Abstract
Purpose
The importance of the BRCA gene products in maintaining genomic stability led us to hypothesize that BRCA-associated and sporadic ovarian cancers would have distinctive genetic profiles despite similarities in histologic appearance.
Experimental Design
A whole-genome copy number analysis of fresh, frozen, papillary serous ovarian cancer DNA was performed using the Affymetrix 50K Xba Mapping Array, using each patient’s normal genomic DNA as the matched control. Loss of heterozygosity and copy number abnormalities were summarized to define regions of amplification, deletion, or uniparental disomy, defined as loss of one allele and duplication of the remaining allele. Genomic abnormalities were compared between BRCA-associated and sporadic tumors.
Results
We compared six BRCA-associated with 14 sporadic papillary serous ovarian carcinomas. Genetic instability, measured by percentage of genome altered, was more pronounced in BRCA-associated tumors (median 86.6%, range 54–100%) than sporadic tumors (median 43.6%, range 2–83%), p=0.009. We used frequency plots to demonstrate the proportion of cases affected by each type abnormality at each genomic region. BRCA-associated tumors demonstrated genome-wide loss of heterozygosity, primarily due to the occurrence of uniparental disomy rather than deletion. Uniparental disomy was found in 100% of the BRCA-associated and 50% of the sporadic tumors profiled.
Conclusions
This study reports on a previously underappreciated genetic phenomenon of uniparental disomy, which occurs frequently in ovarian cancer DNA. We observed distinct genetic patterns between BRCA-associated versus sporadic ovarian cancers, suggesting these papillary serous tumors arise from different molecular pathways.
Keywords: Ovarian cancer, BRCA1, BRCA2, Whole genome analysis, Loss of heterozygosity, Uniparental disomy
INTRODUCTION
Solid tumor DNA undergoes a wide range a genetic changes, including translocations, inversions, duplications, amplifications, deletions, and alterations in chromosome number (1–3). Like other solid tumors, ovarian cancer is genetically characterized by aneuploidy (4), cytogenetic chromosomal abnormalities (5) and deregulation of multiple genetic pathways (6). Among all of the gynecologic malignancies, ovarian cancer has the highest fatality-to-case ratio and it remains the fifth leading cause of all cancer related mortality in women (7). This is due in large part to the advanced-stage, high-grade, papillary serous subtypes.
Approximately 10% of ovarian cancers are attributable to a hereditary predisposition from an inherited germline mutation in BRCA1 or BRCA2 (8). In the decade that has passed since these cancer susceptibilities genes were first identified, an increasing understanding of the molecular and cellular roles of the proteins they encode has emerged and their roles in maintaining genomic integrity and regulating cellular proliferation have been described (9–14). A female BRCA mutation carrier’s lifetime risk of developing ovarian cancer is approximately 16–44% with a BRCA1 mutation and to 16–27% with a BRCA2 mutation (15–17). The majority of ovarian cancers arising in this high-risk subpopulation of women are advanced-stage, high-grade, papillary serous carcinomas (18).
The important role of the BRCA proteins in maintaining genomic stability led us to hypothesize that the molecular pathways leading to ovarian carcinogenesis in BRCA-associated tumors would differ compared to those in sporadic carcinomas, even despite similarities in histologic appearance. A new array-based technology containing single nucleotide polymorphisms (SNPs) has emerged, which allows for a high-density, genome-wide analysis of allelic imbalance and loss of heterozygosity (LOH) from a single DNA sample (19–21). This SNP array technique has the advantage of profiling the genomic abnormalities occurring over the entire tumor genome and has been used to study LOH in a variety of tumor types (21–24) but has not yet been applied to study the complex karyotypes occurring in ovarian cancer. By combining LOH with copy number data, this new technology has also allowed for the identification of a previously underappreciated genetic abnormality in cancer cells called uniparental disomy (UPD), the presence of LOH in an area of normal copy number due to deletion of one allele and gain of the other allele (25, 26). The objective of our study was to apply the SNP-based array technology in order to compare the genetic alterations occurring in BRCA-associated versus sporadic papillary serous ovarian carcinomas.
MATERIALS & METHODS
Subjects
We identified 20 patients with papillary serous epithelial ovarian cancer who donated ovarian cancer tissue and blood at the time of primary cytoreductive surgery under an Institutional Review Board approved protocol at Cedars-Sinai Medical Center in Los Angeles, CA. Demographic, clinical, surgical, and histopathological data were collected by review of medical records, inpatient and outpatient charts, operative reports, and pathology reports.
DNA isolation
DNA was isolated from fresh, snap-frozen, ovarian cancer samples collected during primary cytoreductive surgery using the Qiagen DNeasy Tissue Protocol (QIAGEN Inc., Valencia, CA). Briefly, 25 mg of tumor tissue was sequentially treated with lysis buffer and Proteinase K overnight at 55°C, RNaseA (100 mg/ml), buffer, and 100% ethanol. Precipitated DNA was bound to DNeasy mini spin column, washed, and eluted. Genomic DNA was isolated from matched peripheral blood samples using a standard phenol chloroform extraction method.
