Summary of recent advances
Recent advances from our own group and others have defined a novel PML/PTEN/Akt/mTOR/FoxO signaling network, and highlighted its critical importance in oncogenesis as well as in the functional regulation of normal stem cell and cancer-initiating cell (CIC) biology. These findings are of great importance in cancer therapy in view of the fact that this network is amenable to pharmacological modulation at multiple levels. The integrated analysis of these data allows us to propose a new provocative working model whereby the aberrant super-activation of Akt/mTOR signaling elicits built-in cellular fail-safe mechanisms that could be effectively utilized for cancer treatment to extinguish the CICs pool. In this review, we will discuss these recent findings, this working model, and their therapeutic implications.
Introduction
Our review and the working model that we are here discussing unfolds in a conceptual framework that rests on two major assumptions that are open to debate, but have been substantiated by compelling experimental evidence in the context of leukemia biology and treatment. For this reason, we will mostly focus on the normal and aberrant hemopoietic stem cell compartment while briefly mentioning examples pertaining to solid tumor biology. The two assumptions that permeate this review are the following:
Not all the leukemic/cancer cells are created equal in that a selected cellular pool is very effective at recreating the disease when transplanted, while other cells found in the leukemic/tumor bulk are not. These cells have been variably termed leukemia-initiating cells (LICs) or leukemic stem cells in view of their functional resemblance to normal hemopoietic cells [1–5,6**,7]. This concept is currently being tested in solid tumors, where the presence and role of cancer-initiating cells (CICs) is being validated and challenged in several contexts [1,4,8].
The LIC/CIC pool, and particularly those cells from this pool that are in slow-proliferative/quiescent state, are impervious to conventional chemotherapy and undaunted by targeted cancer therapies. When cancer/leukemia relapses following a period of remission, it is believed to be the residual CICs/LICs that drive disease reemergence [2,4,7,8]. Therefore, development of new therapeutic approaches targeting CICs and LICs may have a profound impact on cancer eradication.
The PML/PTEN/Akt/mTOR/FoxO signaling network that we will discuss in this review has a critical and druggable “rehostat” at the intersection bewteen cancer and stem cell biology [9*,10*]. This is to say that physiological regulation and aberrant activation of this signaling newtork has profound consequences on the function of normal stem cells and their aberrant LIC/CIC counterpart.
The Akt/mTOR pathway is highly complex and multi-factorial, such that the linear sequence of kinase activation is further regulated and executed by many regulatory factors and effectors. Critical modulators and effectors of this pathway, such as PTEN (phosphatase with tensin homology, which is deleted on chromosome 10), FoxO (forkhead O) family and PML (Promyelocytic Leukemia), have recently been implicated in stem cell biology, particularly in the hematopoietic system [11–14**,15*,16**,17**]. Analysis of Pten-deficient hematopoietic system revealed that conditional ablation of Pten drives exit from quiescence through PI3K/Akt hyperactivation, and leads to exhaustion of normal HSCs while at the same time initiating acute leukemia [11–13**]. On the other hand, loss of Pml enhances the cycling pool of HSC through an increase in mTOR activity, but does not initiate leukemia [14**]. Critically, the exit from quiescence by loss of PML is significantly more profound in LICs than in HSCs, therefore suggesting that there could be a therapeutic window in targeting PML for therapy for leukemia [14**]. Further, recent studies demonstrate that the FoxO trascription factor, a well-studied nuclear tumor suppressor target of the PI3K/Akt pathway, is also essential for long-term regenerative potential of the HSC compartment through regulation of HSC quiescence and survival in response to physiologic oxidative stress [16**,17**].
These recent advances lead to the notion that this signaling network acts as a critical ‘rheostat’ directing stem cell maintenance by finely tuning the balance of cell quiescence versus cycling and cellular commitment.
On the basis of a critical and integrated analysis of these results, we propose a working model (Figure 1) whereby in the context of normal hemopoiesis, holding this signal OFF/low maintains stem cells in a quiescent state, while the ON-signal induces active cycling in stem cells and their exhaustion. Tight regulation of this network by many modulators is able to maintain the homeostatis of the normal stem cells. In the context of aberrant signaling in cancer (Figure 1, right panel), we hypothesize that excessive signaling output leads to distinct outcomes depending on the genetic make-up of the cells. An aberrant surge in signaling output would in fact evoke failsafe mechanisms (e.g. cellular senescence, apoptosis or terminal differentiation) which would extinguish the pre-malignant stem cell clone. Only by evading these mechanisms can the cells take full advance of the proto-oncogenic signaling surge and become a full blown neoplasia (Figure 1), as in the case of PTEN loss (see below).
Figure 1. Akt/mTOR signaling pathway as a fundamental ‘rheostat’ for maintenance of stem cells.
In the context of normalcy holding this signal OFF/low maintains stem cells in a quiescent state, while the ON-signal induces active cycling in stem cells and their exhaustion (left panel). In the context of cancer, an aberrant surge in signaling output would evoke fail-safe mechanisms (e.g. cellular senescence, apoptosis or terminal differentiation) which would extinguish the pre-malignant stem cell clone. The cells can become a neoplasia only when they escape these mechanisms (right panel).
