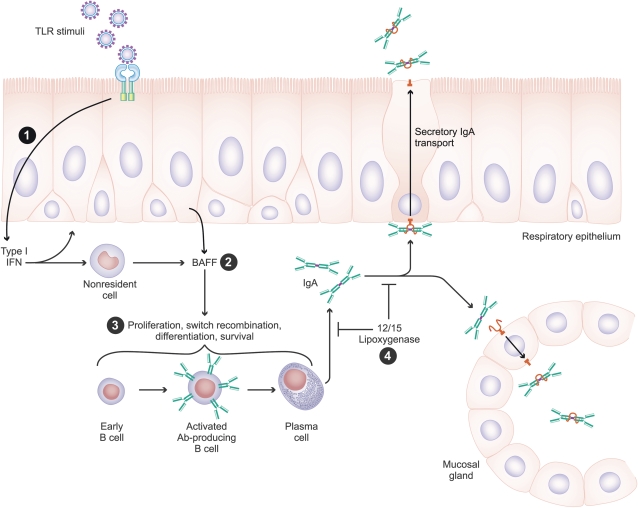
Figure 2.
Hypothesis regarding the role that local cytokine responses play in the recruitment and activation of B lymphocytes in CRS. (1) Activation of epithelium with Toll-like receptor (TLR) agonist (especially TLR3) leads to BAFF production via an autocrine stimulation pathway that involves IFN-β. (2) BAFF is released by epithelial cells as well as hematopoietic lineage cells in CRSwNP. (3) B cells are stimulated to undergo proliferation, class switch recombination, differentiation, and survival by BAFF and other local factors, leading to production of IgA, IgE, and other immunoglobulin isotypes. (4) The processes of local B cell activation and IgA production as well as IgA transport are regulated by the 12/15 lipoxygenase pathway. See text for details.
ALTERATIONS IN BARRIER FUNCTION IN CHRONIC RHINOSINUSITIS
Recent studies of allergic diseases in the skin and lungs such as atopic dermatitis, psoriasis, asthma, and allergic rhinitis have uncovered evidence that there may be deficiencies in the barrier function of the skin or airway mucosal epithelium (46, 47). These studies led us to determine whether several molecules that are known to be members of the epidermal differentiation complex, including S100A7, S100A8, S100A9, and SPINK5, are differentially expressed in the epithelium of patients with CRS. To do this, S. Richer performed real-time RT-PCR analysis on epithelial scraping samples from control subjects and patients, and found a marked decrease in the expression of mRNA for S100A7, S100A8, S100A9, and SPINK5 (48). S100A7 (psoriasin) is an antimicrobial peptide that is elevated in psoriasis and is induced along with other members of the S100 family by IL-22 and related IFN/IL-10 family cytokines (49–51). Interestingly, IL-22 is a major product of Th17 cells and these cells appear to not be elevated in CRS in our studies based on ELISA of the various IL-17 family cytokines. S100 proteins are also nonchemokine chemoattractants of inflammatory cells, and appear to have a role in epithelial cell growth dynamics and repair. The decreased expression of S100 genes in CRS has recently been confirmed at the level of protein expression by D. Tieu (unpublished observations). D. Vermylen and D. Carter have confirmed the reduced levels of SPINK5 by immunohistochemical analysis of samples from patients with CRSwNP (48). SPINK5 is a protease inhibitor that is necessary for barrier function in the skin, as patients lacking this molecule develop Netherton's syndrome, a form of ichthyosis in which dramatic scaling of the cornified layer of the skin occurs (52). J. Norton has been studying polymorphisms of the filaggrin gene in CRS, and his preliminary results to date indicate that known loss of function variants are associated with atopy but may or may not be associated with CRS (J. Norton, unpublished data). These studies suggest a hypothesis that deficiencies of expression of S100 and SPINK5 genes in CRS compromise maintenance of barrier function in the upper airways and sinuses (53). This hypothesis is presently under evaluation.
