Abstract
Asthma is a chronic inflammatory disorder of the airways. Type 2 T helper (Th) cell–dominated inflammation in the lung is a hallmark of asthma. Src homology 2 domain–containing protein tyrosine phosphatase (SHP)-1 is a negative regulator in the signaling pathways of many growth factor and cytokine receptors. However, a direct role of SHP-1 in the IL-4/IL-13 signaling pathway has not been established. In this study, we sought to define the function of SHP-1 in the lung by characterizing the pulmonary inflammation of viable motheaten (mev) mice, and to investigate the molecular mechanisms involved. Pulmonary histology, physiology, and cytokine expression of mev mice were analyzed to define the nature of the inflammation, and the gene-deletion approach was used to identify critical molecules involved. In mev mice, we observed spontaneous Th2-like inflammatory responses in the lung, including eosinophilia, mucus metaplasia, airway epithelial hypertrophy, pulmonary fibrosis, and increased airway resistance and airway hyperresponsiveness. The pulmonary phenotype was accompanied by up-regulation of Th2 cytokines and chemokines. Selective deletion of IL-13 or signal transducer and activator of transcription 6, key genes in the Th2 signaling pathway, significantly reduced, but did not completely eliminate, the inflammation in the lung. These findings suggest that SHP-1 plays a critical role in regulating the IL-4/IL-13 signaling pathway and in maintaining lung homeostasis.
Keywords: Src homology 2 domain–containing protein tyrosine phosphatase-1, protein tyrosine phosphatase, motheaten mouse, type 2 T helper cell inflammation, lung
CLINICAL RELEVANCE
This study revealed a critical role of the phosphatase Src homology 2 domain–containing protein tyrosine phosphatase-1 in maintaining lung homeostasis and in regulating type 2 T helper cell inflammation in the lung. More attention should be given to the process of negative regulation in understanding and controlling pulmonary inflammation.
Asthma affects more than 20 million people in the United States alone. Chronic type 2 T helper (Th) cell–dominated inflammation in the airways is a key feature of asthma. The Th2 cytokines, IL-4 and IL-13, play a central role in mediating allergic inflammatory responses. IL-4 and IL-13 use IL-4 receptor (IL-4R) α for downstream signaling, including activation of signal transducer and activator of transcription (STAT)-6 and transcription of target genes. Although activation of STAT6 and other key components in response to IL-4 and IL-13 has been well documented, the molecular mechanisms responsible for termination of IL-4 and IL-13 signaling are less clear. Some of the molecules involved in the negative regulation of IL-4Rα signaling may include suppressor of cytokine signaling, B-cell lymphoma (BCL)-6, SH2 domain-containing inositol 5-phosphatase (SHIP)-1, and Src homology 2 domain–containing protein tyrosine phosphatase (SHP)-1 (1, 2).
SHP-1 as a negative regulator, has been shown to associate with a variety of cytokine and growth factor receptors and immunoreceptors, including IL-3R, B-cell receptor (BCR), T-cell receptor (TCR), killer-cell immunoglobulin-like receptor (KIR), and Erythropoietin receptor (EPOR). Once phosphorylated and activated, SHP-1 binds to and dephosphorylates its target molecules and terminates the signaling. In the absence or decreased expression of SHP-1, cytokine/growth factor signaling will go unchecked, which may lead to abnormal or pathological responses (3, 4).
SHP-1 has been shown to regulate IL-4 and IL-13 signaling in cultured cells by binding to IL-4Rα and dephosphorylating the downstream target, STAT6, therefore decreasing the transcription of target genes (5). It was also shown in vitro that SHP-1 is a key enzyme for early dephosphorylation of STAT6 after activation by IL-4, and this process largely depends on the binding of functional SHP-1 to the immunoreceptor tyrosine–based inhibitory motif of IL-4Rα (6, 7), which may function as a docking site for phosphatases, including SHP-1. So far, there has been no report that links SHP-1 deficiency directly with allergic disorders in humans. Support for SHP-1 having a role in regulating allergic responses was suggested in a study of an asthma model using heterozygous motheaten (me) mice, which have reduced SHP-1 phosphatase activity. When immunized and challenged with ovalbumin, these mice mounted an enhanced Th2 response and airway hyperresponsiveness (AHR) as compared with wild-type (WT) mice (8). More recently, we reported that SHP-1 deficiency in mice increases cellular and tissue susceptibility to oxidant stress that may lead to enhanced inflammatory responses to aeroallergen stimulation (9).