BRCA Mutation Analysis
Nine patients underwent commercial germline BRCA mutation testing based on characteristics of the clinical and/or family history that suggested an elevated risk for a hereditary ovarian cancer predisposition. For the remaining 11 cases, BRCA1 and BRCA2 mutation status were determined by laboratory evaluation of DNA isolated from peripheral blood. BRCA1 exon 11 and BRCA2 exons 10 and 11 were screened for mutations by protein truncation testing (PTT), which screens for truncating mutations of the two genes. This covers approximately 58% of the coding region of BRCA1 and 50% of the coding region of BRCA2. The three common Ashkenazi Jewish founder mutations, (BRCA1 exon2 185delAG, BRCA1 exon20 5382insC, and BRCA2 exon11 6174delT), were tested by gel electrophoresis. The BRCA1 exon13 ins6kb mutation was also tested by gel electrophoresis. As part of the protocol, all mutations detected by screening protocols are confirmed by DNA sequencing. Laboratory mutation analysis was performed at the Center for Research in Women’s Health at the Sunnybrook Women’s College Health Science Center, University of Toronto, Canada under the direction of S.A.N.
Mapping 50K Array Hybridization
For each case, tumor DNA and matched normal DNA were each hybridized to a Mapping 50K Xba array (Affymetrix, Santa Clara, CA). Briefly, 250 ng of genomic DNA was digested with XbaI restriction enzyme and ligated to common adaptors, allowing one primer PCR amplification of the entire genome. PCR products were fragmented, end-labeled, and hybridized to the array.
Data Analysis
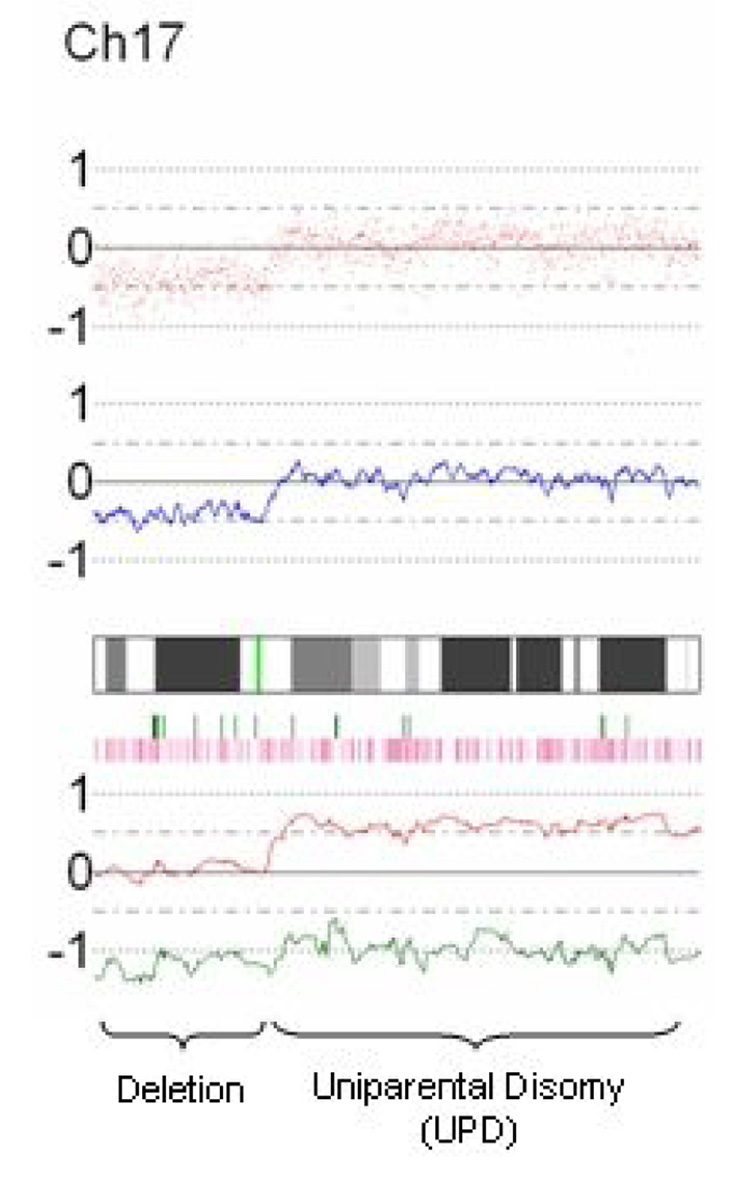
Data was processed and visually displayed using the Copy Number Analyzer for GeneChip (CNAG, University of Tokyo, Japan, http://www.genome.umin.ac.jp) software program (27). Figure 1 demonstrates an example of the CNAG visual output for chromosome 17 of a representative case. LOH and allele-specific plot data are available because matched peripheral blood DNA was used as the reference for each tumor DNA sample. The sum of these plots were summarized and genomic regions were classified as normal, amplified, deleted, or as an area of normal copy number LOH, i.e. uniparental disomy (UPD). UPD with amplification (copy number >2) was found in areas of LOH with deletion of one allele and greater than two copies of the remaining allele. Each genomic abnormality was measured, classified, and schematically represented at its location along the genome. All graphs and statistical analyses were performed with STATA v8 (STATA Corp., College Station, TX).
Fig. 1.
