Abstract
Approaches to the fabrication of surfaces that combine methods for the topographic patterning of soft materials with opportunities for facile, post-fabrication chemical functionalization could contribute significantly to advances in biotechnology and a broad range of other areas. Here, we report methods that can be used to introduce well-defined nano- and microscale topographic features to thin films of reactive polymers containing azlactone functionality using nano-imprint lithography (NIL). We demonstrate that NIL can be used to imprint topographic patterns into thin films of poly(2-vinyl-4,4-dimethylazlactone) and a copolymer of methyl methacrylate and 2-vinyl-4,4-dimethylazlactone using silicon masters having patterns of grooves and ridges ranging in width from 400 nm to 2 μm, demonstrating the potential of this method to transfer patterns to films of these reactive polymers over a range of feature sizes and densities. We demonstrate further that the azlactone functionality of these polymers survives temperatures and pressures associated with NIL, and that topographically patterned films can be readily functionalized post-fabrication by treatment of surface-accessible azlactone functionality with small molecules and polymers containing primary amines. The results of experiments in which NIH-3T3 cells were seeded onto films imprinted with lined patterns having a pitch of 4 μm demonstrated that cells attach and proliferate on these azlactone-containing films and that they align in the direction of the imprinted pattern. Finally, we demonstrate that the treatment of these materials with amine-functionalized poly(ethylene glycol) (PEG) can be used to create regions of topographically patterned films that prevent cell adhesion. The results of this study suggest approaches to the functionalization of topographically patterned surfaces with a broad range of chemical functionality (e.g., peptides, proteins, carbohydrates, etc.) of biotechnological interest. The ability to manipulate and define both the physical topography and chemical functionality of these reactive materials could provide opportunities to investigate the combined effects of substrate topography and chemical functionality on cell behavior and may also be useful in a broad range of other applications.
Introduction
Materials that regulate cellular behaviors such as attachment, growth, differentiation, and gene expression have contributed significantly to advances in both biotechnology and basic biomedical research.1-4 Methods for the chemical functionalization of surfaces have led to significant progress toward the design of functional biomaterials and provide useful tools for understanding the chemical interactions between cells and surfaces that drive or guide cellular response.5-18 More recent work has demonstrated that the micrometer- and nanometer-scale topography of cell substrates can also influence cell behavior significantly.19-22 Although it is now recognized that both surface chemistry and substrate topography can play significant roles in directing cellular interactions with surfaces, it has, in general, proven difficult to investigate and define relationships between both chemical and topographic cues.20,22,23 This is due, in part, to the limited availability of materials and methods that can be used to fabricate surfaces with suitable combinations of well-defined topography and chemical functionality in a convenient and straightforward manner. In this paper, we report a step toward addressing this broader limitation by imprinting nanometer- and micrometer-scale topographical features into thin films of reactive thermoplastic polymers to produce physical patterns that can be further functionalized chemically using a broad range of different amine-functionalized nucleophiles.
The work reported here is based upon the results of numerous past studies describing the influence of surface topography on cell behavior,19-22 including several recent reports by Nealey and Murphy and coworkers demonstrating the effects of nanometer-scale topography on the attachment, spreading, and alignment of corneal epithelial cells and other cell types.24-32 These past studies have established that several factors—including the width, spacing, shape, and depth of surface features—can play roles in guiding the responses of attached cells. For example, corneal epithelial cells seeded on patterns of gratings or grooves with widths ranging from 70 nm to 2 μm generally align in the direction of the features to varying degrees, depending upon the depth, width, or spacing of the pattern and other factors such as shear flow and the composition of growth media.24-28,30,32 Several other reports have also demonstrated synergistic and antagonistic combinations of both chemical functionality and topographic patterning to probe the combined effects of these environmental cues on cell behavior.33-35 For example, Britland et al. characterized the alignment of BHK cells on silicon surfaces having (i) a grooved topography with various micrometer-scale widths and depths and (ii) striped micrometer-scale patterns of cell-adhesive silanes.33 When these topographic and chemical features were fabricated to be parallel to one another, cell alignment was enhanced relative to either topographic or chemical patterning alone. When the silane tracks were orthogonal to the grooves, cells aligned preferentially in the direction of the chemical cues. These studies demonstrate that methods and materials that permit the fabrication of surfaces with both chemical and topographic patterns could play a significant role in advancing an understanding of the interplay between substrate chemistry and substrate topography as signals that guide cellular responses.
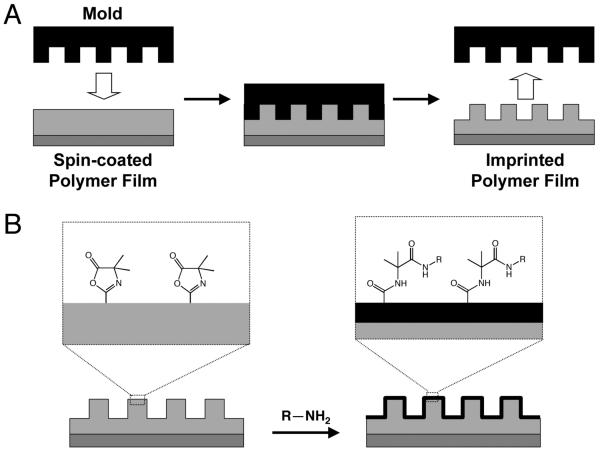
Advances in the field of lithography have yielded methods for the transfer of nanometer-scale features to polymers and other soft materials that could prove useful for addressing and investigating several of the goals outlined above.36-38 Of particular relevance to the study reported here are methods that have been developed for the transfer of nanometer-scale features into soft materials using nano-imprint lithography (NIL).39-42 NIL is a process in which a master (e.g., silicon, typically prepared via conventional lithographic techniques) is pressed into a thermoplastic material heated above its glass transition temperature (Tg). When the thermoplastic is cooled below Tg, the master is removed, leaving the negative relief of the master in the imprinted material (e.g., see Scheme 1A). NIL and other new approaches involving the transfer of a pattern from a silicon master to polymers or other soft materials have reduced certain barriers—such as cost, access to lithographic equipment, or the need for technical expertise—to producing topographically patterned surfaces, because a single silicon master can be used to pattern many samples without loss of fidelity.39-42
Scheme 1.
Schematic illustrations of the approach to topographic and chemical patterning of polymer thin films described in this study. A) Transfer of nanometer- or micrometer-scale topographic features to a thin film of a reactive, azlactone-containing polymer from a silicon master using nano-imprint lithography (NIL). (B) Schematic illustration of the post-fabrication chemical modification of azlactone functional groups present on the surface of a nano-imprinted film by reaction with amine-functionalized nucleophiles.