The me mice and viable me (mev) mice are two natural mutant strains deficient in SHP-1 (10–12). These mice develop spontaneous inflammatory disorders in multiple organs (10, 13, 14). Different terms have been used to describe the lung pathology of these mice, including pneumonitis, unusual pneumonia, and interstitial lung disease (10, 11, 15, 16). However, the exact nature of the inflammation in the lung is still not clear. Furthermore, the molecular mechanisms by which SHP-1 deficiency causes abnormalities in the lung are not defined.
To understand the nature of the me lung pathology, we examined mev mice, performed comprehensive analyses of the histopathology and pulmonary physiology of these mice, and investigated the cellular and molecular mechanisms that are involved. Our studies demonstrate that type 2 inflammation is a prominent feature of the mev lung pathology, and Th2 cytokines, especially IL-13, are up-regulated in the lung. Experiments using cytokine and STAT6 gene knockout (KO) mice revealed that the Th2 signaling pathway is largely responsible for the pulmonary phenotype resulting from the SHP-1 deficiency.
MATERIALS AND METHODS
Animals
The mev (Ptpn6mev) mice and IL-4–null mice on C57BL/6 genetic background were from the Jackson Laboratory (Bar Harbor, Maine). Heterozygous mice (Ptpn6mev/+) were interbred to generate WT, heterozygous, and homozygous mice (Ptpn6mev/Ptpn6mev, hereafter abbreviated as mev). The genotype of these mice was determined using PCR primers and protocols described by the Jackson Laboratory. STAT6-null mice on BALB/c genetic background were purchased from the Jackson Laboratory, and were backcrossed to C57BL/6 background for more than 10 generations. IL-13–null mice were generated as described previously (17), and backcrossed to C57BL/6 background for more than 10 generations. Crossbreeding between mev mice and IL-4, IL-13, or STAT6-null mice was performed to generate mev mice on respective gene KO background. Mice were used for experiments at 7 to 9 weeks of age, unless indicated otherwise. All mice were housed in cages with microfilters in the specific pathogen-free environment. All procedures performed on mice were in accordance with the National Institutes of Health guidelines for humane treatment of animals and were approved by the Institutional Animal Care and Use Committee of Johns Hopkins University.
Lung and Bronchoalveolar Lavage Samples
Lung tissue and bronchoalveolar lavage (BAL) samples were obtained as previously described by our laboratories (18, 19). Briefly, mice were anesthetized; the trachea was isolated by blunt dissection; and a small-caliber tubing was inserted and secured in the airway. Three successive volumes of 0.75 ml of PBS with 0.1% BSA were instilled and gently aspirated and pooled. BAL samples were centrifuged, and supernatants were stored at −70°C until assayed. Cells in 100 μl aliquots were counted. A total of 100,000 viable BAL cells were centrifuged onto slides by a Cytospin 3 (Thermo Shandon Ltd, Runcorn, UK) and stained with Hema 3 System (Fisher Scientific Co., Newark, DE). The numbers and types of cells in the pellet were determined. The lung was perfused with cold PBS through the right ventricle with cut vena cava until the pulmonary vasculature was cleared of blood. The whole lung was either excised for RNA and protein analyses or inflated with fixatives for histology.
Histology Evaluation
Hematoxylin and eosin, periodic acid Schiff (PAS), Masson's trichrome, and Alcian blue (AB) stains were performed on lung sections after fixation with Streck solution (Streck Laboratories, St. La Vista, NE), as previously described (19). The same microscopic magnification was used for the sample slides from WT and mev mice under comparison.
Assessment of Pulmonary Physiology
To assess pulmonary physiology, invasive pulmonary function tests were performed. The baseline airway resistance and AHR to methacholine (MCh) challenge were assessed. Mice were anesthetized with intraperitoneal injection of 100 μl/30 g of a mixture of ketamine (15 mg/ml) and xylazine (15 mg/ml). A tracheotomy was performed using an 18-gauge intravenous adaptor, and a polyethylene cannula was inserted into the distal trachea. The mice were connected to a computer-controlled small animal ventilator (FlexiVent; SCIREQ, Montreal, PQ, Canada) and ventilated at a frequency of 150 breaths/minute and a volume of 6 ml/kg, with a positive end-expiratory pressure of 3 cm H2O. Airway resistance was measured by using the forced oscillation technique (20). Dose–response curves to inhaled MCh (Sigma, St. Louis, MO) were determined. MCh was diluted in sterile PBS to 0, 1.56. 3.12, 6.25, and 12.5 mg/ml, and delivered through an in-line nebulizer. After each dose, data were collected at 1-minute intervals and then averaged. The values for airway resistance (cm H2O/ml/s) were plotted as a function of MCh doses.
mRNA Analysis
Total cellular RNA from lung tissues was obtained using Trizol reagent (Invitrogen, Carlsbad, CA). The mRNA of specific genes was evaluated by RT-PCR using specific primers, as previously described (19). The mRNA of β-actin was used as an internal control.