Representative CNAG output. Elements from top to bottom include SNP plot (each red dot represents a SNP, baseline n=2), copy-number plot (blue line represents average copy number over consecutive SNPs, baseline n=2), chromosomal cytoband, heterozygosity calls (each green vertical bar represents a heterozygous, i.e. A/B, SNP call), loss of heterozygosity calls (each pink vertical bar represents LOH in tumor DNA compared to normal, i.e. A/A or B/B in tumor DNA; A/B in normal DNA), and allele-specific plot (red and green lines each represent specific allele copy number, baseline n=1). This example on chromosome 17 demonstrates deletion of the p-arm (copy number <2, one allele deleted, LOH) and UPD of the q-arm (copy number 2, one allele deleted, one allele duplicated, LOH).
A variable was created to represent the percentage of the genome altered in each tumor. The length of each abnormality was summed and the total was divided by 2917.4 MB, the length of genome coverage on the 50K Array. Frequency plots were generated to display the frequency of each type of genomic abnormality along each chromosome. The genomic abnormalities occurring in ovarian cancer DNA were compared between sporadic and BRCA-associated groups.
RESULTS
Patient characteristics
Among 20 patients with papillary serous epithelial ovarian cancer, 18 patients (90%) were Caucasian, 9 (45%) were Jewish, and median age at diagnosis was 62 years (range, 45–72). All patients underwent surgical cytoreduction; 19/20 patients were optimally cytoreduced to less than 1 mm residual disease. Stage distribution included 2 stage IIC, 3 stage IIIB, 11 stage IIIC, and 4 stage IV ovarian cancers. Following surgery, all patients received systemic adjuvant chemotherapy with a platinum-based regimen (18 Carboplatin/Paclitaxel, 2 Cisplatin/Cytoxan). All 20 patients recurred with a median PFS of 14 months (range, 0.1–39.2 months). Median overall survival (OS) was 42.9 months (range, 11.5–90.5 months).
Nine patients with suspicious clinical or family histories underwent commercial BRCA mutation testing and six tested positive. None of the eleven remaining cases tested in the laboratory were found to have an additional germline mutation. Among the six patients, there were three with BRCA1 mutations, two with BRCA2 mutations, and one patient with a mutation in both BRCA genes. The spectrum of BRCA mutations are detailed in Table 1.
Spectrum of BRCA mutations.
| Case | BRCA1 mutation | BRCA2 mutation |
|---|---|---|
| 1 | 185delAG | |
| 2 | 185delAG | |
| 3 | 185delAG | |
| 4 | 2699delT | |
| 5 | 6174delT | |
| 6 | L598X | 8765delAG |
Genetic instability of ovarian cancer DNA
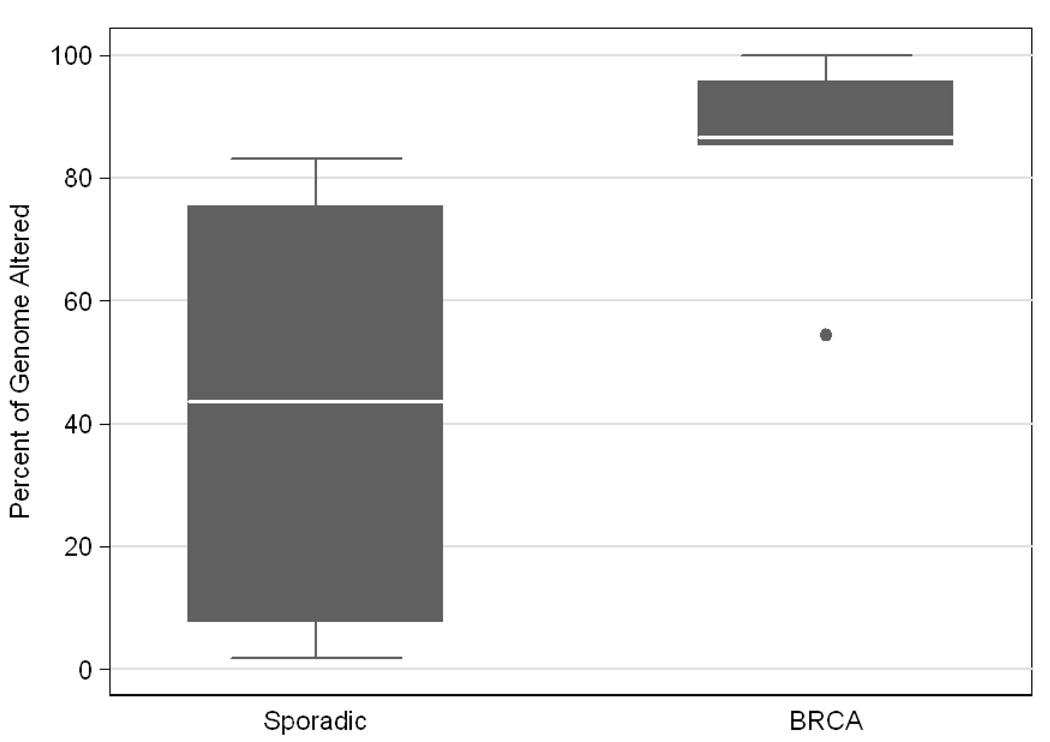
Ovarian cancer DNA was found to have a great degree of genetic instability as well as heterogeneity between cases. The degree of genetic instability was measured as a percentage of the genome altered, inclusive of all areas with copy number changes and/or LOH. Among the 20 cases, the percent of genome altered varied from 4–100%, with a median of 67.4%. Patients with germline BRCA mutations had tumors with more genetic alterations (median 86.6%, range 54–100%) compared to BRCA negative patients (median 43.6%, range 2–83%), p=0.009 (Figure 2).