Although the transfer of micrometer- and nanometer-scale topographic patterns to soft materials is facilitated by NIL and other novel lithographic approaches, methods to immobilize molecules of biological relevance on topographically patterned surfaces have remained more limited. This is due, in part, to the relatively inert nature of materials that are amenable to traditional lithographic techniques (e.g., silicon) or the nature of the soft materials typically used for NIL or soft lithographic approaches [e.g., thermoplastics such as poly(methyl methacrylate) (PMMA) used for NIL or polyurethanes used in replica molding]. The chemical functionalization of these types of materials often involves the post-fabrication deposition of one or more layers of metals (e.g., gold) onto the surface, followed by the deposition of a self-assembled monolayer, which may also require further reaction steps to link a particular molecule or present the desired chemical functionality.43 This is a versatile and straightforward approach that can be used conveniently in a laboratory setting, and it has contributed significantly to advances in basic biological research in vitro.7,16,43 However, concerns surrounding the long term stability and biocompatibility of these materials raise important questions about the general suitability of this approach for the generation of chemically patterned materials for use in vivo and in other applications.44 The development of methods for the generation of topographic patterns in soft materials that can be readily and directly functionalized chemically post-fabrication would provide new opportunities to investigate the complex interplay between chemical and topographical cues on cell behavior at surfaces in both fundamental and applied contexts. More generally, such methods and materials could also contribute to advances in other applications, such as the fabrication of materials for biosensing or the design of organic electronic devices.
We reasoned that the transfer of micro- or nano-scale topography into thin films of chemically reactive polymers could provide a platform for the generation of topographically patterned surfaces that could subsequently be functionalized chemically for a broad range of applications (e.g., see Scheme 1B). We note in this context that Embrechts et al. recently reported an approach to the nano-imprinting and subsequent functionalization of thin films of a reactive block copolymer containing N-hydroxysuccinimide functionality.45 This current study sought to evaluate the feasibility of this approach using NIL and polymers containing azlactone functionality, which have been shown in past studies to react readily with a wide variety of nucleophiles.46 Here, we report that NIL can be used to transfer micrometer- and nanometer-scale lined patterns into thin films of poly(2-vinyl-4,4-dimethylazlactone) (polymer 1) and of a copolymer of methyl methacrylate and 2-vinyl-4,4-dimethylazlactone (polymer 2). We show that it is possible to imprint topography with depths on the order of hundreds of nanometers and line widths ranging from 400 nm to 2 μm, demonstrating the applicability of this method to transfer patterns to films of these polymers over a broad range of feature sizes and densities. We demonstrate further that the azlactone functionality of these polymers survives the NIL process and that surface-accessible azlactone groups can react with primary amines to functionalize imprinted films with both small molecules and macromolecules. Experiments in which mammalian cells were seeded on films of polymer 2 imprinted with silicon masters having patterns of ridges and troughs reveal that cells attach and align on these materials in the direction of the imprinted pattern. Finally, we demonstrate that imprinted films can be functionalized with amine-terminated poly(ethylene glycol) (PEG), and that PEG-treated areas of these films resist the attachment of cells. The results of this study suggest the basis of an approach that could, with further development, provide a platform for the design of surfaces to interface cells with well-defined combinations of chemical functionality and topographic patterns.
Materials and Methods
Materials
2-Vinyl-4,4-dimethylazlactone (VDMA) monomer was a kind gift from Dr. Steven M. Heilmann (3M Corporation, Minneapolis, MN). Methyl methacrylate (MMA) was purchased from Aldrich Chemical Company (Milwaukee, WI) and passed through an inhibitor removal resin. Poly(2-vinyl-4,4-dimethylazlactone) (PVDMA, MW = 130,000, PDI = 3.1) and copolymers with methyl methacrylate [Poly(MMA-co-VDMA), 80% MMA as determined by NMR, MW = 35,000, PDI = 2.8] were synthesized as previously described.47 Test grade n-type silicon wafers were purchased from Si-Tech, Inc. (Topsfield, MA). Poly(methyl methacrylate) (MW = 135,000) was purchased from Polymer Laboratories, Ltd. (Shrewsbury, UK). 3-(Diethoxymethylsilyl)propylamine (APMDS) was purchased from TCI (Tokyo). O-(2-Aminoethyl)-O′-methylpolyethylene glycol 5000 (PEG-NH2) was purchased from Fluka. Poly(ethylene glycol) (MW = 5000) was purchased from Polysciences, Inc. (Warrington, PA). Tetramethylrhodamine cadaverine was purchased from AnaSpec, Inc. (San Jose, CA). Calcein AM was purchased from Invitrogen (Carlsbad, CA). Ethyl acetate was purchased from Burdick 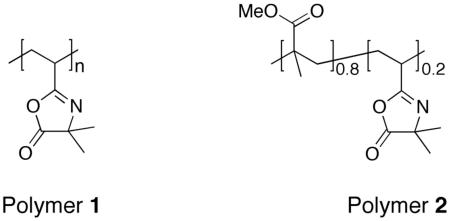 & Jackson (Muskegon, MI). Absolute ethanol was purchased from Aaper Alcohol (Shelbyville, KY). All commercially available materials were used as received without further purification unless otherwise noted. Silicon masters for imprinting were generously provided by the group of Prof. Paul F. Nealey at the University of Wisconsin-Madison. One pattern consisted of lines with a 4-μm pitch (1:1 ridge:groove) and a height of 355 nm. A second master contained a region patterned with lines having an 800-nm pitch (1:1 ridge:groove) and a height of approximately 400 nm.27 Silicon masters were treated with tridecafluoro-1,1,2,2-tetrahydrooctyl trichlorosilane as a release layer.
& Jackson (Muskegon, MI). Absolute ethanol was purchased from Aaper Alcohol (Shelbyville, KY). All commercially available materials were used as received without further purification unless otherwise noted. Silicon masters for imprinting were generously provided by the group of Prof. Paul F. Nealey at the University of Wisconsin-Madison. One pattern consisted of lines with a 4-μm pitch (1:1 ridge:groove) and a height of 355 nm. A second master contained a region patterned with lines having an 800-nm pitch (1:1 ridge:groove) and a height of approximately 400 nm.27 Silicon masters were treated with tridecafluoro-1,1,2,2-tetrahydrooctyl trichlorosilane as a release layer.