Cytokines and Chemokines
The levels of cytokines and chemokines in the BAL were determined using commercially available ELISA kits per the manufacturer's instructions (R&D Systems, Minneapolis, MN; eBioscience, San Diego, CA).
Western Blot Analysis
Antibody to MUC5AC was purchased from Neomarker (Fremont, CA). Antibodies to STAT6 and phosphorylated STAT6 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Statistical Analysis
Most of the data were assessed by Student's t test and expressed as mean (± SEM). Differences between groups with P values of 0.05 or less were considered statistically significant.
RESULTS
Pulmonary Inflammation
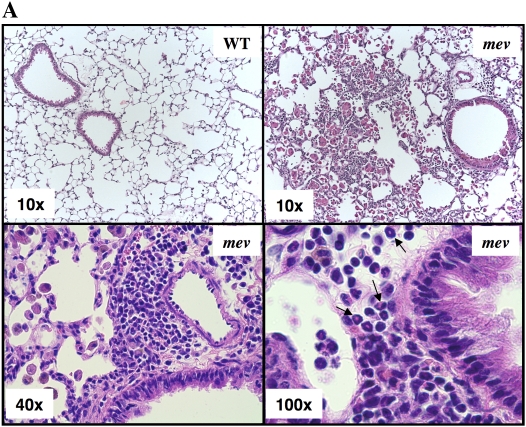
To begin to define the nature of the inflammation seen in the lung of mev mice, we compared the lung histopathology of WT and mev mice. All the mice examined in this study were 7 to 9 weeks old. Lung sections from WT mice had no infiltration of inflammatory cells. In contrast, patchy cellular infiltration of the airways and lung parenchyma was readily seen in lung sections from mev mice, which ranged from mild infiltrates to total lobar consolidation (Figure 1A). Under higher magnification, clusters of eosinophils (arrows) and enlarged macrophages were readily seen in the airways and lung parenchyma of mev mice (Figure 1A). These macrophages had crystalline inclusions typical of murine Th2 inflammation, which we and others have previously shown to correspond to the chitinase-related protein, Ym1/2 (21, 22). Consistent with these findings, BAL total cell counts and differential analysis in mev mice showed significant increases in all cell types, including macrophages, eosinophils, lymphocytes, and neutrophils, as compared with WT mice (Figure 2B). These results indicate that the pulmonary inflammation seen in mev mice is a characteristic type 2 inflammatory response.
Figure 1.
Comparison of lung histology and bronchoalveolar lavage (BAL) cellularity of viable motheaten (mev) mice and wild-type (WT) littermate controls. (A) Representative hematoxylin and eosin–stained lung sections from 9-week-old WT and mev mice. Arrows indicate eosinophils. (B) BAL samples were obtained from WT and mev mice (n = 6 each), and total cell counts and differentials were determined (*P < 0.05). Eos, eosinophils; Lym, lymphocytes; Mac, macrophages; Neu neutrophils. Data represent mean and SEM values.
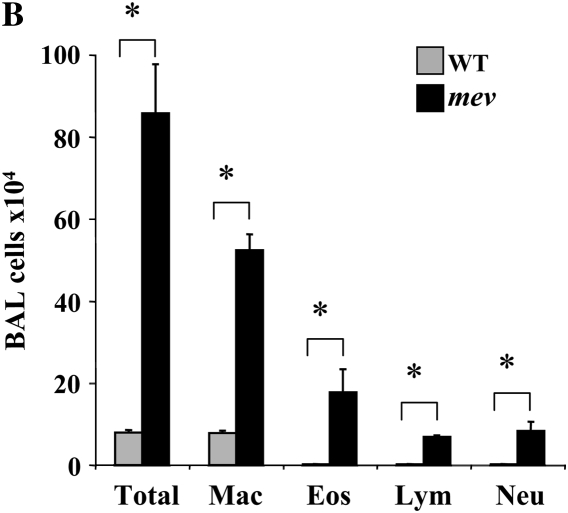
Figure 2.
Mucous metaplasia and up-regulation of MUC5AC in the lung of mev mice. (A) Lung sections from WT and mev mice were stained with periodic acid Schiff for neutral mucin, and with Alcian blue (AB) for acidic mucin. (B) RT-PCR analysis of MUC5AC expression in the lungs of WT and mev mice. (C) Western slot blot of MUC5AC in BAL fluids from WT and mev mice.