Fig. 2.
Box and whisker plot demonstrates significantly higher degree of genetic instability among BRCA-associated cases compared to sporadic cases, p=0.009. Median is represented by the white line, 25th and 75th percentiles (interquartile range, IQR) by the grey box, data range by the whiskers, and outliers (defined as more than 1.5IQR beyond the first or third quartile) are plotted as individual data points.
Patterns of genomic abnormalities differ among BRCA-associated tumors
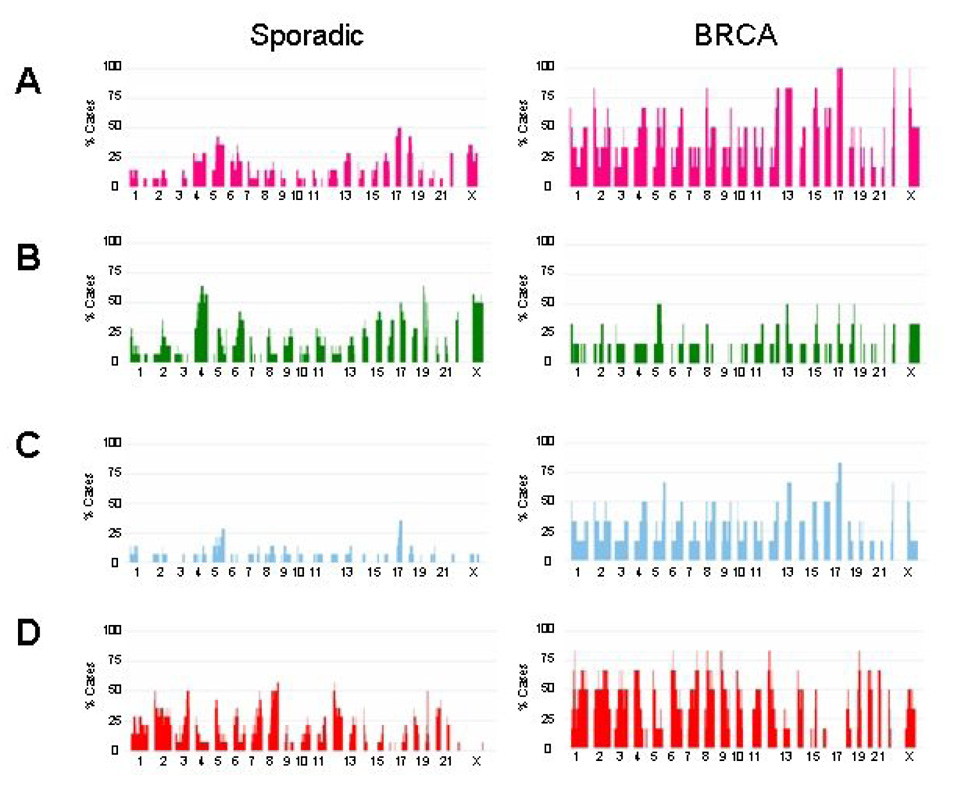
We created a frequency plot summarizing the specific types of genetic changes occurring in sporadic versus BRCA-associated tumors (Figure 3). LOH (demonstrated in Fig 3A, pink bars) occurs in genomic regions that have undergone hemizygous deletion (Fig 3B, green bars) or uniparential disomy (Fig 3C, blue bars).
Fig. 3.
Frequency plot summarizing the specific types of genetic changes occurring in sporadic versus BRCA-associated tumors: LOH (A, pink bars), deletions (B, green bars), uiparental disomy (C, blue bars), amplifications (D, red bars). In each panel, the percentage of cases affected by a specific type of genomic abnormality at each region is represented as a vertical bar along the y-axis. The genomic location is represented along the x-axis with chromosome 1 to the left and the X chromosome on the right.
Sporadic tumors undergo a lower level of LOH compared to the BRCA tumors and the areas undergoing LOH are largely represented by hemizygous deletions. In contrast, the BRCA-associated tumors have a dramatic degree of LOH affecting the majority of the genome. With the exception of chromosomes 7 and 20, every other chromosome is affected by LOH in at least 50% of the cases. In the BRCA cases, LOH occurs 100% of the time on regions of chromosomes 17, 22 and the X-chromosome. This increased level of LOH is largely due to UPD rather than deletion. Among the sporadic tumors, UPD occurs at a lower frequency along the entire genome, with the exception of a higher frequency of UPD occurring on chromosome 17.
Amplifications (Fig 3D, red bars) occur at greater frequency among the BRCA-associated tumors and are relatively evenly distributed across the genome. Exceptions occur at chromosomes 13, 16, and 17, where very rare amplifications occur in the BRCA- associated tumors.