General Considerations
Silicon and glass substrates (typically ∼12 × 15 mm) were rinsed successively with acetone, ethanol, methanol, and water and then dried under a stream of compressed air passed through a 0.2 μm filter. For films to be used in experiments involving cell staining, substrates were then treated with an aminosilane to stabilize the films against the repeated drying and rehydration steps associated with the staining procedure. Silanization was performed by immersion in a 1 wt% solution of APMDS in ethanol for 30 minutes, followed by rinsing in ethanol and drying under filtered air prior to use. Atomic force microscopy (AFM) images were acquired in tapping mode on a Nanoscope Multimode atomic force microscope (Veeco/Digital Instruments, Santa Barbara, CA), using scan rates of 0.8 – 5 μm/s to obtain 256 × 256 pixel images. Silicon cantilevers with a spring constant of 40 N/m were used (model NSC15/NoAl, MikroMasch USA, Inc., Portland, OR). Height data were flattened using a 2nd-order fit. The height and pitch of features were calculated from image sections using the Nanoscope software (Veeco/Digital Instruments, Santa Barbara, CA). Optical micrographs of imprinted films were acquired using an Olympus C-4000 Zoom digital camera mounted on an Olympus BX60 microscope in reflective mode. Fluorescence microscopy images were acquired using an Olympus IX70 microscope and were analyzed using the Metavue version 7.1.2.0 software package (Molecular Devices; Toronto, Canada). Polarization-modulation infrared reflectance-absorbance spectroscopy (PM-IRRAS) was conducted in analogy to previously reported methods.48,49 Briefly, gold-coated silicon substrates coated with spin-coated films of polymers 1 or 2 were placed at an incident angle of 83° in a Nicolet Magna-IR 860 Fourier transform infrared spectrophotometer equipped with a photoelastic modulator (PEM-90, Hinds Instruments, Hillsboro, OR), a synchronous sampling demodulator (SSD-100, GWC Technologies, Madison, WI), and a liquid-nitrogen-cooled mercury cadmium telluride detector. The modulation was set at 1500 cm−1, and 500 scans were obtained for each sample at a resolution of 2 cm−1. The differential reflectance infrared spectra were then normalized and converted to absorbance spectra using a previously reported procedure.50 Measurements of Tg were conducted on a DSC Q100 digital scanning calorimeter (TA Instruments, New Castle, DE) and are presented as the mean values from the final two of three consecutive scans between 25 °C and 200 °C at a rate of 10 °C/minute.
Spin-Coating of Polymer Films
Solutions of PVDMA and poly(MMA-co-VDMA) used for spin-coating were prepared at 4 wt% in ethyl acetate. Films were spin-coated using these solutions (filtered through a 0.2 μm membrane syringe filter) at speeds ranging from 2500 to 4000 RPM for 60 seconds in the clean room facilities at the Wisconsin Center for Applied Microelectronics (WCAM) at the University of Wisconsin-Madison. After spin-coating, films were baked on a hot plate at 180 °C for 5 minutes. The thickness of films deposited on silicon were measured with a Filmetrics F-20 Optical Reflectometer (San Diego, CA), using refractive index data for PMMA. For each sample, at least four measurements were taken and are reported as approximate mean values (e.g., rounded to the nearest 5 nm).
Nanoimprinting of Polymer Films
Nanoimprinting experiments were conducted using an ObducatAB NIL-2.5 nanoimprinter in the WCAM facilities using an automated process under PC control. The nanoimprinting of spin-coated polymer films was conducted in the following general manner. Film-coated substrates were placed on the sample stage of the nanoimprinter, a silicon master etched with the desired pattern was inverted and placed on top of the film-coated sample, and three sheets of an aluminum alloy foil (3 mils thick, oil-free) were placed on top of the substrate/master assembly. The stage was then heated to 180 °C, and pressure (40 bar) was applied. Pressure and temperature were maintained for five minutes, after which time the samples were air-cooled. Once the temperature reached 50 °C, the pressure was released.
Characterization and Chemical Functionalization of Nanoimprinted Films
Nanoimprinted PVDMA and poly(MMA-co-VDMA) films were characterized by optical microscopy and AFM, as described above under General Considerations. The chemical functionalization of imprinted films with small-molecule fluorescent dyes was conducted by placing a drop of TMR cadaverine (1 μL; 2 mg/mL in H2O) on the surface of an imprinted film and allowing the spot to sit for 1 minute. The film was then soaked in water to remove unreacted fluorophore and dried under filtered air. Chemical functionalization of surfaces with PEG was conducted by placing a drop of amine-functionalized PEG (1 μL; 10 mg/mL in H2O) on the surface of an imprinted film. The spot was allowed to sit for 150 minutes prior to rinsing. Control samples were prepared by treatment of imprinted films with either a drop of a solution of PEG that was not amine functionalized or a drop of water free of PEG. These samples were either characterized by fluorescence microscopy or used directly in cell culture experiments.
Characterization of Cell Attachment and Alignment on Nanoimprinted Films
Substrates coated with nanoimprinted poly(MMA-co-VDMA) films were placed film-side up in the bottoms of the wells of 6- or 12-well polystyrene tissue culture plates. NIH-3T3 cells (American Type Culture Collection; Manassas, VA) were seeded on top of the films by placing a sufficient volume of a suspension of cells (130,000 to 190,000 cells/mL in approximately 400 μL of Dulbecco's modified Eagle medium supplemented with 10% newborn calf serum and 100 units/mL of penicillin and 100 μg/mL of streptomycin) to cover the surface of the film without spilling over into the rest of the well. After allowing 30 minutes for cells to begin to attach, 1-2 mL of additional growth medium was added, and the culture plate was placed in an incubator. After approximately 1 day of incubation, the cells were either stained using Calcein AM and then imaged or directly imaged without staining by fluorescence or phase contrast microscopy, respectively. Substrates treated with amine-functionalized PEG were prepared as described above; scratch marks on the sides of the glass substrate were used to aid in identifying the locations of treated spots by optical microscopy.
Results and Discussion
The development of methods that can be used to imprint topographic features into surfaces that can be chemically modified post-fabrication would provide new opportunities to understand complex relationships between cell contact guidance based on topography and the effects of surface chemistry on cells at surfaces. This study sought to determine the feasibility of an approach to transferring micrometer- and nanometer-scale topography to thin films of reactive polymers by NIL to create topographically patterned surfaces that can subsequently be functionalized to present desired chemical species at the surface. Although various reactive polymers have been used in the context of immobilizing biomolecules at the surfaces of thin films,51-57 the ideal polymers for the process investigated here should have at least several, if not all, of the following properties: (i) the polymers should have a Tg that is relatively low (e.g., ∼50 to 150 °C) to facilitate the imprinting of topographic features using NIL, (ii) the polymers should be straightforward to synthesize and be produced from monomers that can be readily copolymerized with other monomers typically used for NIL, and (iii) the polymers should react with a variety of chemical functional groups, preferably in a manner that is rapid and that occurs without the generation of byproducts.
To explore the feasibility of this approach and demonstrate proof of concept, we selected polymers 1 and 2, both of which contain azlactone functionality. Polymers containing azlactone groups are well-suited for the general approach described above because they react with a wide variety of nucleophiles (e.g., amines, thiols, alcohols) through a ring-opening reaction that proceeds rapidly without the formation of byproducts and, in many cases, without the need for a catalyst.46 In addition, polymers 1 and 2 can be cast as thin films and have glass transition temperatures of approximately 101 °C and 96 °C, respectively, which are in the range of temperatures commonly used for NIL.39-42 We selected methyl methacrylate as a comonomer to prepare polymers for use in these initial studies (e.g., polymer 2) because of its established use both in NIL41,42 and as a substrate for cell culture and the design of new biomedical materials.58 On the basis of these considerations, we sought to determine whether NIL could be used to transfer topographic patterns to thin films of polymers 1 and 2 and, subsequently, whether the imprinted films could be functionalized post-fabrication via reaction of the pendant azlactone groups with amine-functionalized molecules.