Mucous Metaplasia
To determine whether mucous metaplasia occurred in the airways, we analyzed lung sections from WT and mev mice using PAS and AB staining for neutral and acidic mucins, respectively. No PAS- or AB-positive staining was found in the small airways in the lung sections of WT mice, with occasional positive cells in the large airways (Figure 3A). In contrast, markedly increased PAS-positive and AB-positive goblet cells were readily seen in the airways of mev mice (Figure 3A). To further confirm this observation, we assessed the expression of mucin gene, MUC5AC, at mRNA and protein levels in the lung. RT-PCR results showed that low levels of MUC5AC mRNA were seen in the lung of WT mice, whereas increased MUC5AC mRNA was found in the lung of mev mice (Figure 3B). Western slot blot using anti-MUC5AC antibody showed markedly increased MUC5AC in the BAL fluid from mev mice as compared with that of WT mice (Figure 3C). These results indicate that SHP-1 deficiency resulted in goblet cell metaplasia, mucous hyperproduction, and up-regulation of MUC5AC gene expression in the airways.
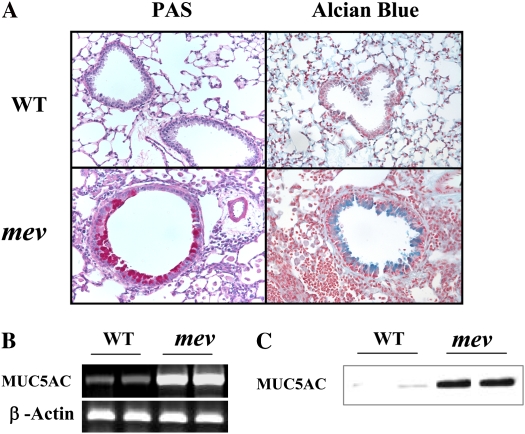
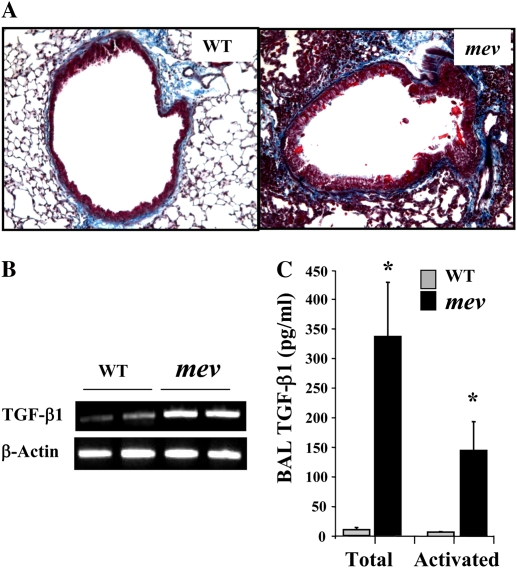
Figure 3.
Pulmonary fibrosis. (A) Masson's trichrome staining of lung sections from WT and mev mice. (B) RT-PCR analysis of transforming growth factor (TGF)-β1 expression in the lungs of WT and mev mice. (C) Protein levels of total and active TGF-β1 in BAL fluids from WT and mev mice determined by ELISA (n = 6 for each group; *P < 0.05). Data represent mean and SEM values.
Fibrosis in the Lung
As part of the airway remodeling, there is increased airway wall thickness, which includes an increase in connective tissue. To assess fibrosis in the lung, Masson's trichrome staining was used to detect collagen deposition. As shown in Figure 4A, trichrome-positive–stained material can be seen around the airways of WT mice (Figure 4A). However, significantly increased collagen deposition was readily seen around the airways and in the parenchyma of mev mice (Figure 4A). To understand the molecular mechanisms of the fibrotic change, we compared the expression of transforming growth factor (TGF)-β1 in the airways of WT and mev mice by ELISA. As shown in Figure 4B, either total or activated TGF-β1 was barely detectable in the BAL from WT mice. In contrast, TGF-β1 protein was highly increased in the lung of mev mice, including spontaneously activated TGF-β1 (Figure 4B). These data indicate that SHP-1 deficiency resulted in pulmonary fibrosis.
Figure 4.