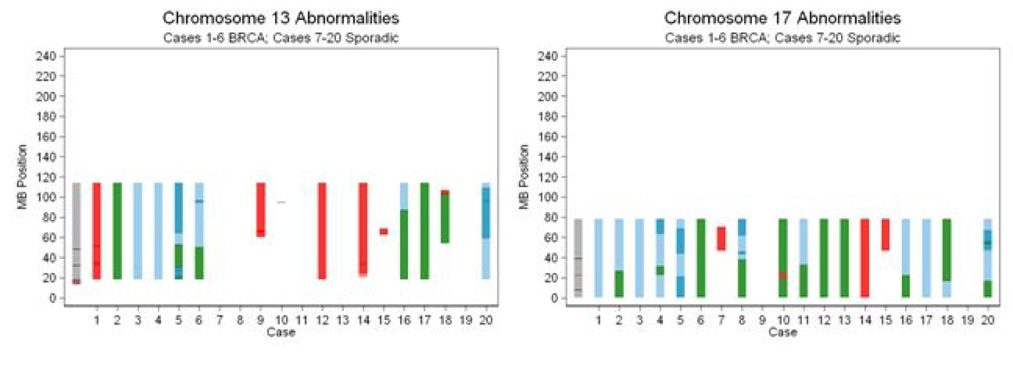
Genetic alterations in higher resolution
We mapped the specific types of abnormalities occurring on Chromosomes 13 and 17 (Figure 4). Cases 1–6 correspond to the tumors from BRCA mutation carriers (case number corresponds to BRCA mutations described in Table 1) while cases 7–20 represent the sporadic tumors. Among the six BRCA-associated cases, the entirety of chromosomes 13 and 17 are affected, almost exclusively by UPD or deletion. We also determined the genetic alterations occurring at the loci containing important tumor suppressor genes. On chromosome 13, BRCA2 (located at 31.7 MB) and RB1 (located at 47.7 MB) are affected by deletion/UPD in 5/6 (83%) BRCA-associated cases compared to 3/14 (21%) sporadic cases, p=0.01 by Fisher Exact test. On chromosome 17, TP53 (located at 7.5 MB) and BRCA1 (located at 38.5 MB) are affected by deletion/UPD in 6/6 (100%) of the BRCA-associated cases compared to 9/14 (64%) of the sporadic cases, p=0.1.
Fig. 4.
Genomic abnormalities on chromosomes 13 and 17 are demonstrated at higher resolution. Cases 1–6 correspond to the tumors from BRCA mutation carriers (see Table 1 for mutation details) while cases 7–20 represent the sporadic tumors. The types of abnormalities characterized include amplification (red), hemizygous deletion (green), UPD with diploid copy number (light blue), and UPD with copy number > 2 (dark blue). The locations of important tumor suppressor genes are shown with black hatch marks on the cytoband on the left edge of the graphs: BRCA2 (chromosome 13 at 31.7 MB), RB1 (chromosome 13 at 47.7 MB), TP53 (chromosome 17 at 7.5 MB) and BRCA1 (chromosome 17 at 38.5 MB).
Analysis of the genomic events occurring at the BRCA1 and BRCA2 loci among BRCA1/2 mutation carriers demonstrates UPD to be a frequent cause of LOH (Fig. 4). Among the four patients with germline BRCA1 mutations (cases 1, 2, 3, 6), LOH at the BRCA1 locus (chromosome 17, 38.5 MB) occurs by UPD in 3 cases and by deletion in 1 case. Among the three patients with germline BRCA2 mutations (cases 4, 5, 6), LOH at the BRCA2 locus (chromosome 13, 31.7 MB), occur by UPD in one case and by deletion in 2 cases.
Details of the genetic alterations occurring on all 22 autosomal chromosomes and the X chromosome can be found in the supplemental materials. Visual comparison of the six BRCA-associated tumors compared to the 14 sporadic tumors further demonstrates the higher degree of genetic instability in the BRCA-associated cases. Additionally, color changes represent copy number transitions, demonstrating multiple areas of chromosomal breakage, but no areas representing recurrent breakpoints in either group.
Patterns of Uniparental Disomy
Using the SNP-based array platform, we found uniparental disomy in 13 of the 20 (65%) ovarian cancer genomes profiled. UPD occurred in all 6 BRCA-associated tumors (100%) and in 7 of the 14 sporadic tumors (50%). The BRCA-associated tumors had a significantly higher percentage of the genome affected by UPD (range 7.8 to 99.9%, median 19%, mean 30%) than the sporadic tumors (range 0 to 23.2%, median 0.7%, mean 6%), p=0.009.
We found several different types of UPD events: (1) UPD extending to the telomere, (2) UPD involving the entire chromosome and (3) UPD buried within the chromosome. Additionally, there were UPD events with diploid copy number and those with greater than diploid copy number. We found frequent areas with adjacent UPD blocks with varying copy numbers. Each change in copy number was considered a discrete UPD locus as each copy number change implied a unique chromosomal breakage event. Among the 6 BRCA-associated tumors, there were 118 discrete UPD loci; 49 (41%) extended to the telomere, 22 (19%) involved the entire chromosome, and 47 (40%) were buried within the chromosome. Among the UPD events within the chromosome, 23 (20%) were within a run of adjacent UPD events that extended to the telomere and 24 (20%) were not. We found a total of 77 discrete UPD loci in the 14 sporadic tumors. 34 (44%) extended to the telomere, 3 (4%) involved the entire chromosome, and 40 (52%) were buried within the chromosome. Of those buried within the chromosome, 10 (13%) were within a run of UPD extending to the telomere and 30 (39%) were not.