Since this signaling network is eminently druggable, understanding how the PI3K/Akt/mTOR signaling pathway contributes to stem cell biology and tumorigenesis is of paramount importance. Here we thus focus on the role of PML/PTEN/Akt/mTOR/FoxO network in HSCs along this conceptual framework, and summarize the current knowledge of this tumor suppressors/signaling network in stem cell biology and tumorigenesis.
PML downregulation is effective for LIC eradication
Chronic myeloid leukemia (CML), which is characterized by the presence of the Philadelphia chromosome (Ph+) and the BCR-ABL aberrant kinase [18,19], is one of the most extensively investigated and paradigmatic stem cell disorders [7]. In CML, the LIC-pool is not eradicated by current therapy, leading to disease relapse on drug-discontinuation [20**,21]. Surviving leukemia stem and progenitor cells are thought to be the source of relapse in many malignancies, but above all in CML. For this reason, CML patients must remain on imatinib for life to keep their cancer at bay.
PML is a tumor suppressor cloned at the breakpoint of the t(15;17) translocation of Acute Promyelocytic Leukemia (APL). PML epitomizes a distinct macromolecular sub-nuclear structure termed the PML nuclear body (PML-NB) [22**,23**]. Loss of PML is associated with the pathogenesis of a variety of hemopoietic malignancies and solid tumors. PML exerts its tumor suppressive role by modulating a number of tumor suppressive functions including the suppression of neoangiogenesis through the negative regulation of mTOR activity [24*].
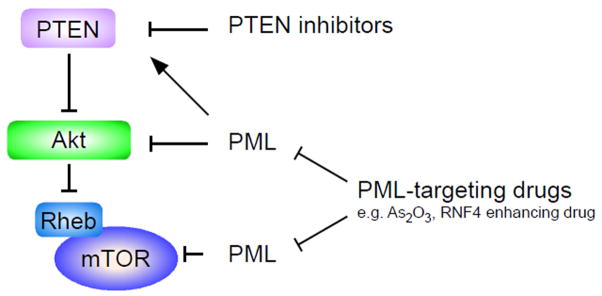
Recently, we defined a critical role for PML in LIC-maintenance in CML disease models and presented a new therapeutic approach for targeting quiescent LICs by pharmacological inhibition of PML [14**]. Studies from our lab had previously identified PML as a negative regulator of the Akt/mTOR pathway at multiple levels. In particular, PML has been reported to oppose the function of nuclear Akt (Figure 2) [15*]. Also, PML was identified as a repressor of mTOR through inhibition of Rheb-mTOR interaction in hypoxia (Figure 2) [24**]. Further, PML regulates PTEN-localization in the hematopoietic system through modulation of a DAXX/HAUSP deubiquitination network (Figure 3) [25*]. Thus, PML might oppose mTOR activity by controlling Akt, Rheb-mTOR interaction and/or PTEN in stem cells (Figure 2).
Figure 2. PML might be a negative regulator of the Akt/mTOR pathway at multiple levels.
PML could regulate mTOR activity through nuclear Akt function, mTOR-Rheb interaction and PTEN localization [15**,24**,25**]. IRS and TSC is insulin receptor substrate and tuberous sclerosis complex, respectively.
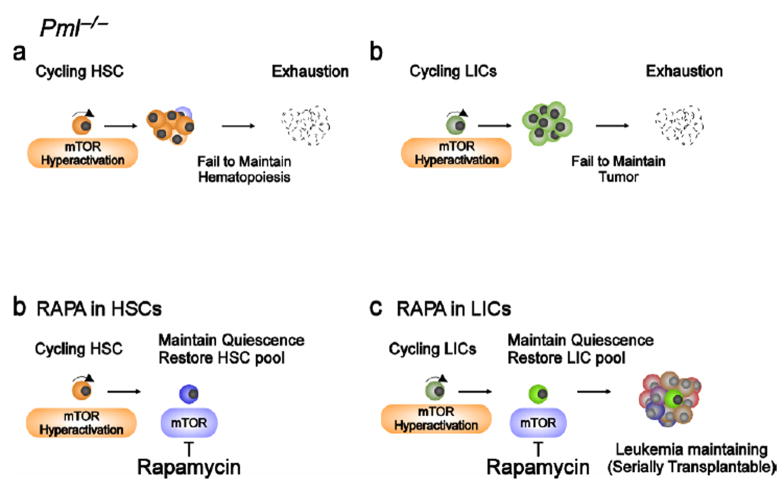
Figure 3. Critical role of PML in stem cells and the effect of rapamycin in Pml−/− stem cells.
a, Loss of Pml leads to enhances the cycling HSC pool through mTOR activation without initiating leukemia [13**]. b, Pml-deficient LICs also have defect of maintenance of quiescence, resulting in their exhaustion [13**]. c,d, Inhibition of mTOR by rapamycin rescues the phenotype of both Pml-deficient HSCs and LICs [13**].