Nano-Imprinting of Thin Films of Azlactone-Containing Polymers 1 and 2
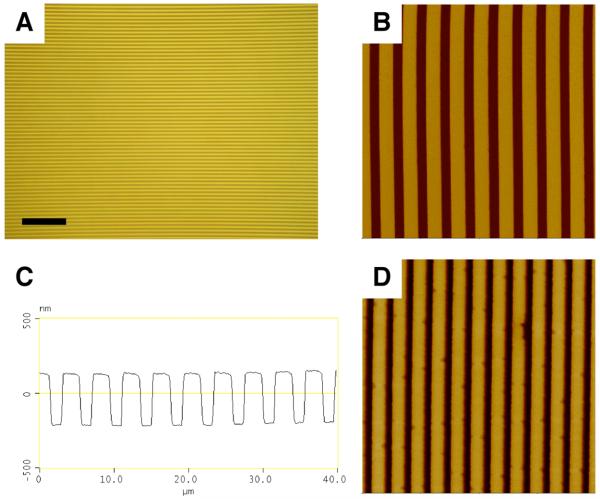
We conducted an initial set of experiments to determine whether thin films of polymers 1 and 2 could be imprinted with features having micrometer- and nanometer-scale dimensions. Thin films of polymers 1 and 2 (e.g., ∼200 nm thick) were spin-coated from solutions in ethyl acetate onto planar silicon and glass substrates and imprinted using methods for NIL similar to those described in past studies (see Materials and Methods section for details of film casting and imprinting).39-42 Figure 1 shows several representative images of imprinted thin films of polymer 1. Figures 1A-B show a film imprinted using a master having a lined pattern with a 4 μm pitch, 2 μm line width, and 355 nm height. Figure 1A is an optical microscopy image (354 μm by 265 μm) acquired in reflective mode. Inspection of this image reveals ridges and troughs at regular intervals without observable large-scale defects, and demonstrates qualitatively that NIL can be used to pattern polymer 1 over relatively large areas. To determine quantitatively the fidelity of pattern transfer and characterize the sizes and shapes of the features imprinted in the film, we also characterized imprinted films by atomic force microscopy (AFM). Figure 1B shows a height image acquired in tapping mode of a 40 μm by 40 μm area of the same film shown in Figure 1A. Figure 1C shows a cross-sectional view of the line drawn through the AFM image in Figure 1B. Measurements of the pitch, width, and height of the lines (4.06 μm, 2.03 μm, and 346 nm, respectively) closely match the corresponding values of the silicon master (as described above).
Figure 1.
(A-C) A film of polymer 1 imprinted with a silicon master patterned with lines at a 4-μm pitch (see text), imaged by (A) reflective optical microscopy (scale bar = 50 μm) and (B,C) AFM (40 μm × 40 μm, z scale = 1 μm). (D) 10 μm × 10 μm AFM image of a film of polymer 1 imprinted with a silicon master patterned with lines at a 800-nm pitch (z scale = 800 nm). See text for additional details describing imprinting and acquisition of images by optical microscopy and AFM.
To explore the range of pattern sizes that can be accessed using this approach, we imprinted spin-coated films with a second silicon master patterned with lines 400 nm wide, having a pitch of 800 nm and a height of ∼400 nm. Figure 1D shows a 10 μm by 10 μm AFM image of a film of polymer 1 that was imprinted using this master. Cross-sectional analysis of this image yielded a pitch, line width, and height of 820 nm, 469 nm, and 445 nm, all of which were in close agreement with the corresponding values for the master. These results demonstrate that NIL can be used to faithfully transfer to thin films of polymer 1 topographic patterns with features ranging from the nanometer scale to the micrometer scale. Characterization of films of polymer 2 imprinted with the masters described above by optical microscopy and AFM yielded results indistinguishable from those shown in Figure 1 for polymer 1 (data not shown).
Chemical Functionalization of Imprinted Films with an Amine-Functionalized Fluorophore
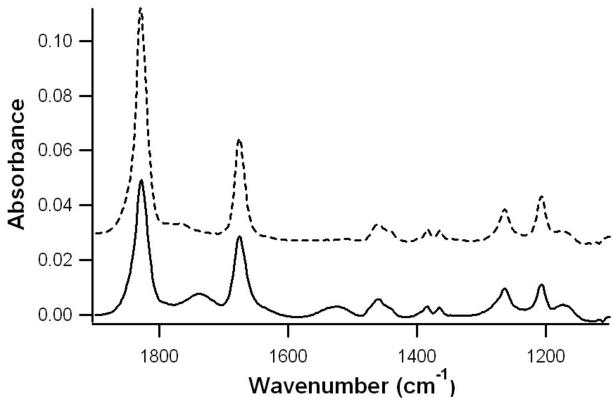
The results described above are similar to those of previous reports describing the use of NIL to transfer topographic features into thin films of conventional polymers such as poly(styrene) or poly(methyl methacrylate).39-42 In contrast to these previous studies, however, the polymers used in this study contain reactive azlactone groups, and, thus, provide additional opportunities to chemically functionalize the imprinted films post-fabrication. We conducted several experiments to determine (i) whether the azlactone groups in topographically patterned films of polymers 1 and 2 could survive the relatively high temperatures and pressures associated with the NIL process and (ii) whether imprinted films could be further functionalized with amine-functionalized molecules. To determine whether azlactone groups remained present in the topographically patterned films (i.e., that they did not all react or otherwise degrade substantially during imprinting), an ultrathin film (∼30 nm) of polymer 1 was spin-coated on a gold-coated substrate and imprinted using a flat master (i.e., subjected to the same cycle of temperature and pressure as other imprinted films, but pressed against a flat, unpatterned piece of silicon wafer rather than a master containing micrometer- or nanometer-scale patterns) and characterized by polarization-modulation infrared reflectance-adsorbance spectroscopy (PM-IRRAS). Inspection of the IR spectrum of the imprinted film (Figure 2, solid line) reveals an absorbance peak at 1828 cm−1 characteristic of the carbonyl group of the azlactone functionality in polymer 1.46 Further inspection reveals two additional smaller absorbance peaks at 1737 cm−1 and 1525 cm−1 that were not present in the IR spectrum of the films prior to treatment (Figure 2, dashed line). Additional control experiments using films treated with water or aqueous base demonstrated that these two small peaks correspond to hydrolyzed azlactone groups. The results of these experiments demonstrate that the azlactone functionality of polymer 1 remains substantially intact upon exposure to the temperatures and pressures used for NIL, although a small amount of the azlactone groups does appear to react (most likely with ambient water vapor) during this process.