DISCUSSION
There are substantial differences in the frequency and types of genetic abnormalities occurring in BRCA-associated versus sporadic ovarian cancer. Compared to sporadic ovarian cancers, BRCA-associated tumors are characterized by increased genomic instability. The types of genomic alterations also differ between tumor types. BRCA-associated ovarian cancers demonstrate more frequent amplifications, as well as, LOH due to UPD, but do not appear to have a greater frequency of allelic loss due to deletions.
These findings are compatible with the known function of the BRCA proteins in maintaining genomic integrity. The BRCA1 and BRCA2 genes encode large nuclear proteins that are widely expressed during the S and G2 phases of the cell cycle (12). BRCA1 has been implicated in a wide number of cellular processes, including chromatin remodeling, transcriptional regulation, and DNA repair (9, 13, 28). BRCA2 has been found to have a more limited role in DNA recombination and repair, mainly acting as a regulator of RAD51 activity (12, 28, 29).
Deficiency in the BRCA1 or BRCA2 proteins results in the loss of homologous recombination, a conservative DNA repair process that repairs double strand DNA breaks during the S/G2 phases of the mitotic cell cycle by means of recombination between sister chromatids (12, 28). Loss of homologous recombination results in the inappropriate repair of double strand DNA breaks using non-conservative and error-prone mechanisms, such as non-homologous end joining and single-strand annealing, which produce genomic instability through an increase in deletions and/or translocations (12, 28, 30, 31). Spontaneous chromosomal instability has been observed in murine cells deficient in BRCA1 (13) or BRCA2 (32, 33), as well as, in clinical samples from human cancer cells (12, 13, 32–35), supporting the vulnerability of these cells to undergo neoplastic transformation. The gross chromosomal rearrangements occurring in BRCA deficient cells, such as translocations, deletions, and abnormal fusions, have been attributed to a defect in mitotic recombination (12). Interestingly, in this study, we have found UPD to be one of the hallmark features of BRCA-associated ovarian carcinogenesis.
UPD is a genetic alteration resulting from deletion of one allele and duplication of the other allele, resulting in LOH in an area of normal copy number. The magnitude of this genetic change has been underappreciated in the past as technologies such as array comparative genomic hybridization have been unable to detect signals in areas of normal copy number. Use of a SNP-based array platform, which combines LOH with copy number data, has allowed us to identify UPD as a novel finding within the ovarian cancer genome, most significantly in those tumors arising on the background of a germline BRCA mutation.
Since the advent of the SNP array technology, several other groups have demonstrated UPD to be a frequent somatic event occurring in various cancer types (25, 26, 36, 37). The distribution of UPD was found to frequently extend to the telomere, implying a mechanism of somatic recombination as the cause of normal copy-number homozygosity (26, 38). We have found a similar high occurrence of UPD extending to the telomere, occurring in 41% of the UPD events in the tumors of BRCA mutation carriers and in 44% of the UPD events in sporadic tumors. However, we also found a high frequency of UPD events buried within chromosomes, as well as tandemly arranged with other UPD events with various copy number changes. The complexity of the UPD patterns seen in ovarian carcinoma signals the possibility of other mechanisms underlying these events, such as structural alterations resulting from interchromosomal recombinations due to defects in the double-strand repair-recombination machinery (39, 40).
Genomic regions that undergo UPD are likely to contain important genes that drive the carcinogenic process. One of the two daughter cells may acquire a growth advantage due to differences in alleles between the involved gene copies, resulting in a tumor cell population dominated by the descendents of the selected clone (41). Indeed, this has been demonstrated to be the case in several hematologic processes. In myeloproliferative disorders, the acquired activating mutation of the JAK2 tyrosine kinase is homozygous in a subset of patients due to somatic recombination (42) and in acute myeloid leukemia, homozygous mutations of the WT1, FLT3, CEBPA, and RUNX1 genes have been found, implying that mutation precedes mitotic recombination, which acts as the “second-hit” that removes the normal, wild-type allele (43).
This study demonstrates the complexity of the genomic abnormalities occurring during the ovarian cancer carcinogenic process. Distinct differences exist in the molecular pathways leading to BRCA-associated versus sporadic ovarian cancers. In tumors arising from a background of BRCA-deficiency, the loss of the homologous recombination DNA repair process results in genomic instability. Use of the 50K SNP array to profile the genetic changes occurring in ovarian cancer DNA demonstrates frequent UPD in BRCA-associated tumors, supporting a link between loss of BRCA function and the occurrence of somatic recombination events. Further work in a larger dataset may penetrate the background noise generated by genomic instability to reveal the non-random genetic events occurring in BRCA-associated ovarian cancers and lead to the identification of critical genes in the carcinogenic process.
Supplementary Material
Details of the genetic alterations occurring on all 22 autosomal chromosomes and the X chromosome in 20 papillary serous ovarian cancer cases. Cases 1–6 correspond to the tumors from BRCA mutation carriers (case number corresponds to BRCA mutations described in Table 1) while cases 7–20 represent the sporadic tumors. The types of abnormalities characterized include amplification (red), hemizygous deletion (green), homozygous deletion (black), UPD with diploid copy number (light blue), and UPD with copy number > 2 (dark blue).