In the hemopoietic system, we found that the expression of PML is high in HSC and declines while cells commit to differentiate. Moreover and surprisingly, we also found that PML is highly expressed in cells from CML patients; and contrary to what was described in solid tumors, loss of PML predicts favorable outcome [14**], suggesting that PML expression may be selected for and not against in CML. This surprising finding was explained by the analysis of the role of PML in CML and in HSC/LIC function. With a CML serial bone marrow (BM) transplantation mouse model, we revealed that PML plays an indispensable role in LICs quiescence in a cell autonomous manner. Loss of Pml perturbs the quiescence of both HSCs and LICs (Figure 3a, b), although faster proliferation of HSCs in the sole absence of Pml does not lead to leukemia, as Pml null mice do not develop leukemia during their life span [14**]. Indeed, in both HSCs and LICs loss of Pml leads to cell exhaustion at some point or another, as demonstrated by serial transplantation experiments. In agreement with our previous results indicating that in hypoxic condition PML inhibits mTOR activity [24**], we observed that HSCs, which are maintained in a hypoxic environment [26,27], have higher mTOR activity in the absence of Pml [24**]. Moreover, since the mTOR inhibitor rapamycin dramatically prevents the exhaustion of both Pml−/− HSCs and LICs (Figure 3c, d), we conclude that in these cellular compartments Pml acts mainly as a repressor of mTOR activity and that mTOR super-activation impairs normal or leukemic hematopoietic stem cells maintenance [14**]. Our conclusions have been recently confirmed by another study showing that acute Tsc1 inactivation in hematopoietic stem cells causes mTOR hyper-activation, exit from quiescence, increased proliferation and exhaustion of HSCs without leading to leukemia [28**], a phenotype similar to what we observed in Pml−/− mice.
Importantly, cell cycle entry and exit from quiescence in response to Pml ablation is significantly more profound in LICs than in HSCs [14**]. LICs have an overpowered cell cycle engine and aberrantly elevated signaling output due to the presence of activated oncogenes (e.g. BCR-ABL). PML likely represents a brake for strong signaling output and increased proliferation; therefore, once LICs are released from this blockade due to PML-loss, they exit from quiescent and enter vigorous proliferation followed by exhaustion.
Importantly, these findings have an immediate therapeutic applicability. To mimic loss of Pml we utilized As2O3, a drug that downregulates PML through proteosomal-dependent degradation [29,30**,31**] and that is currently used for the treatment of APL with limited toxicity [32]. Indeed, the exquisite efficacy of As2O3 in APL treatment is precisely due to its ability to target the PML-RARα fusion oncoprotein for degradation through its PML moiety [29]. Recently, it has been shown that the RNF4 (RING finger protein 4) is essential for As2O3-induced catabolism of both PML and PML-RARα [30**,31**] Similar to genetic inactivation of Pml, inhibition of Pml by low doses of As2O3 disrupts LICs maintenance. Even more importantly, As2O3 also increased the efficacy of anti-leukemic therapy for LICs by inducing exit from a quiescent state and sensitizing both mouse and human LICs to pro-apoptotic stimuli [14**]. On the basis of our findings, we propose that As2O3 or novel PML lowering drugs could be transiently utilized at leukemia onset along with or followed by a standard of care regimens, as we will discuss in the following paragraphs.
Pten-deletion results in generation of LICs accompanied by depletion of normal HSCs
The PTEN gene encodes one of the most relevant tumor suppressors genes which is mutated, genetically lost or silenced in multiple sporadic tumor types as well as in patients with cancer predisposition syndromes such as Cowden disease [33,34]. PTEN is a multifunctional phosphatase whose major substrate is phosphatidylinositol–3,4,5-triphosphate (PIP3), which activates numerous downstream targets including the serine-threonine kinase Akt and mTOR [35].
PTEN itself has been reported as critical regulators of both normal stem cell and LICs/CICs function in both solid tumors and leukemia (see also following paragraphs). Recent studies using acute Pten inactivation in the bone marrow have revealed that PTEN may differentially regulate normal hematopoietic and leukemic cells [11–13**]. Acute deletion of Pten leads to HSC proliferation, followed by a myeloproliferative disease within days, and by the development of transplantable leukemias within weeks (Figure 4a) [11–13**]. At the same time, Pten deletion leads to depletion of HSCs via a cell-autonomous mechanism (Figure 4a) [11**,12**]. Rapamycin in this model is found to inhibit leukemia development and generation of LICs while the function of HSCs is normalized (Figure 4b) [11**]. However, rapamycin treatment does not eradicate these Pten-deficient leukemias after leukemia onset (Figure 4c) [11**]. Moreover, it should be more clearly demonstrated how rapamycin affects the long-term Pten-deficient LICs. Further examination, such as analysis at single cell level of enriched LIC compartment and investigation of the effect of long-term treatment with rapamycin in serial transplantation models, would reveal the LIC-specific effect of mTOR-inhibition in Pten-deleted mice. Even more importantly, functional analysis of the consequence of PTEN-reduction in human LICs and investigation of rapamycin effects in human PTEN-deficient leukemia/LICs remains to be performed.