CONCLUSIONS
The current review suggests the hypothesis that CRS results, at least in part, from a dysfunction of the nasal epithelium in its ability to orchestrate appropriate and regulated immune responses to foreign matter. As an implied corollary, defects in the mechanical barrier and innate immune response would result in appropriate recruitment and persistence of adaptive immune responses with development of the clinical symptoms characteristic of the disease. As is clear from this review and the hypotheses that we have advanced based on our preliminary results, this is very much a work in progress and a great deal is yet to be learned about CRS, what causes it and how to treat it better. We hope that testing of these and other hypotheses will lead to some new concepts regarding the pathogenesis and treatment of CRS as well as other diseases of the airways and will give some insight into the normal innate and adaptive immune responses that protect us from microorganisms that might otherwise inhabit our upper airways and cause disease.
Acknowledgments
The authors thank the NIH, the Bazley Trust, our collaborators, and the individuals mentioned throughout the text of this manuscript for making this work possible.
Supported by the NIH and the Ernest S. Bazley Grant to Northwestern Memorial Hospital and Northwestern University Feinberg School of Medicine.
Conflict of Interest Statement: R.P.S. has participated in various scientific meetings organized by pharmaceutical companies (Sepracor, VentiRx, Altana, Genentech, ScheringPlough, Wyeth) and has received consultancy money as stated above. A.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.C.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.E.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.A.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.C.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.C.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.C.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, Adinoff AD, Bachert C, Borish L, Chinchilli VM, et al. Rhinosinusitis: developing guidance for clinical trials. J Allergy Clin Immunol 2006;118:S17–S61. [DOI] [PubMed] [Google Scholar]
- 2.Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, Lanza DC, Marple BF, Osguthorpe JD, Stankiewicz JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg 2003;129:S1–S32. [DOI] [PubMed] [Google Scholar]
- 3.Hamilos DL, Lund VJ. Etiology of chronic rhinosinusitis: the role of fungus. Ann Otol Rhinol Laryngol Suppl 2004;193:27–31. [DOI] [PubMed] [Google Scholar]
- 4.Schubert MS. A superantigen hypothesis for the pathogenesis of chronic hypertrophic rhinosinusitis, allergic fungal sinusitis, and related disorders. Ann Allergy Asthma Immunol 2001;87:181–188. [DOI] [PubMed] [Google Scholar]
- 5.Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol 2001;107:607–614. [DOI] [PubMed] [Google Scholar]
- 6.Bachert C, Gevaert P, Howarth P, Holtappels G, van Cauwenberge P, Johansson SG. IgE to Staphylococcus aureus enterotoxins in serum is related to severity of asthma. J Allergy Clin Immunol 2003;111:1131–1132. [PubMed] [Google Scholar]
- 7.Bachert C, Gevaert P, van Cauwenberge P. Staphylococcus aureus enterotoxins: a key in airway disease? Allergy 2002;57:480–487. [DOI] [PubMed] [Google Scholar]
- 8.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, Bachert C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 2006;61:1280–1289. [DOI] [PubMed] [Google Scholar]
- 9.Drake-Lee AB. Histamine and its release from nasal polyps: preliminary communication. J R Soc Med 1984;77:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by Toll-like receptor agonists. Am J Respir Cell Mol Biol 2004;31:358–364. [DOI] [PubMed] [Google Scholar]
- 11.Lane AP, Truong-Tran QA, Schleimer RP. Altered expression of genes associated with innate immunity and inflammation in recalcitrant rhinosinusitis with polyps. Am J Rhinol 2006;20:138–144. [PMC free article] [PubMed] [Google Scholar]