Figure 2.
PM-IRRAS spectra of thin films of polymer 1 before (dashed line, offset by 0.03 absorbance units for clarity) and after (solid line) being subjected to the NIL process using a flat silicon master (see text).
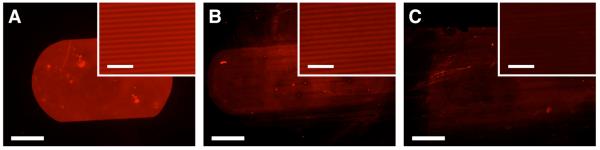
Although the results of the experiments above demonstrate that imprinted films of polymers 1 and 2 retain their azlactone groups, they do not necessarily indicate that these reactive groups are presented at the surface of the films or that they are accessible for further reaction. To determine whether the azlactone groups of imprinted films were available for reaction at the surface (e.g., as opposed to being buried in the interiors of the film) after imprinting, films of polymers 1 and 2 were imprinted with the pattern of lines 2 μm wide described above and were subsequently exposed to a small molecule fluorophore functionalized with a primary amine group. Figures 3A-B show fluorescence micrographs of imprinted films of polymers 1 and 2, respectively, that were treated with a drop of an aqueous solution of tetramethylrhodamine (TMR) cadaverine for one minute and then soaked in deionized water for three hours to remove unreacted fluorophore. Inspection of these images reveals bright spots of red fluorescence that are oblong in shape due to asymmetric wetting along the lines of the patterned surfaces. A comparison of Figures 3A and 3B reveals that the fluorescence associated with the film of polymer 1 (shown in Figure 3A) appears brighter than the fluorescence associated with the film of polymer 2 (Figure 3B). This result is consistent with the large difference in the amount of azlactone groups in the homopolymer (polymer 1) relative to the copolymer (polymer 2), and suggests that it may be possible to tune the amount or density of reactive functional groups at the surfaces of these films for particular applications by control over the structures and compositions of the polymers used to form the films.
Figure 3.
Fluorescence micrographs of films of (A) polymer 1, (B) polymer 2, and (C) PMMA imprinted with a silicon master patterned with lines at a 4-μm pitch after exposure to TMR cadaverine (see text). Scale bar = 500 μm (20 μm in inset). To aid in visualization of fluorescence, the brightness and contrast in (B) and (C) have been enhanced (to identical extents for both images).
The images in Figures 3A and 3B confirm the presence of TMR in or on the fluorophore-treated films and are consistent with the immobilization of TMR at the surface resulting from chemical reaction with the azlactone groups of polymers 1 and 2. It remains possible, however, that the red fluorescence in these images could arise from TMR that is either adsorbed on the surface or absorbed into the films rather than TMR that is covalently attached at the surface. To investigate this possibility further, we conducted an additional experiment using a film of PMMA imprinted and treated with TMR cadaverine as described above for the films of polymers 1 and 2. A fluorescence micrograph of this TMR-treated PMMA film is shown in Figure 3C. Inspection of this image reveals a low level of red fluorescence. The intensity of fluorescence is significantly lower than the fluorescence in the images in either Figure 3A or 3B, and presumably arises from small amounts of TMR that are physically adsorbed onto the surface, since PMMA should not react readily with amine functionality under the conditions used in this experiment. The greater intensities of fluorescence in Figures 3A and 3B in comparison to the fluorescence intensity in Figure 3C suggest that in films of polymers 1 and 2, TMR has chemically reacted with the azlactone groups and is therefore present in larger quantities than would be present through physical association in the absence of a chemical reaction. Although it is well known that azlactone groups react rapidly with amines to form amide bonds,46 direct observation of amide-based reaction products at the surfaces of these treated films using IR or other methods was complicated by the thickness of the films (i.e., the small number of reacted groups at the surface relative to the amount of azlactone groups in the bulk of the film) and additionally by difficulties arising from the topography at the surface of imprinted films.
Investigation of Attachment and Alignment of Cells Seeded on Imprinted Films of Polymer 2
The results described in the previous two sections establish (i) that films of reactive polymers 1 and 2 can be imprinted with topography having micrometer- and nanometer-scale features and (ii) that imprinted films retain azlactone functionality and can subsequently be functionalized by reaction with amine-functionalized nucleophiles. These results provide a basis for the design of surfaces with unique combinations of chemistry and topography that could provide a platform for the investigation of the combined influence of chemical and topographic cues on the responses of cells at surfaces. During initial experiments investigating the behavior of films of these polymers in biologically relevant contexts, we observed that films of polymer 1 wrinkled and peeled significantly upon prolonged exposure to aqueous media. In contrast, films of polymer 2 were more stable, remained attached, and were largely free of wrinkles (data not shown). It may ultimately prove possible to enhance the stability of thin films of polymer 1 (e.g., by modifying the chemical functionality of the substrate upon which it is spin-coated) and thus permit the use of imprinted films of polymer 1 in aqueous environments. However, in the following sections, we focus our attention specifically on the evaluation and characterization of the suitability of polymer 2 as a platform for the investigation of the interactions between cells and chemically functionalized, topographically patterned surfaces.
To determine whether mammalian cells could attach and grow on imprinted films of polymer 2, and to provide an initial indication of the potential biocompatibility of these surfaces, we seeded NIH-3T3 fibroblasts on a film imprinted with the pattern of lines 2 μm wide used to imprint the film shown in Figures 1A-C. After incubation for 16 h, cells were stained using calcein AM. Calcein AM is a small molecule that becomes fluorescent when cleaved by esterases in viable cells, and, in addition to providing an indication of cell viability, also aids in the identification of cells and the characterization of cell morphology and alignment using fluorescence microscopy.59,60 Figures 4A and 4B show representative phase contrast and fluorescence microscopy images of cells incubated on an imprinted film of polymer 2. Inspection of these images reveals that cells are attached to the surface of the film and that they are viable, as indicated by the green fluorescence visible in virtually every cell. Although additional studies will be required to characterize more completely the broader biocompatibility of polymer 2, these results demonstrate that the reactive azlactone groups on the surfaces of these films do not promote significant cell death or prevent cell attachment.
Figure 4.
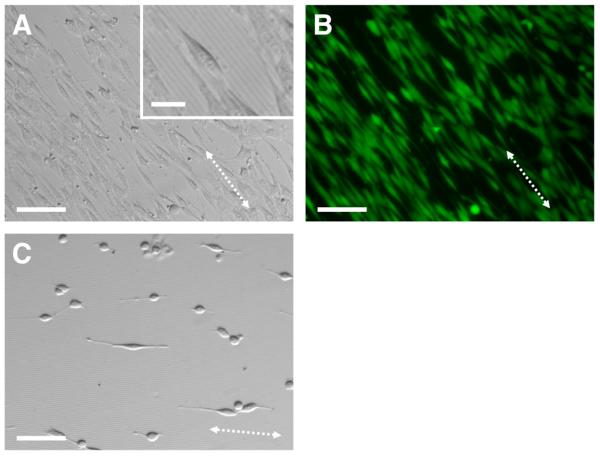
(A) Phase contrast and (B) fluorescence microscopy images of NIH-3T3 fibroblasts (stained with calcein AM) seeded on a film of polymer 2 imprinted with a silicon master patterned with lines at a 4-μm pitch (see text). (C) Phase contrast image of NIH-3T3 fibroblasts seeded on a film of PMMA imprinted with the same pattern. The dotted arrows indicate the direction of the topographic features. Scale bar = 100 μm (25 μm for inset).