Figure 4. The function of Pten in HSCs and leukemia-initiation and the effect of rapamycin in Pten-deficient stem cells.
a, In the absence of Pten, the proliferation of HSCs increases and leukemia develops through hyperactivity of PI3K/Akt pathway, resulting in survival and proliferation advantage. Additional mutations and/or block of lineage committment lead to development of transplantable leukemia. At the same time, Pten-deletion induces excessive proliferation in HSCs, resulting in their exhaustion [11–13**]. b, Treatment with rapamycin before leukemia development normalizes HSC function and prevents the development of leukemia in Pten-deleted mice [11**]. c, Administration of rapamycin after leukemia onset, it has survival advantage by growth inhibitory effect but can not cure leukemia [11**].
Nevertheless, it could be argued that these findings are seemingly at odds with what we observed when inactivating Pml. Indeed, rapamycine restores LIC maintenance in the Pml model, while opposing leukemogenesis and restoring normal HSC function in the case of Pten loss. As we will discuss in greater detail in subsequent paragraphs, we believe that these findings are in fact in agreement with a model whereby the fail-safe mechanisms that super activation of the Akt/mTOR pathway elicits must be evaded for leukemogenesis to occur (Figure 1). According to this working model, in normal HSCs, or in LICs that still retain intact fail-safe mechanisms, rapamycin protects both HSC and LIC pools from exhaustion by normalizing the signaling output (hence becoming tumor promoting). Whenever these fail-safe mechanisms are evaded leading to leukemia, the shut down of the pathway becomes in fact tumor suppressive. Indeed, Guo et al. recently demonstrated that multiple genetic or molecular events, such as β-catenin activation, c-myc overexpression and t(14;15) chromosomal translocation, collaboratively contribute to T-ALL formation upon Pten deletion [13**]. Therefore, leukemia could be triggered by any of these mechanisms that could rapidly override the fail-safe mechanisms elicited by Akt/mTOR superactivation.
We therefore explain the difference between the Pml loss/CML model and the Pten loss/AML model within this conceptual framework. This in turn begs the obvious corollary question on why upon Pten loss in the HSC compartment these failsafe mechanisms would be rapidly evaded, unlike in the case of Pml loss and CML. We will discuss this below.
Consequence of FoxO deletion in the HSC compartment
The FoxO subfamily (FoxO1, FoxO3, FoxO4, and FoxO6) of transcription factors, is required for diverse cellular processes that include tumor suppression and cell death [36]. FoxOs operate under the negative regulation of the PI3K/Akt pathway: when in the nucleus, Akt directly phosphorylates FoxO family members leading to their 14-3-3 dependent export into the cytoplasm [36]. Recently, it has been demonstrated that FoxOs are critical for the long-term maintenance of HSCs. Mice in which FoxO1, FoxO3 and FoxO4 are conditionally and concomitantly deleted in the adult hematopoietic system display a marked reduction of HSC number and function in response to physiologic oxidative stress [16**]. Furthermore, aged FoxO3a knockout animals also show a reduction of the HSC pool and a deficient repopulating capacity in serial transplantation assays [17**], similarly to Pml null mutants.
Increased exit from quiescence and enhanced apoptosis, two of the features observed in FoxO-deficient mutants, could act in concert to decrease the pool size of HSCs available for self-renewal. FoxO−/− HSCs are driven out of quiescence into cell cycle, possibly due to cell cycle defects. Indeed, FoxO members play an important role at the G0-G1, G1-S, and G2-M checkpoints by direct transcriptional modulation of proteins such as p21, p27, and cyclin D [37**]. In addition, loss of FoxO1, FoxO3, and FoxO4 in the adult hematopoietic system results in significantly increased levels of apoptosis in HSCs. This has been argued to be due to defective regulation of FasL production and increased ROS (reactive oxygen species) production [37**].
Differences and similarities in stem cell and LICs biology among PML, PTEN and FoxO models
Pten-deficient HSCs have many phenotypic similarities to Pml−/− and FoxOs−/− HSCs, in that similar perturbations of quiescence of the HSC compartment leads to its exhaustion. However, the hematological phenotypes observed in Pml-, FoxOs- and Pten-deficient stem cells are quite different. Pml−/− and FoxOs−/− mice do not develop acute leukemia whilst abrogation of Pten function in adult hematopoietic cells leads to a brief myeloproliferative disorder (MPD), followed by the development of acute leukemia which is serially transplantable (Figure 4a) [11**,12**]. By contrast, Pml-deficient LICs undergo intensive cell cycling, resulting in impairment of LIC maintenance (Figure 3c) [14**].
Importantly, a recent report demonstrates that hyper-activation of mTOR seems to be relevant in regulating HSCs, but is not sufficient to trigger leukemia. Tsc1 deletion in the HSC compartment drives stem cells proliferation which results in their exhaustion, but not in leukemia initiation [28**]. It can be therefore speculated that different levels of mTOR activation in Pml-null, Tsc1-null and Pten-null stem cells might result in different outcomes. Furthermore, chronic mTOR activation, as obtained in Pml−/− HSCs, could be different from a burst of mTOR activity upon acute Pten loss.
It has been argued that the PI3K/Akt network regulates HSC activity mainly through Akt/FoxOs instead of mTOR [16**,17**]. Pml plays a dual role of concomitantly opposing mTOR and Akt/FoxOs as well [14*]. However, the quantitative output of Pten and Pml loss in the HSC pool towards mTOR and Akt/FoxOs could be once again distinct.