Acknowledgments
Financial Support: This work was supported by grants from the L & S Milken Foundation (CSW, BYK), the Parker Hughes Fund (HPK), and the American Cancer Society California Division-Early Detection Professorship (BYK)
Footnotes
Statement of Clinical Relevance:
Ovarian cancer continues to present significant clinical challenges. Despite initial success achieved with surgical debulking and platinum-based chemotherapy, the majority of epithelial ovarian cancers will recur and lead to death due to chemoresistant disease. There is a critical need to expand our treatment armamentarium against this highly fatal gynecologic malignancy. Towards this end, an understanding of the genetic events driving the growth of these tumors will ultimately lead to the development of more effective targeted therapies. The completion of the draft human genome has led to technologies that are accelerating the pace of these discoveries. In this study, we use a single nucleotide polymorphism-based mapping array to study the genetic events occurring in papillary serous ovarian cancers. Despite histologic similarities, we have found distinct genetic profiles that distinguish BRCA1/2-associated and sporadic ovarian carcinomas. The “BRCA-signature” is defined by uniparental disomy, a genetic event which was previously under-recognized due to the difficulty of its identification with older genotyping technologies. BRCA-associated and sporadic ovarian cancers appear to arise out of distinct molecular pathways. These findings have important clinical implications for future studies defining the molecular oncogenesis of ovarian cancer and targeted treatment approaches.
REFERENCES
- 1.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 2.Albertson DG, Collins C, McCormick F, Gray JW. Chromosome aberrations in solid tumors. Nat Genet. 2003;34:369–376. doi: 10.1038/ng1215. [DOI] [PubMed] [Google Scholar]
- 3.Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 4.Hoglund M, Gisselsson D, Hansen GB, Sall T, Mitelman F. Ovarian carcinoma develops through multiple modes of chromosomal evolution. Cancer Res. 2003;63:3378–3385. [PubMed] [Google Scholar]
- 5.Taetle R, Aickin M, Yang JM, et al. Chromosome abnormalities in ovarian adenocarcinoma: I. Nonrandom chromosome abnormalities from 244 cases. Genes Chromosomes Cancer. 1999;25:290–300. [PubMed] [Google Scholar]
- 6.Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 8.Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartman AR, Ford JM. BRCA1 induces DNA damage recognition factors and enhances nucleotide excision repair. Nat Genet. 2002;32:180–184. doi: 10.1038/ng953. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy RD, D'Andrea AD. DNA repair pathways in clinical practice: lessons from pediatric cancer susceptibility syndromes. J Clin Oncol. 2006;24:3799–3808. doi: 10.1200/JCO.2005.05.4171. [DOI] [PubMed] [Google Scholar]
- 11.Welcsh PL, King MC. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum Mol Genet. 2001;10:705–713. doi: 10.1093/hmg/10.7.705. [DOI] [PubMed] [Google Scholar]
- 12.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Weaver Z, Linke SP, et al. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoformdeficient cells. Mol Cell. 1999;3:389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Jeffrey PD, Miller J, et al. BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science. 2002;297:1837–1848. doi: 10.1126/science.297.5588.1837. [DOI] [PubMed] [Google Scholar]
- 15.Abeliovich D, Kaduri L, Lerer I, et al. The founder mutations 185delAG and 5382insC in BRCA1 and 6174delT in BRCA2 appear in 60% of ovarian cancer and 30% of early-onset breast cancer patients among Ashkenazi women. Am J Hum Genet. 1997;60:505–514. [PMC free article] [PubMed] [Google Scholar]
- 16.Levy-Lahad E, Catane R, Eisenberg S, et al. Founder BRCA1 and BRCA2 mutations in Ashkenazi Jews in Israel: frequency and differential penetrance in ovarian cancer and in breast-ovarian cancer families. Am J Hum Genet. 1997;60:1059–1067. [PMC free article] [PubMed] [Google Scholar]
- 17.Moslehi R, Chu W, Karlan B, et al. BRCA1 and BRCA2 mutation analysis of 208 Ashkenazi Jewish women with ovarian cancer. Am J Hum Genet. 2000;66:1259–1272. doi: 10.1086/302853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogg R, Friedlander M. Biology of epithelial ovarian cancer: implications for screening women at high genetic risk. J Clin Oncol. 2004;22:1315–1327. doi: 10.1200/JCO.2004.07.179. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy GC, Matsuzaki H, Dong S, et al. Large-scale genotyping of complex DNA. Nat Biotechnol. 2003;21:1233–1237. doi: 10.1038/nbt869. [DOI] [PubMed] [Google Scholar]
- 20.Lindblad-Toh K, Tanenbaum DM, Daly MJ, et al. Loss-of-heterozygosity analysis of small-cell lung carcinomas using single-nucleotide polymorphism arrays. Nat Biotechnol. 2000;18:1001–1005. doi: 10.1038/79269. [DOI] [PubMed] [Google Scholar]
- 21.Wong KK, Tsang YT, Shen J, et al. Allelic imbalance analysis by high-density single-nucleotide polymorphic allele (SNP) array with whole genome amplified DNA. Nucleic Acids Res. 2004;32:e69. doi: 10.1093/nar/gnh072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoque MO, Lee CC, Cairns P, Schoenberg M, Sidransky D. Genome-wide genetic characterization of bladder cancer: a comparison of high-density single-nucleotide polymorphism arrays and PCR-based microsatellite analysis. Cancer Res. 2003;63:2216–2222. [PubMed] [Google Scholar]
