Abstract
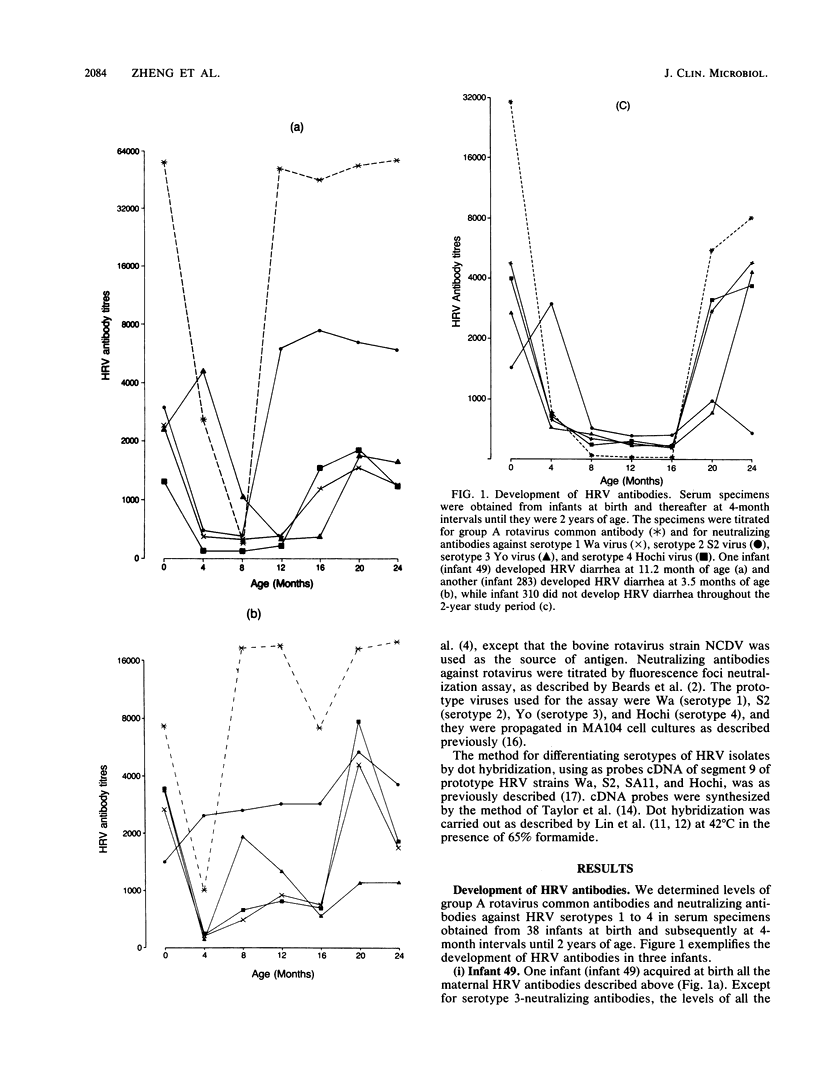
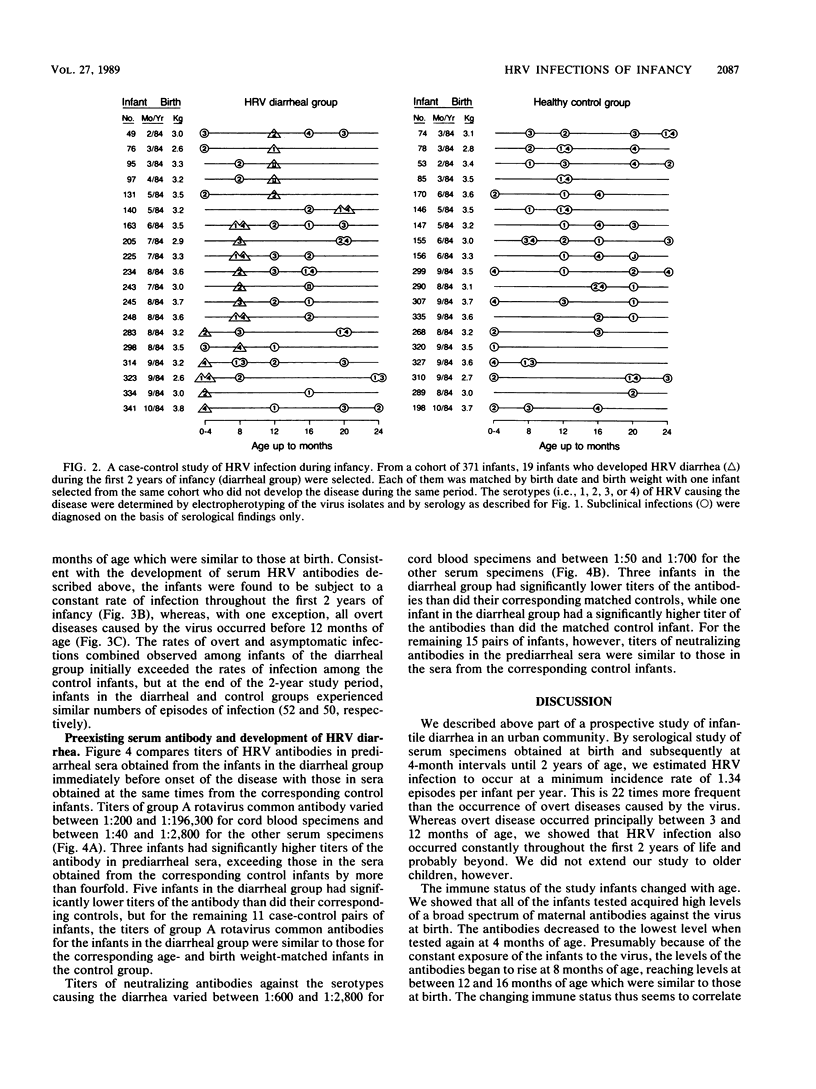
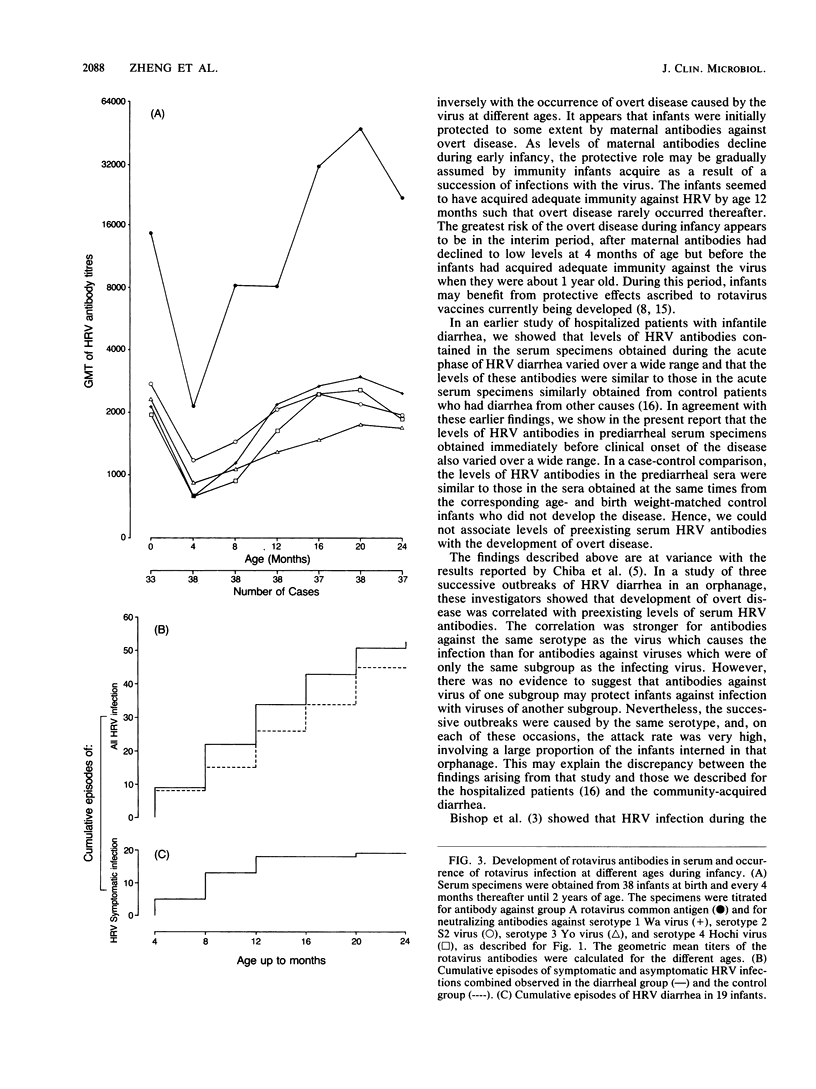
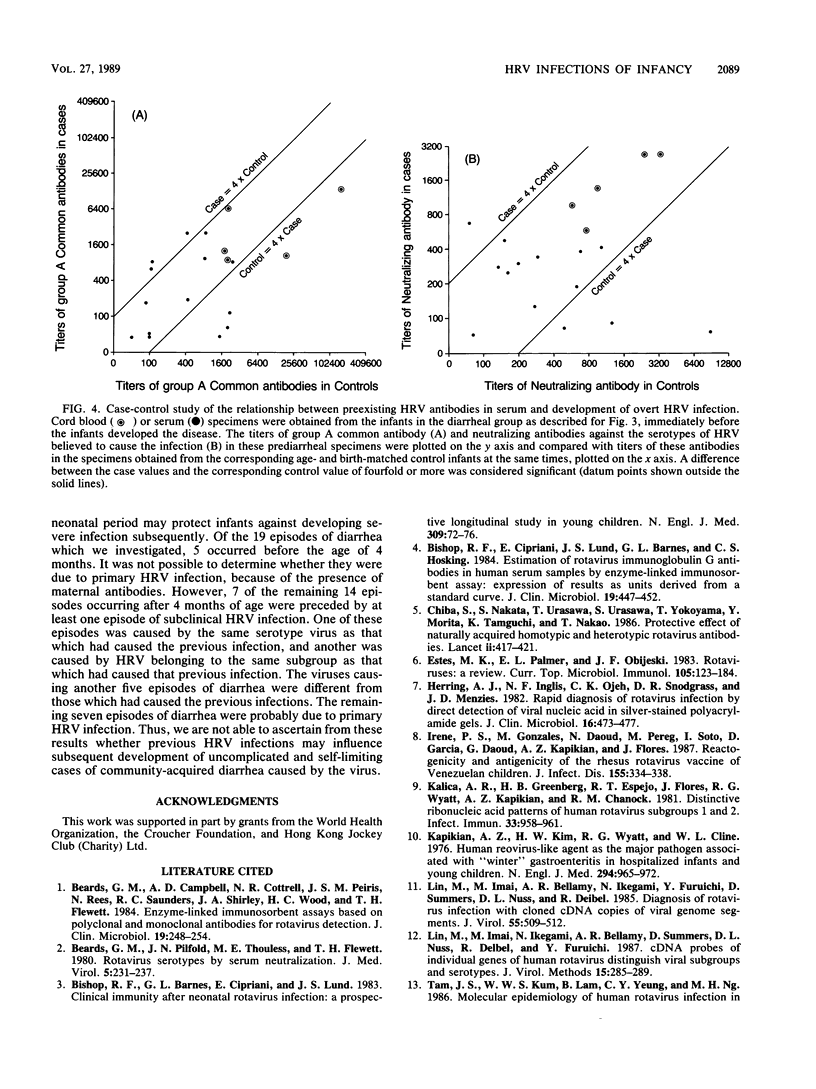
We determined titers of group A rotavirus common antibodies and neutralizing antibodies against serotypes 1 to 4 of prototype human rotavirus (HRV) in cord blood and serum specimens obtained from 38 infants at 4-month intervals from birth until 2 years of age. Nineteen of the infants developed one episode of HRV diarrhea each, and they were matched by age and birth weight with the other 19 infants, who did not develop HRV diarrhea during the follow-up period. We estimated the incidence rate of HRV infection for the two groups of infants combined to be a minimum of 1.34 episodes per infant per year, which is 22 times more common than the occurrence of overt disease caused by the virus in this community. The infection occurred constantly throughout the first 2 years of infancy, whereas all but one of the 19 episodes of overt disease occurred before 12 months of age. Seven of these overt episodes were preceded by at least one episode of subclinical infection earlier, and the other seven were probably due to primary HRV infection. The remaining five episodes occurred before 4 months of age, so that we could not ascertain whether they were due to primary infections because of the presence of maternal antibodies. We showed that