The different outcomes of deleting Pml or Pten may be also explained by the fact that the expression pattern of Pml and Pten may not be completely overlapping, although both genes are expressed in the HSCs. Importantly, PML and PTEN certainly exert additional non overlapping functions that may justify this differential outcome. For instance, it has been suggested that Pten exerts multiple roles in the prevention of leukemogenesis [12**]: 1. Pten regulates the decision of maintaining quiescence (G0) versus entry in G1 in HSC; 2. Pten controls proliferation speed after HSC have undergone the decision to proliferate; 3. Pten also plays a role in hematopoietic lineage fate. Pten-deficiency blocks the development of cells in the prepro-B-lympoid stage, leads to increases in the myeloid and T lineages but decreases in the B lineage. Thus, Pten may be more pleiotropic than Pml within the stem cell compartment, and some of these mTOR-independent roles could favor leukemia initiation.
However, another fundamental difference between these two models could reside in the nature of LICs pools involved in the process. As CML is a stem cell disorder in which all hematopoietic lineages are involved, the CML-initiating cells very likely originate from the HSC pool. Therefore, HSCs and CML-initiating cells should be regulated by common mechanisms. On the other hand, there is no clear evidence that the AML/ALL caused by Pten-loss originates from a HSC. Indeed, the LIC characterized in Pten-deficient T-ALL compartment (c-KitmidCD3+Lin−) is a phenotypically different compartment from the HSCs pool [13**]. Pten-deficiency might induce HSC exit from quiescence leading to its exhaustion and at the same time trigger secondary ‘hits’ through genomic instability, leading to generation of LICs in more committed cells. It can be therefore speculated that leukemias that harbour PTEN mutations are driven by LICs that do not originate from the stem cell pool. This hypothesis is supported by the fact that PTEN is unaltered in patients with CML and MDS, which are considered to be diseases originating at the pluripotent stem cell level [38].
PML has also been implicated in the control of genomic stability and DNA repair. Furthermore, we demonstrated that functional inactivation of Pml leads to exit from quiescence in stem cells, predictive of an increased DNA mutation rate in Pml−/− stem cells than wt control. However, spontaneous leukemia development was never observed in Pml−/− mice [39]. Further, leukemia is not triggered by As2O3 treatment neither in the mouse nor in humans [40].
Perhaps one of the most striking differences, however, is the effect of the mTOR inhibitor, rapamycin, on leukemic cells in the two models. Rapamycin inhibits the development of leukemia derived from Pten-deficiency (Figure 4b) but maintains the leukemia-initiation capacity of Pml−/− LICs even after serial BMT (Figure 3c) [11**,14**]. As we have discussed before, we propose that this difference is due to the fact that BCR-ABL-positive LICs in CML chronic phase are extremely responsive to super activation of Akt/mTOR because the oncogene already activates signaling output, and yet they retain the fail-safe mechanims (terminal differentiation, apoptosis or cellular senescence), that in response to a further elevation of signaling output would affect their maintenance ability. In this scenario, Pten-deficiency driven leukemia would have evaded this response through additional mutations, in turn leading to bypass of cellular senescence, apoptosis or to a block in cellular differentiation. In this respect, it would be extremely interesting to understand whether when CML evolves to blast crisis and morphs into an acute leukemia, LICs in this phase are still responsive to pathway superactivation leading to their exhaustion. On the contrary, it could be hypothesized that the accumulation of additional genetic hits (e.g. p53 loss, often observed in blast crisis) in this phase would prevent LICs exhaustion, and that therefore PML loss or its pharmacological inactivation may be in this context tumor promoting, and hence detrimental.
Lesson from the analysis of solid tumors and CICs biology
In remains to be established to which extent the notion that acute loss of tumor suppressors such as PTEN or PML leads to exhaustion of LICs can be exported to solid tumorigenesis and CICs at large. An indication that this may be the case in other cellular contexts comes from our analysis of the role of Pten in prostate cancer.
That PTEN is a powerful tumor suppressor is unequivocal, as demonstrated by the extensive variety of mutations identified in many different tumor types, the augmented tumor susceptibility of individuals harboring germline PTEN mutations and multiple tumors identified in Pten knockout mouse models [41*]. Furthermore, analysis of Pten heterozygous inactivation in the mouse prostate reveals that Pten is haploinsufficient in suppressing tumorigenesis in the prostatic epithelium, as demonstrated by the fact that Pten+/− mice develop prostatic intraepithelial neoplasia in the absence of Pten loss of heterozygosity [42].
By contrast, it is currently clear that complete loss of PTEN function is not obligatory for tumor development. Indeed, we have surprisingly demonstrated that complete Pten loss does not favor tumor growth at all in the prostate; instead it activates a p53-dependent cellular senescence program, which acts as a ‘brake’ on tumor growth [43]. Therefore, combined inactivation of Pten and Trp53 is required for maximal tumor growth as demonstrated in mouse models that develop rapidly fatal prostate cancer [43]. This is coherent with the fact that in human epithelial cancers (e.g. prostate cancer and breast cancer), complete PTEN loss is rarely observed and only described in very advanced cancers which have also suffered functional or genetic loss of p53. These findings also highlight the importance of cellular senescence as a fail-safe tumor suppressive mechanism activated in response to maximal and aberrant activation of the PI3K signaling pathway, suggesting that pharmacological manipulation of this response could be beneficial for cancer prevention and therapy.