Past studies have demonstrated that azlactones react readily with the lysine residues of proteins.61-63 Although PMMA is commonly used as a biomaterial suitable for in vivo applications,58 we speculate that, in addition to any role played by the PMMA component of polymer 2 in promoting cell attachment, the reaction of serum proteins in the cell culture media with the azlactone groups at the surfaces of these films could also influence or help promote the attachment of cells. Indeed, comparison of the image in Figure 4A with the image in Figure 4C, which shows cells seeded on an imprinted film of PMMA, reveals that cells attached and aligned to a greater degree on imprinted films of polymer 2 than on imprinted films of PMMA. We note that, in addition to potential reactions with serum proteins, any residual unreacted azlactone groups at the surfaces of films of polymer 2 would also likely hydrolyze shortly after the incubation of these films in cell culture media. Finally, we note that the images shown in Figure 4 reveal a high degree of cell alignment and extension parallel to the imprinted lines (these imprinted lines are visible in the inset of Figure 4A). Cells attached to unpatterned portions of the film (i.e., regions outside of the area into which the silicon master was imprinted) did not exhibit a preferred orientation (data not shown). These results are consistent with those of past studies demonstrating the contact guidance of cells by topographically patterned substrates fabricated using traditional approaches.24-32
Imprinted Films Functionalized Using Amine-Terminated Poly(ethylene glycol) Prevent Cell Attachment
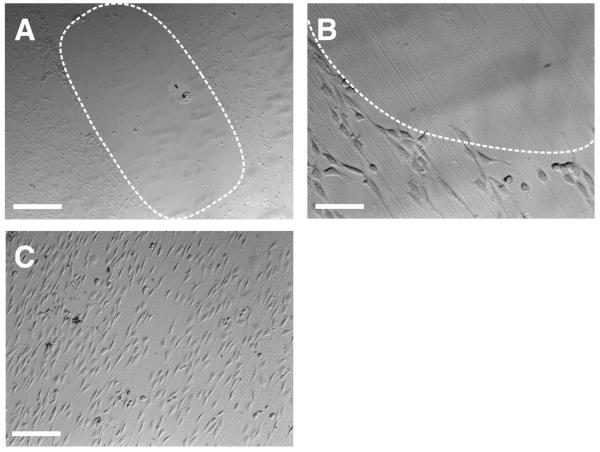
The chemical reactivity of the azlactone groups of the materials investigated above provides a materials-based platform that could be used to investigate the combined effects of surface topography and chemical functionalization on the responses of cells at surfaces. As a first step toward the design of surfaces with patterns of topography and chemical functionality, we sought to determine whether methods commonly used to confer resistance to non-specific protein adsorption or cell attachment on the surfaces of other commonly used materials [e.g., attachment of poly(ethylene glycol) (PEG)64-67 or short oligomers of ethylene glycol68,69] could be used to prevent cell attachment to localized regions of imprinted films of polymer 2. To investigate the feasibility of this approach, a film of polymer 2 imprinted with lines 2 μm wide was treated with a drop of a solution containing PEG functionalized with a primary amine at one terminus (PEG-NH2, MW = 5000) for ∼2.5 hours and then rinsed with water prior to the seeding of cells. Figures 5A-B show phase contrast optical micrographs of this film 40 hours after seeding NIH-3T3 fibroblasts on the surface. Inspection of these images reveals an oblong region in which very few cells are attached, surrounded by areas in which cells are attached and elongated with a high degree of alignment, similar to the results shown in Figure 4 for a film that was not functionalized. These results suggest that the immobilization of PEG at the surface through reaction of the amine terminal groups of PEG-NH2 with the azlactone groups of polymer 2 occurs to an extent that is sufficient to resist the attachment of cells.
Figure 5.
Phase contrast microscopy images of NIH-3T3 fibroblasts seeded on a film of polymer 2 imprinted with a silicon master patterned with lines at a 4-μm pitch. Prior to cell seeding, the imaged areas were treated with a drop of (A,B) PEG-NH2 and (C) PEG. The dotted lines are added as visual guides to indicate the approximate area of the film in A and B functionalized with PEG-NH2. Scale bars are (A) 500 μm, (B) 50 μm, and (C) 200 μm.
Figure 5C shows a phase contrast image of cells seeded on a topographically patterned film of polymer 2 that was treated with a drop of an aqueous solution of PEG (without amine functionality, MW = 5000) similar to the procedure described above for the film treated with a solution of PEG-NH2. Inspection of Figure 5C reveals that cells are attached and elongated over the entire area of the image. The result of this control experiment provides support for the view that the resistance to cell adhesion illustrated in Figures 5A-B results from the covalent attachment of PEG to the surface of the film rather than the physical adsorption of PEG or the presence of acid functionality resulting from the hydrolysis of azlactone groups. These results, when combined, demonstrate that films of polymer 2 imprinted with micrometer-scale topography can be chemically functionalized to create regions that resist the attachment of cells to the surfaces of the films. The approach described here for the fabrication of surfaces with combinations of topographic patterns and chemical functionality suggests opportunities to functionalize topographically patterned surfaces with more complex chemical functionality (e.g., peptides, proteins, carbohydrates, etc.) and with more complex patterns of functionality. This could potentially extend to the ability to functionalize different surfaces of the topographic features (e.g., the tops of ridges vs. sidewalls and grooves) with different chemical species. Studies to this end are currently underway.