levels of HRV antibodies in serum specimens obtained before clinical onset of diarrhea varied widely, and, for most infants in the diarrheal group, levels of these antibodies were similar to those in the serum specimens obtained at the same times from the corresponding age- and birth weight-matched control infants. Nevertheless, the age at which overt disease caused by HRV was most prevalent coincided with the time when the maternal antibodies had declined to low levels but the infants had not yet acquired high titers of these antibodies in their sera.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beards G. M., Campbell A. D., Cottrell N. R., Peiris J. S., Rees N., Sanders R. C., Shirley J. A., Wood H. C., Flewett T. H. Enzyme-linked immunosorbent assays based on polyclonal and monoclonal antibodies for rotavirus detection. J Clin Microbiol. 1984 Feb;19(2):248–254. doi: 10.1128/jcm.19.2.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards G. M., Pilfold J. N., Thouless M. E., Flewett T. H. Rotavirus serotypes by serum neutralisation. J Med Virol. 1980;5(3):231–237. doi: 10.1002/jmv.1890050307. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Barnes G. L., Cipriani E., Lund J. S. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983 Jul 14;309(2):72–76. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Cipriani E., Lund J. S., Barnes G. L., Hosking C. S. Estimation of rotavirus immunoglobulin G antibodies in human serum samples by enzyme-linked immunosorbent assay: expression of results as units derived from a standard curve. J Clin Microbiol. 1984 Apr;19(4):447–452. doi: 10.1128/jcm.19.4.447-452.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Yokoyama T., Nakata S., Morita Y., Urasawa T., Taniguchi K., Urasawa S., Nakao T. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet. 1986 Aug 23;2(8504):417–421. doi: 10.1016/s0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Espejo R. T., Flores J., Wyatt R. G., Kapikian A. Z., Chanock R. M. Distinctive ribonucleic acid patterns of human rotavirus subgroups 1 and 2. Infect Immun. 1981 Sep;33(3):958–961. doi: 10.1128/iai.33.3.958-961.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Kim H. W., Wyatt R. G., Cline W. L., Arrobio J. O., Brandt C. D., Rodriguez W. J., Sack D. A., Chanock R. M., Parrott R. H. Human reovirus-like agent as the major pathogen associated with "winter" gastroenteritis in hospitalized infants and young children. N Engl J Med. 1976 Apr 29;294(18):965–972. doi: 10.1056/NEJM197604292941801. [DOI] [PubMed] [Google Scholar]
- Lin M., Imai M., Bellamy A. R., Ikegami N., Furuichi Y., Summers D., Nuss D. L., Deibel R. Diagnosis of rotavirus infection with cloned cDNA copies of viral genome segments. J Virol. 