Although it is tempting to speculate that similar fail-safe mechanisms are operational also in the HSCs compartment, it remains to be proven that this is indeed the case, and to which extent tissue specificity may affect the biological nature of this response. Indeed, more apoptosis is found in FoxO-deficient HSCs than WT control [16**], while to date we failed to detect a significant increase in apoptosis [14**] or senescence in the HSC compartment from young Pml−/− mice (Ito K et al., unpublished data). It remains to be determined whether Pml−/− HSCs are more prone to terminally differentiate as a consequence of their enhanced proliferative potential. On the other hand, nothing is known to date regarding the consequence of acute Pten inactivation in the HSC compartment at the cell biological level (i.e. we do not currently know why the HSC pool exhausts upon acute Pten inactivation).
It is also important to point out that acute Akt/mTOR pathway superactivation may have diverse consequences on the basis of tissue/cell type specific differences. Some cell types may tolerate better or worse a surge in Akt/mTOR signaling, or the consequence of PML/PTEN/FOXO inactivation. Hence the outcome may be different in distinct tissues. To this end, activation of PTEN/mTOR pathway is required for viability and maintenance in breast cancer stem-like cells [44]. In neurons, for instance, PTEN negatively regulates neural stem cell proliferation. Loss of PTEN, leads to hyperphosphorylation of Akt and increases the pool of self-renewing neural stem cells [45], and promotes their escape from the homeostatic mechanisms of proliferation control, possibly leading to promotion of tumor progression by providing tumor initiating cells a self-renewal mechanism [46,47]. Thus, it is possible that neuronal stem cells simply proliferate more and more efficiently in the absence of Pten. Alternatively, once again, it could be proposed that this proliferative response is preceded by a senescence/apoptosis response phase that is subsequently evaded.
Therapeutic Implications and Future Directions
Our findings have straighforward and clinically testable therapeutic implications as the new signaling network that we have described is eminently “druggable” (11–14**,15*,16**,17**,37**):
By slowing down proliferation and by normalizing signaling output, rapamycin prolongs maintenance of both wt and Pml-null CML-initiating cells in serial transplantation models [14**]. Pml−/− LICs treated with rapamycin retained the potential to develop CML-like disease even after serial BMT by maintaining quiescence, although a delayed leukemia onset was observed in first BMT. Notably, rapamycin also accelerated CML-like disease driven by Pml+/+ LICs after serial BMT [14**]. Taken together, these findings suggest that great caution should be used when considering clinical trials with rapamycin in CML. In addition, PTEN loss in CML is infrequently observed [44]. Importantly, the study of Morrison and colleagues demonstrates that rapamycin is not curative after leukemia development in a Pten null setting [11**] (Figure 4c). Hence, even if CML blasts experience PTEN loss during disease evolution, rapamycin would not represent an effective treatment, while the maintenance of CML LICs with functionally normal PTEN could be promoted by rapamycin favoring disease recurrence.
-
PML-targeting drugs such as arsenic trioxide, or RNF4 enhancing drugs in the future, could eradicate LICs and synergize with standard of care treatments such as imatinib mesylate in CML (Figure 5). Destabilization of Pml by arsenic treatment was successful in suppressing LIC maintenance in the mouse model of CML, providing strong support to the notion that CML patients might benefit from administration of arsenic-mediated therapy [14**]. However, much work remains to be done to translate these findings from basic/preclinical research to the clinic. In particular, the tyrosine kinase inhibitor imatinib mesylate represents to date the first-choice treatment for all newly diagnosed CML patients [48]. The ability of arsenic to synergize with this standard of care therapy towards disease eradication has to be rigorously assessed in clinically relevant mouse models.
It is speculated that in the LIC compartment, inhibition of BCR-ABL activity by imatinib mesylate would inhibit signaling output (inlcuding PI3K/Akt signaling) and thus result in reactivation of FoxOs and induction of quiescence, in turn failing to eradicate the LIC population [37**]. Therefore, it is possible that sequential rather than combinatorial administration of these two drugs will prove more effective for eradication of LICs in CML. Priming the cells with arsenic therapy prior to imatinib therapy could represent a rational approach. Alternatively, discontinuation of imatinib mesylate could trigger cell cycle entry effectively in LIC which would be exacerbated by a pulse of arsenic therapy through superactivation of PI3K/Akt signaling. However, the effect of this short-term imatinib discontinuation(s) on disease outcome should be investigated at first in a preclinical setting in faithful mouse models of CML. Finally, the potential toxicity of this combination and the effect on the phenotypic characteristics of LICs remains to be thouroughly assessed. To this end, it will be essential to soon perform formal phase I and II clinical trials in human CML patients. Indeed such clinical trails are currently being designed and developed in several countries.