- 23.Zhao X, Li C, Paez JG, et al. An integrated view of copy number and allelic alterations in the cancer genome using single nucleotide polymorphism arrays. Cancer Res. 2004;64:3060–3071. doi: 10.1158/0008-5472.can-03-3308. [DOI] [PubMed] [Google Scholar]
- 24.Wang ZC, Lin M, Wei LJ, et al. Loss of heterozygosity and its correlation with expression profiles in subclasses of invasive breast cancers. Cancer Res. 2004;64:64–71. doi: 10.1158/0008-5472.can-03-2570. [DOI] [PubMed] [Google Scholar]
- 25.Teh MT, Blaydon D, Chaplin T, et al. Genomewide single nucleotide polymorphism microarray mapping in basal cell carcinomas unveils uniparental disomy as a key somatic event. Cancer Res. 2005;65:8597–8603. doi: 10.1158/0008-5472.CAN-05-0842. [DOI] [PubMed] [Google Scholar]
- 26.Raghavan M, Lillington DM, Skoulakis S, et al. Genome-wide single nucleotide polymorphism analysis reveals frequent partial uniparental disomy due to somatic recombination in acute myeloid leukemias. Cancer Res. 2005;65:375–378. [PubMed] [Google Scholar]
- 27.Nannya Y, Sanada M, Nakazaki K, et al. A robust algorithm for copy number detection using high-density oligonucleotide single nucleotide polymorphism genotyping arrays. Cancer Res. 2005;65:6071–6079. doi: 10.1158/0008-5472.CAN-05-0465. [DOI] [PubMed] [Google Scholar]
- 28.Turner N, Tutt A, Ashworth A. Hallmarks of 'BRCAness' in sporadic cancers. Nat Rev Cancer. 2004;4:814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 29.Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med. 2002;8:571–576. doi: 10.1016/s1471-4914(02)02434-6. [DOI] [PubMed] [Google Scholar]
- 30.Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 31.Tutt A, Bertwistle D, Valentine J, et al. Mutation in Brca2 stimulates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequences. Embo J. 2001;20:4704–4716. doi: 10.1093/emboj/20.17.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel KJ, Yu VP, Lee H, et al. Involvement of Brca2 in DNA repair. Mol Cell. 1998;1:347–357. doi: 10.1016/s1097-2765(00)80035-0. [DOI] [PubMed] [Google Scholar]
- 33.Yu VP, Koehler M, Steinlein C, et al. Gross chromosomal rearrangements and genetic exchange between nonhomologous chromosomes following BRCA2 inactivation. Genes Dev. 2000;14:1400–1406. [PMC free article] [PubMed] [Google Scholar]
- 34.Tirkkonen M, Johannsson O, Agnarsson BA, et al. Distinct somatic genetic changes associated with tumor progression in carriers of BRCA1 and BRCA2 germ-line mutations. Cancer Res. 1997;57:1222–1227. [PubMed] [Google Scholar]
- 35.Gretarsdottir S, Thorlacius S, Valgardsdottir R, et al. BRCA2 and p53 mutations in primary breast cancer in relation to genetic instability. Cancer Res. 1998;58:859–862. [PubMed] [Google Scholar]
- 36.Andersen CL, Wiuf C, Kruhoffer M, Korsgaard M, Laurberg S, Orntoft TF. Frequent occurrence of uniparental disomy in colorectal cancer. Carcinogenesis. 2007;28:38–48. doi: 10.1093/carcin/bgl086. [DOI] [PubMed] [Google Scholar]
- 37.Kawamata N, Ogawa S, Zimmermann M, et al. Molecular allelokaryotyping of pediatric acute lymphoblastic leukemias by high-resolution single nucleotide polymorphism oligonucleotide genomic microarray. Blood. 2008;111:776–784. doi: 10.1182/blood-2007-05-088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bignell GR, Huang J, Greshock J, et al. High-resolution analysis of DNA copy number using oligonucleotide microarrays. Genome Res. 2004;14:287–295. doi: 10.1101/gr.2012304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiagalingam S, Laken S, Willson JK, et al. Mechanisms underlying losses of heterozygosity in human colorectal cancers. Proc Natl Acad Sci U S A. 2001;98:2698–2702. doi: 10.1073/pnas.051625398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson DO, Sekiguchi JM, Chang S, et al. The nonhomologous end-joining pathway of DNA repair is required for genomic stability and the suppression of translocations. Proc Natl Acad Sci U S A. 2000;97:6630–6633. doi: 10.1073/pnas.110152897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson WP. Mechanisms leading to uniparental disomy and their clinical consequences. Bioessays. 2000;22:452–459. doi: 10.1002/(SICI)1521-1878(200005)22:5<452::AID-BIES7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 42.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgibbon J, Smith LL, Raghavan M, et al. Association between acquired uniparental disomy and homozygous gene mutation in acute myeloid leukemias. Cancer Res. 2005;65:9152–9154. doi: 10.1158/0008-5472.CAN-05-2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of the genetic alterations occurring on all 22 autosomal chromosomes and the X chromosome in 20 papillary serous ovarian cancer cases. Cases 1–6 correspond to the tumors from BRCA mutation carriers (case number corresponds to BRCA mutations described in Table 1) while cases 7–20 represent the sporadic tumors. The types of abnormalities characterized include amplification (red), hemizygous deletion (green), homozygous deletion (black), UPD with diploid copy number (light blue), and UPD with copy number > 2 (dark blue).