- 12.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol 2005;175:3463–3468. [DOI] [PubMed] [Google Scholar]
- 13.Peters AT, Kato AT, Carr T, Suh L, Norton J, Grammer LC, Harris KE, Chandra R, Conley DB, Kern R, Schleimer RP. Analysis of the Th 17 pathway in chronic rhinosinusitis. J Allergy Clin Immunol 2009;123:S145. [Google Scholar]
- 14.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med 2007;357:1608–1619. [DOI] [PubMed] [Google Scholar]
- 15.Schleimer RP. Glucocorticoids suppress inflammation but spare innate immune responses in airway epithelium. Proc Am Thorac Soc 2004;1:222–230. [DOI] [PubMed] [Google Scholar]
- 16.Zhang N, Truong-Tran QA, Tancowny B, Harris KE, Schleimer RP. Glucocorticoids enhance or spare innate immunity: effects in airway epithelium are mediated by CCAAT/enhancer binding proteins. J Immunol 2007;179:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Power CA, Church DJ, Meyer A, Alouani S, Proudfoot AE, Clark-Lewis I, Sozzani S, Mantovani A, Wells TN. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3alpha from lung dendritic cells. J Exp Med 1997;186:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman RM, Inaba K. Myeloid dendritic cells. J Leukoc Biol 1999;66:205–208. [DOI] [PubMed] [Google Scholar]
- 19.Leonard WJ. TSLP: finally in the limelight. Nat Immunol 2002;3:605–607. [DOI] [PubMed] [Google Scholar]
- 20.Kato A, Favoreto S Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol 2007;179:1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002;3:673–680. [DOI] [PubMed] [Google Scholar]
- 22.Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol 2005;174:8183–8190. [DOI] [PubMed] [Google Scholar]
- 23.Sung SS, Fu SM, Rose CE Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol 2006;176:2161–2172. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Segura A, Brieva JA, Rodriguez C. T lymphocytes that infiltrate nasal polyps have a specialized phenotype and produce a mixed TH1/TH2 pattern of cytokines. J Allergy Clin Immunol 1998;102:953–960. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein JM, Ballow M, Schlievert PM, Rich G, Allen C, Dryja D. A superantigen hypothesis for the pathogenesis of chronic hyperplastic sinusitis with massive nasal polyposis. Am J Rhinol 2003;17:321–326. [PubMed] [Google Scholar]
- 26.Van Zele T, Gevaert P, Watelet JB, Claeys G, Holtappels G, Claeys C, van Cauwenberge P, Bachert C. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol 2004;114:981–983. [DOI] [PubMed] [Google Scholar]
- 27.Tripathi A, Conley DB, Grammer LC, Ditto AM, Lowery MM, Seiberling KA, Yarnold PA, Zeifer B, Kern RC. Immunoglobulin E to staphylococcal and streptococcal toxins in patients with chronic sinusitis/nasal polyposis. Laryngoscope 2004;114:1822–1826. [DOI] [PubMed] [Google Scholar]
- 28.Conley DB, Tripathi A, Ditto AM, Reid K, Grammer LC, Kern RC. Chronic sinusitis with nasal polyps: staphylococcal exotoxin immunoglobulin E and cellular inflammation. Am J Rhinol 2004;18:273–278. [PubMed] [Google Scholar]
- 29.Seiberling KA, Conley DB, Tripathi A, Grammer LC, Shuh L, Haines GK III, Schleimer R, Kern RC. Superantigens and chronic rhinosinusitis: detection of staphylococcal exotoxins in nasal polyps. Laryngoscope 2005;115:1580–1585. [DOI] [PubMed] [Google Scholar]
- 30.Conley DB, Tripathi A, Seiberling KA, Schleimer RP, Suh LA, Harris K, Paniagua MC, Grammer LC, Kern RC. Superantigens and chronic rhinosinusitis: skewing of T-cell receptor V beta-distributions in polyp-derived CD4+ and CD8+ T cells. Am J Rhinol 2006;20:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leckie MJ, Jenkins GR, Khan J, Smith SJ, Walker C, Barnes PJ, Hansel TT. Sputum T lymphocytes in asthma, COPD and healthy subjects have the phenotype of activated intraepithelial T cells (CD69+ CD103+). Thorax 2003;58:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurosawa S, Myers AC, Chen L, Wang S, Ni J, Plitt JR, Heller NM, Bochner BS, Schleimer RP. Expression of the costimulatory molecule B7–H2 (inducible costimulator ligand) by human airway epithelial cells. Am J Respir Cell Mol Biol 2003;28:563–573. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Myers AC, Chen L, Pardoll DM, Truong-Tran QA, Lane AP, McDyer JF, Fortuno L, Schleimer RP. Constitutive and inducible expression of B7 family of ligands by human airway epithelial cells. Am J Respir Cell Mol Biol 2005;33:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinecke L, Proud D, Sanders S, Schleimer RP, Kim J. Induction of B7–H1 and B7-DC expression on airway epithelial cells by the Toll-like receptor 3 agonist double-stranded RNA and human rhinovirus infection: in vivo and in vitro studies. J Allergy Clin Immunol 2008;121:1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameron L, Hamid Q, Wright E, Nakamura Y, Christodoulopoulos P, Muro S, Frenkiel S, Lavigne F, Durham S, Gould H. Local synthesis of epsilon germline gene transcripts, IL-4, and IL-13 in allergic nasal mucosa after ex vivo allergen exposure. J Allergy Clin Immunol 2000;106:46–52. [DOI] [PubMed] [Google Scholar]
- 37.Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol 1997;158:2406–2413. [PubMed] [Google Scholar]
- 38.Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy 2005;60:71–79. [DOI] [PubMed] [Google Scholar]
- 39.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, Conley D, Grammer LC, Kern R, Schleimer RP. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2008;121:1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol 2006;177:7164–7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol 2003;21:231–264. [DOI] [PubMed] [Google Scholar]
- 42.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, Mizoguchi E, Geha RS. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci USA 2004;101:3903–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato A, Xiao H, Chustz RT, Liu MC, Schleimer RP. Local release of B cell-activating factor of the TNF family after segmental allergen challenge of allergic subjects. J Allergy Clin Immunol 2009;123:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hajek AR, Lindley AR, Favoreto S Jr, Carter R, Schleimer RP, Kuperman DA. 12/15-Lipoxygenase deficiency protects mice from allergic airways inflammation and increases secretory IgA levels. J Allergy Clin Immunol 2008;122:633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patadia M, Conley D, Chandra R, Tripathi-Peters A, Suh L, Kato A, Carter R, Harris KE, Grammer L, Kern R, et al. Evaluation of the presence of B cell attractant chemokines in chronic rhinosinusitis [internet]. Warwick, NY:American Rhinologic Society; 2008. Available from: http://www.american-rhinologic.org [DOI] [PMC free article] [PubMed]
- 46.Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat Rev Immunol 2004;4:978–988. [DOI] [PubMed] [Google Scholar]
- 47.Holgate ST. The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol 2007;28:248–251. [DOI] [PubMed] [Google Scholar]
- 48.Richer SL, Truong-Tran AQ, Conley DB, Carter R, Vermylen D, Grammer LC, Peters AT, Chandra RK, Harris KE, Kern RC, et al. Epithelial genes in chronic rhinosinusitis with and without nasal polyps. Am J Rhinol 2008;22:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 2006;36:1309–1323. [DOI] [PubMed] [Google Scholar]
- 50.Bryborn M, Adner M, Cardell LO. Psoriasin, one of several new proteins identified in nasal lavage fluid from allergic and non-allergic individuals using 2-dimensional gel electrophoresis and mass spectrometry. Respir Res 2005;6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol 2005;6:57–64. [DOI] [PubMed] [Google Scholar]
- 52.Walley AJ, Chavanas S, Moffatt MF, Esnouf RM, Ubhi B, Lawrence R, Wong K, Abecasis GR, Jones EY, Harper JI, et al. Gene polymorphism in Netherton and common atopic disease. Nat Genet 2001;29:175–178. [DOI] [PubMed] [Google Scholar]
- 53.Kern R, Conley D, Walsh W, Chandra R, Kato A, Tripathi-Peters A, Grammer L, Schleimer RP. Perspecitves on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol 2008;22:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]