Finally, an even more provocative approach to be tested in the future is represented by testing the efficay of PTEN reversible inhibitors as “Akt/mTOR enhancing drugs” towards LIC/CIC eradication. Such small molecule inhibitors are currently at hand, having been developed for the treatment of diabetes [49], and could be assessed singly or in combination with PML targeting drugs such as arsenic. To this end, much preclinical work will be needed, as well as a genetic analysis of the consequence of compound Pml/Pten inactivation in the stem cell compartment. This will allow a determination of whether concomitant reduction in Pml and Pten function attenuates or accelerates disease pathogenesis in a tissue/cell specific setting, and to identify specific signaling pathways by which Pml, Pten and FoxO can regulate the quiescent status of LICs. Future work focusing on further defining the PI3K/Akt/mTOR molecular network regulating stem cell quiescence and biology will enable a more rational design of novel drugs to eradicate cancer and the cancer-initiating cells (Figure 5).
Figure 5. The therapeutic implication of Akt/mTOR signaling.
PML-targeting drugs such as arsenic trioxide and RNF4 enhancing drugs could eradicate LICs and synergize with standard of care treatment. A transient super-activation of Akt/mTOR signaling might also have therapeutic potential.
Acknowledgments
We thank A. Morotti, S. Matsuoka, Y. Ikeda, J. Rosenblatt, D.E. Avigan and J. Teruya-Feldstein and all members of the Pandolfi laboratory for help, valuable comments and critical discussion K.I. was supported by a JSPS postdoctoral fellowship for research abroad. R.B. has been supported by a K01 NIH grant and is now supported by an Armenise/Harvard career development grant. This work was supported by NIH grants to P.P.P.
Footnotes
Conflict of interest
The authors hereby state that they have no financial or other conflicting relationship with people or organizations that can influence their work
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Paper of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Bruce WR, Van Der Gaag H. A Quantitative Assay for the Number of Murine Lymphoma Cells Capable of Proliferation in Vivo. Nature. 1963;199:79–80. doi: 10.1038/199079a0. [DOI] [PubMed] [Google Scholar]
- 2.Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 3.Scadden DT. Cancer stem cells refined. Nat Immunol. 2004;5:701–703. doi: 10.1038/ni0704-701. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 6**.Holtz M, Forman SJ, Bhatia R. Growth factor stimulation reduces residual quiescent chronic myelogenous leukemia progenitors remaining after imatinib treatment. Cancer Res. 2007;67:1113–1120. doi: 10.1158/0008-5472.CAN-06-2014. A good example demonstrating administration of growth factors reduces quiescence in the marrow and in turn reduces minimal residual disease (MRD) after imatinib treatment. [DOI] [PubMed] [Google Scholar]
- 7.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 9*.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 10*.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. Papers [9, 10] are excellent recent reviews of the role of mTOR pathway in tumorigenesis. [DOI] [PubMed] [Google Scholar]
- 11**.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 12**.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. Papers [12,13] are key papers for critical role of PTEN/AKT in HSC and LICs; the authors show that deletion of the Pten gene in murine hematopoietic system results in generation of LICs but depletion of normal HSCs. [DOI] [PubMed] [Google Scholar]
- 13**.Guo W, Lasky JL, Chang CJ, Mosessian S, Lewis X, Xiao Y, Yeh JE, Chen JY, Iruela-Arispe ML, Varella-Garcia M, et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature. 2008;453:529–533. doi: 10.1038/nature06933. An important paper demonstrating that multi-genetic events contribute to Pten-null leukemia formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, Rosenblatt J, Avigan DE, Teruya-Feldstein J, Pandolfi PP. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453:1072–1078. doi: 10.1038/nature07016. Particularly important for the novelty and clinical implication in cancer/leukemia stem cell model. This paper presents a new therapeutic approach for targeting quiescent LICs by pharmacological inhibition of PML. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441:523–527. doi: 10.1038/nature04809. A key paper demonstrating PML/Akt tumor suppressor network. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. A critical paper showing requirement of FoxO-dependent signaling for long-term regenerative potential of the HSC-compartment through regulation of HSC response to physiologic oxidative stress, quiescence, and survival. [DOI] [PubMed] [Google Scholar]
- 17**.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. An important paper demonstrating essential role of FoxO 3A in stem cell maintenance. [DOI] [PubMed] [Google Scholar]
- 18.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 19.de Klein A, van Kessel AG, Grosveld G, Bartram CR, Hagemeijer A, Bootsma D, Spurr NK, Heisterkamp N, Groffen J, Stephenson JR. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]
- 20**.Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O, Blanchet O, Marit G, Gluckman E, Reiffers J, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109:58–60. doi: 10.1182/blood-2006-03-011239. A good example showing CML-relapse in cases including those showing good responses without signs of disease progression after therapy is discontinued. This result suggests that small number of surviving LICs is a potential source for relapse. [DOI] [PubMed] [Google Scholar]