Summary and Conclusions
We have reported an approach to the introduction of nanoscale and microscale topographic features into thin films of reactive, azlactone-containing polymers. Our results demonstrate that (i) nano-imprint lithography (NIL) can be used to imprint films of reactive polymers 1 and 2 using methods developed for the imprinting of nanoscale and microscale topographic features into conventional, non-reactive polymers, (ii) that the azlactone groups in these materials do not degrade or react substantially during the imprinting process, and (iii) that the resulting topographically patterned films can be chemically functionalized post-fabrication by treatment with either small molecules or polymers containing primary amine groups. These results, when combined, suggest the basis of approaches to the fabrication of topographically patterned surfaces that can be further functionalized with a broad range of chemical functionality (e.g., peptides, proteins, carbohydrates, etc.) of biotechnological interest. The ability to manipulate and define both the physical topography and the chemical functionality of these reactive materials also provides future opportunities to investigate the combined effects of substrate topography and chemical functionality on cell behavior. As a first step toward evaluating the general feasibility of this approach, we have demonstrated that thin films of polymer 2 can support the attachment and proliferation of NIH 3T3 fibroblasts, and that when cells are cultured on the surfaces of films imprinted with lined topographic patterns having a pitch of 4 μm, they align and elongate in the direction of the imprinted pattern. We have also demonstrated that the treatment of imprinted films of polymer 2 with amine-terminated PEG can be used to create regions of these topographically patterned films that completely prevent cell adhesion. The results of this investigation provide opportunities for the printing of more complex micrometer- or nanometer-scale patterns of functionality that could prove useful for the investigation of the interplay between chemical and topographic cues on cell behavior. The general approach reported here for the imprinting of nanometer- and micrometer-scale topography into reactive, azlactone-containing polymers could also contribute to advances in a broad range of other areas, including biotechnology, microfluidics, and microelectronics.
Acknowledgment
Financial support was provided by the National Science Foundation (DMR-0520527) through a grant to the Materials Research Science and Engineering Center (MRSEC) at the University of Wisconsin – Madison, the Alfred P. Sloan Foundation, and the 3M Corporation. This research made use of facilities associated with the Wisconsin Center for Applied Microelectronics (WCAM) and the UW Soft Materials Laboratory, which are funded in part by the NSF through the UW MRSEC and the UW Nanoscale Research Center (NSEC). We thank Prof. Paul F. Nealey for providing the silicon masters used in this study, Prof. Christopher J. Murphy for many helpful discussions, and Sean Delcambre and Rebecca Bauer for assistance with nano-imprinting equipment. We thank Christopher M. Jewell, Eric M. Saurer, Ryan M. Flessner, Shane L. Bechler, and Selin Aytar for assistance with cell culture experiments. N. J. F. acknowledges the NSF for a Graduate Research Fellowship. M. E. B. was funded in part by an NIH Chemistry-Biology Interface Training Grant (NIGMS T32 GM008505). D. M. L. is a Research Fellow of the Alfred P. Sloan Foundation.
References
- 1.Curtis A, Wilkinson C. Biomaterials. 1997;18(24):1573–1583. doi: 10.1016/s0142-9612(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 2.Hubbell JA. Curr. Opin. Biotechnol. 1999;10(2):123–129. doi: 10.1016/s0958-1669(99)80021-4. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JM. Annu. Rev. Mater. Res. 2003;31:81–110. [Google Scholar]
- 4.Stevens MM, George JH. Science. 2005;310(5751):1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 5.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Nature. 1997;385(6616):537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 6.Hern DL, Hubbell JA. J. Biomed. Mater. Res. A. 1998;39(2):266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Biomaterials. 1999;20:2363–2376. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 8.Mrksich M. Chem. Soc. Rev. 2000;29(4):267–273. [Google Scholar]
- 9.Ostuni E, Chapman RG, Liang MN, Meluleni G, Pier G, Ingber DE, Whitesides GM. Langmuir. 2001;17(20):6336–6343. [Google Scholar]
- 10.Yousaf MN, Houseman BT, Mrksich M. Proc. Natl. Acad. Sci. USA. 2001;98(11):5992–5996. doi: 10.1073/pnas.101112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dertinger SKW, Jiang X, Li Z, Murthy VN, Whitesides GM. Proc. Natl. Acad. Sci. USA. 2002;99(20):12542–12547. doi: 10.1073/pnas.192457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy WL, Mooney DJ. J. Am. Chem. Soc. 2002;124(9):1910–1917. doi: 10.1021/ja012433n. [DOI] [PubMed] [Google Scholar]
- 13.Rowley JA, Mooney DJ. J. Biomed. Mater. Res. 2002;60(2):217–223. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 14.Segura T, Shea LD. Bioconj. Chem. 2002;13(3):621–629. doi: 10.1021/bc015575f. [DOI] [PubMed] [Google Scholar]
- 15.Shin H, Jo S, Mikos AG. Biomaterials. 2003;24(24):4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 16.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Annu. Rev. Biomed. Eng. 2003;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 17.Khademhosseini A, Suh KY, Yang JM, Eng G, Yeh J, Levenberg S, Langer R. Biomaterials. 2004;25(17):3583–3592. doi: 10.1016/j.biomaterials.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Science. 2004;303(5662):1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 19.Curtis A, Wilkinson C. J. Biomater. Sci. Polym. Ed. 1998;9:1313–1329. doi: 10.1163/156856298x00415. [DOI] [PubMed] [Google Scholar]
- 20.Curtis A, Wilkinson C. Trends Biotechnol. 2001;19(3):97–101. doi: 10.1016/s0167-7799(00)01536-5. [DOI] [PubMed] [Google Scholar]
- 21.Sniadecki NJ, Desai RA, Ruiz SA, Chen CS. Ann. Biomed. Eng. 2006;34:59–74. doi: 10.1007/s10439-005-9006-3. [DOI] [PubMed] [Google Scholar]
- 22.Lim JY, Donahue HJ. Tissue Eng. 2007;13:1879–1891. doi: 10.1089/ten.2006.0154. [DOI] [PubMed] [Google Scholar]
- 23.Craighead HG, James CD, Turner AMP. Curr. Opin. Solid State Mater. Sci. 2001;5(23):177–184. [Google Scholar]