1985 Aug;55(2):509–512. doi: 10.1128/jvi.55.2.509-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Imai M., Ikegami N., Bellamy A. R., Summers D., Nuss D. L., Deibel R., Furuichi Y. cDNA probes of individual genes of human rotavirus distinguish viral subgroups and serotypes. J Virol Methods. 1987 Mar;15(4):285–289. doi: 10.1016/0166-0934(87)90151-0. [DOI] [PubMed] [Google Scholar]
- Perez-Schael I., Gonzalez M., Daoud N., Perez M., Soto I., Garcia D., Daoud G., Kapikian A. Z., Flores J. Reactogenicity and antigenicity of the rhesus rotavirus vaccine in Venezuelan children. J Infect Dis. 1987 Feb;155(2):334–338. doi: 10.1093/infdis/155.2.334. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E., Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984 May 5;1(8384):977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- Zheng B. J., Han S. X., Yan Y. K., Liang X. R., Ma G. Z., Yang Y., Ng M. H. Development of neutralizing antibodies and group A common antibodies against natural infections with human rotavirus. J Clin Microbiol. 1988 Aug;26(8):1506–1512. doi: 10.1128/jcm.26.8.1506-1512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B. J., Lam W. P., Yan Y. K., Lo S. K., Lung M. L., Ng M. H. Direct identification of serotypes of natural human rotavirus isolates by hybridization using cDNA probes derived from segment 9 of the rotavirus genome. J Clin Microbiol. 1989 Mar;27(3):552–557. doi: 10.1128/jcm.27.3.552-557.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