- 21.Ghanima W, Kahrs J, Dahl TG, 3rd, Tjonnfjord GE. Sustained cytogenetic response after discontinuation of imatinib mesylate in a patient with chronic myeloid leukaemia. Eur J Haematol. 2004;72:441–443. doi: 10.1111/j.1600-0609.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 22**.Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 2007;8:1006–1016. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- 23**.Bernardi R, Papa A, Pandolfi PP. Regulation of apoptosis by PML and the PML-NBs. Oncogene. 2008;27:6299–6312. doi: 10.1038/onc.2008.305. Great reviews [23,24] of current knowledge of PML and nuclear body. [DOI] [PubMed] [Google Scholar]
- 24**.Bernardi R, Guernah I, Jin D, Grisendi S, Alimonti A, Teruya-Feldstein J, Cordon-Cardo C, Simon MC, Rafii S, Pandolfi PP. PML inhibits HIF-1alpha translation and neoangiogenesis through repression of mTOR. Nature. 2006;442:779–785. doi: 10.1038/nature05029. A key paper demonstrating the involvement of PML is AKT/mTOR signaling pathway. This study shows that PML acts as a negative regulator of mTOR in neoangiogenesis. [DOI] [PubMed] [Google Scholar]
- 25*.Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J, Pandolfi PP. The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature. 2008;455:813–817. doi: 10.1038/nature07290. An important paper showing PML/HAUSP network in PTEN regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubota Y, Takubo K, Suda T. Bone marrow long label-retaining cells reside in the sinusoidal hypoxic niche. Biochem Biophys Res Commun. 2008;366:335–339. doi: 10.1016/j.bbrc.2007.11.086. [DOI] [PubMed] [Google Scholar]
- 28**.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. A particularly important paper showing that acute Tsc1 inactivation in HSCs induces mTOR hyper-activation, leading to exit from quiescence and exhaustion of HSCs without leading to leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lallemand-Breitenbach V, Zhu J, Puvion F, Koken M, Honore N, Doubeikovsky A, Duprez E, Pandolfi PP, Puvion E, Freemont P, et al. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation. J Exp Med. 2001;193:1361–1371. doi: 10.1084/jem.193.12.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Tatham MH, Geoffroy MC, Shen L, Plechanovova A, Hattersley N, Jaffray EG, Palvimo JJ, Hay RT. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat Cell Biol. 2008;10:538–546. doi: 10.1038/ncb1716. [DOI] [PubMed] [Google Scholar]
- 31**.Lallemand-Breitenbach V, Jeanne M, Benhenda S, Nasr R, Lei M, Peres L, Zhou J, Zhu J, Raught B, de The H. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol. 2008;10:547–555. doi: 10.1038/ncb1717. Papers [30,31] are particularly important for revealing the nature of the degradation pathway of PML in the response to arsenic trioxide. These papers show that As2O3-induced PML degradation is mediated by RNF4, a SUMO-dependent E3 ubiquitin ligase. [DOI] [PubMed] [Google Scholar]
- 32.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 33.Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 35.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 36.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 37**.Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1:140–152. doi: 10.1016/j.stem.2007.07.017. A great review of current knowledge of the role of Foxo in stem cell biology. [DOI] [PubMed] [Google Scholar]
- 38.Aggerholm A, Gronbaek K, Guldberg P, Hokland P. Mutational analysis of the tumour suppressor gene MMAC1/PTEN in malignant myeloid disorders. Eur J Haematol. 2000;65:109–113. doi: 10.1034/j.1600-0609.2000.90181.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang ZG, Delva L, Gaboli M, Rivi R, Giorgio M, Cordon-Cardo C, Grosveld F, Pandolfi PP. Role of PML in cell growth and the retinoic acid pathway. Science. 1998;279:1547–1551. doi: 10.1126/science.279.5356.1547. [DOI] [PubMed] [Google Scholar]
- 40.Rego EM, Wang ZG, Peruzzi D, He LZ, Cordon-Cardo C, Pandolfi PP. Role of promyelocytic leukemia (PML) protein in tumor suppression. J Exp Med. 2001;193:521–529. doi: 10.1084/jem.193.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Suzuki A, Nakano T, Mak TW, Sasaki T. Portrait of PTEN: messages from mutant mice. Cancer Sci. 2008;99:209–213. doi: 10.1111/j.1349-7006.2007.00670.x. A great review paper summarizing current knowledge PTEN in tumorigenesis and phenotype of conditional knockout mice of Pten. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van Dyke T, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E, 3rd, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 46.Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, Geschwind DH, Liu X, Kornblum HI, Wu H. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci U S A. 2006;103:111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Liu F, Salmonsen RA, Turner TK, Litofsky NS, Di Cristofano A, Pandolfi PP, Jones SN, Recht LD, Ross AH. PTEN in neural precursor cells: regulation of migration, apoptosis, and proliferation. Mol Cell Neurosci. 2002;20:21–29. doi: 10.1006/mcne.2002.1115. [DOI] [PubMed] [Google Scholar]
- 48.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 49.Rosivatz E, Matthews JG, McDonald NQ, Mulet X, Ho KK, Lossi N, Schmid AC, Mirabelli M, Pomeranz KM, Erneux C, et al. A small molecule inhibitor for phosphatase and tensin homologue deleted on chromosome 10 (PTEN) ACS Chem Biol. 2006;1:780–790. doi: 10.1021/cb600352f. [DOI] [PubMed] [Google Scholar]