- 24.Teixeira AI, Abrams GA, Murphy CJ, Nealey PF. J. Vac. Sci. Technol. B. 2003;21:683–687. [Google Scholar]
- 25.Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. J. Cell Sci. 2003;116:1881–1892. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teixeira AI, Nealey PF, Murphy CJ. J. Biomed. Mater. Res. A. 2004;71A:369–376. doi: 10.1002/jbm.a.30089. [DOI] [PubMed] [Google Scholar]
- 27.Karuri NW, Liliensiek S, Teixeira AI, Abrams GA, Campbell S, Nealey PF, Murphy CJ. J. Cell Sci. 2004;117:3153–3164. doi: 10.1242/jcs.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diehl KA, Foley JD, Nealey PF, Murphy CJ. J. Biomed. Mater. Res. A. 2005;75A:603–611. doi: 10.1002/jbm.a.30467. [DOI] [PubMed] [Google Scholar]
- 29.Foley JD, Grunwald EW, Nealey PF, Murphy CJ. Biomaterials. 2005;26:3639–3644. doi: 10.1016/j.biomaterials.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 30.Teixeira AI, McKie GA, Foley JD, Bertics PJ, Nealey PF, Murphy CJ. Biomaterials. 2006;27:3945–3954. doi: 10.1016/j.biomaterials.2006.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karuri NW, Porri TJ, Albrecht RM, Murphy CJ, Nealey PF. IEEE Trans. Nanobiosci. 2006;5:273–280. doi: 10.1109/tnb.2006.886570. [DOI] [PubMed] [Google Scholar]
- 32.Liliensiek SJ, Campbell S, Nealey PF, Murphy CJ. J. Biomed. Mater. Res. A. 2006;79A:185–192. doi: 10.1002/jbm.a.30744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Britland S, Morgan H, Wojiak-Stodart B, Riehle M, Curtis A, Wilkinson C. Exp. Cell Res. 1996;228:313–325. doi: 10.1006/excr.1996.0331. [DOI] [PubMed] [Google Scholar]
- 34.Charest JL, Eliason MT, García AJ, King WP, Talin AA, Simmons BA. J. Vac. Sci. Technol. B. 2005;23:3011–3014. [Google Scholar]
- 35.Charest JL, Eliason MT, García AJ, King WP. Biomaterials. 2006;27:2487–2494. doi: 10.1016/j.biomaterials.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Marrian CRK, Tennant DM. J. Vac. Sci. Technol. B. 2003;21(5):S207–S215. [Google Scholar]
- 37.Bratton D, Yang D, Dai J, Ober CK. Polym. Adv. Technol. 2006;17(2):94–103. [Google Scholar]
- 38.Cui Z. Micro-Nanofabrication: Technologies and Applications. Higher Education Press; Beijing: 2005. [Google Scholar]
- 39.Chou SY, Krauss PR, Renstrom PJ. J. Vac. Sci. Technol. B. 1996;14:4129–4133. [Google Scholar]
- 40.Zankovych S, Hoffmann T, Seekamp J, Bruch JU, Torres CMS. Nanotechnology. 2001;12:91–95. [Google Scholar]
- 41.Guo LJ. J. Phys. D: Appl. Phys. 2004;37:R123–R141. [Google Scholar]
- 42.Guo LJ. Adv. Mater. 2007;19:495–513. [Google Scholar]
- 43.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Chem. Rev. 2005;105(4):1103–1170. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 44.Flynn NT, Tran TNT, Cima MJ, Langer R. Langmuir. 2003;19(26):10909–10915. [Google Scholar]
- 45.Embrechts A, Feng CL, Mills CA, Lee M, Bredebusch I, Schnekenburger J, Domschke W, Vancso GJ, Schönherr H. Langmuir. 2008;24:8841–8849. doi: 10.1021/la800770y. [DOI] [PubMed] [Google Scholar]
- 46.Heilmann SM, Rasmussen JK, Krepski LR. J. Polym. Sci. Polym. Chem. 2001;39:3655–3677. [Google Scholar]
- 47.Guichard B, Nöel C, Reyx D, Thomas M, Chevalier S, Senet JP. Macromol. Chem. Phys. 1998;199:1657–1674. [Google Scholar]
- 48.Yang KL, Cadwell K, Abbott NL. J. Phys. Chem. B. 2004;108:20180–20186. [Google Scholar]
- 49.Zhang J, Fredin NJ, Lynn DM. J. Polym. Sci. Polym. Chem. 2006;44:5161–5173. [Google Scholar]
- 50.Frey BL, Corn RM, Weibel SC, Chalmers JM, Griffiths PR. Handbook of Vibrational Spectroscopy. Wiley; New York: 2002. p. 1042. [Google Scholar]
- 51.Pompe T, Zschoche S, Herold N, Salchert K, Gouzy MF, Sperling C, Werner C. Biomacromolecules. 2003;4(4):1072–1079. doi: 10.1021/bm034071c. [DOI] [PubMed] [Google Scholar]
- 52.Sperling C, Salchert K, Streller U, Werner C. Biomaterials. 2004;25(21):5101–5113. doi: 10.1016/j.biomaterials.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Feng CL, Vancso GJ, Schönherr H. Adv. Funct. Mater. 2006;16:1306–1312. [Google Scholar]
- 54.Feng CL, Embrechts A, Vancso GJ, Schönherr H. Eur. Phys. J. 2006;42:1954–1965. [Google Scholar]
- 55.Feng CL, Embrechts A, Bredebusch I, Schnekenburger J, Domschke W, Vancso GJ, Schönherr H. Adv. Mater. 2007;19:286–290. [Google Scholar]
- 56.Feng CL, Vancso GJ, Schönherr H. Langmuir. 2007;23:1131–1140. doi: 10.1021/la0615185. [DOI] [PubMed] [Google Scholar]
- 57.Alberti K, Davey RE, Onishi K, George S, Salchert K, Seib FP, Bornhauser M, Pompe T, Nagy A, Werner C, Zandstra PW. Nat. Methods. 2008;5(7):645–650. doi: 10.1038/nmeth.1222. [DOI] [PubMed] [Google Scholar]
- 58.Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science: An Introduction to Materials in Medicine. 2nd ed. Elsevier Academic Press; San Diego, CA: 2004. [Google Scholar]
- 59.Lichtenfels R, Biddison W, Schulz H, Vogt AB, Martin R. J. Immunol. Metho. 1994;172:227–239. doi: 10.1016/0022-1759(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 60.Bischof JC, Padanilam J, Holmes WH, Ezzell RM, Lee RC, Tompkins RG, Yarmush ML, Toner M. Biophys. J. 1995;68:2608–2614. doi: 10.1016/S0006-3495(95)80445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coleman PL, Walker MM, Milbrath DS, Stauffer DM, Rasmussen JK, Krepski LR, Heilmann SM. J. Chromatogr. 1990;512:345–363. [Google Scholar]
- 62.Xie S, Svec F, Fréchet JM. Biotechnol. Bioeng. 1999;62:30–35. doi: 10.1002/(sici)1097-0290(19990105)62:1<30::aid-bit4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 63.Cullen SP, Mandel IC, Gopalan P. Langmuir. 2008;24(23):13701–13709. doi: 10.1021/la8024952. [DOI] [PubMed] [Google Scholar]
- 64.Jeon SI, Lee JH, Andrade JD, de Gennes PG. J. Colloid Interface Sci. 1991;142:149–158. [Google Scholar]
- 65.Golander CG, Herron JN, Lim K, Claesson P, Stenius P, Andrade JD. In: Poly(Ethylene Glycol) Chemistry: Biotechnical and Biomedical Appliications. Harris JM, editor. Plenum Press; New York: 1992. pp. 221–246. [Google Scholar]
- 66.Sofia SJ, Premnath V, Merrill EW. Macromolecules. 1998;31(15):5059–5070. doi: 10.1021/ma971016l. [DOI] [PubMed] [Google Scholar]
- 67.Kenausis GL, Voros J, Elbert DL, Huang N, Hofer R, Ruiz-Taylor L, Textor M, Hubbell JA, Spencer ND. J. Phys. Chem. B. 2000;104(14):3298–3309. [Google Scholar]
- 68.Pale-Grosdemange C, Simon ES, Prime KL, Whitesides GM. J. Am. Chem. Soc. 1991;113(1):12–20. [Google Scholar]
- 69.Prime KL, Whitesides GM. Science. 1991;252(5009):1164–1167. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]