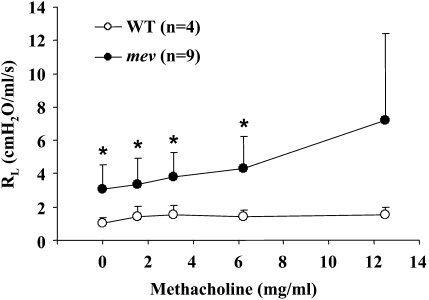
Airway resistance and airway hyperresponsiveness of WT and mev mice. Invasive pulmonary function tests were performed on anesthetized WT and mev mice using the FlexiVent System, as described in Materials and Methods. Airway resistance in response to increasing concentrations of methacholine through inhalation was recorded and analyzed (*P < 0.05). Data represent mean and SEM values.
Airway Epithelial Hypertrophy
One of the pathological changes in the lung of patients with asthma is airway epithelial hypertrophy. Comparing the airways of mev mice with those of similarly sized WT mice, it was noticed that the airway epithelium in the lung of mev mice was thickened (Figures 1A, 2A, and 3A).
Abnormal Pulmonary Physiology
Analysis of the lung mechanics using the forced oscillation technique demonstrated that, in the absence of any stimulation, mev mice already had significantly increased baseline airway resistance compared with that of WT mice (Figure 4). When challenged, mev mice showed further increases in airway resistance in response to increasing concentrations of MCh, whereas WT mice only showed small increments (Figure 4). The variation of airway resistance within the mev group significantly increased when the concentration of MCh was 12.5 mg/ml, and some mev mice died at 25 mg/ml of MCh. Thus, no consistent data could be obtained at concentrations higher than 12.5 mg/ml.
Expression of Cytokines and Activation of Th2 Signaling Pathway
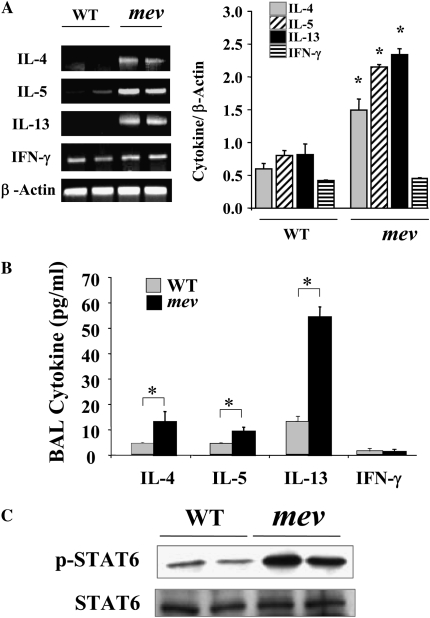
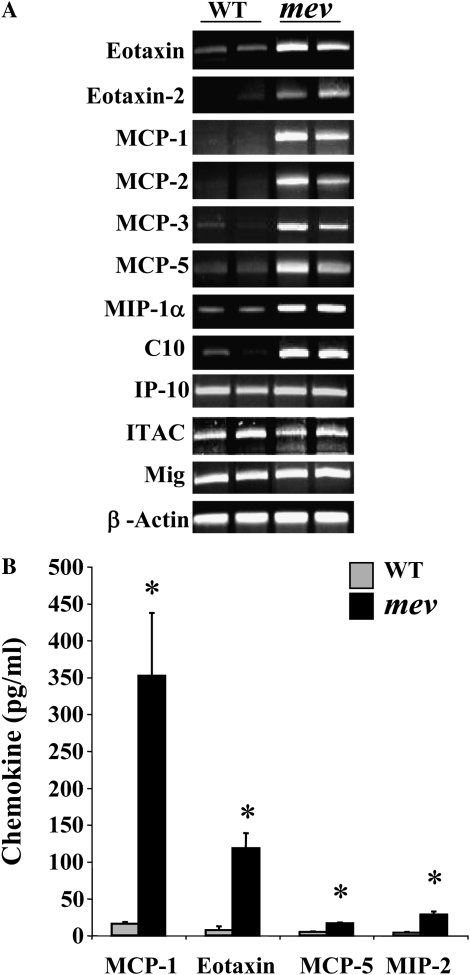
To understand the underlying molecular mechanisms of the pulmonary inflammation in mev mice, we investigated the cytokine expression profiles in the lung. As determined by RT-PCR in the lung of mev mice, the mRNA levels of IL-4, IL-5, and IL-13 were increased, whereas that of IFN-γ was not (Figure 5A). Measurement using ELISA showed that both IL-4 and IL-13 were significantly increased in the BAL of mev mice (Figure 5B). IL-5 levels were increased in mev mice, but no differences were seen in IFN-γ levels between WT and mev mice (Figure 5B). To determine if the Th2 signaling pathway was activated, we examined the activation status of STAT6 in the lung tissue using antibodies against STAT6 and phosphorylated STAT6 by Western blot. As shown in Figure 5C, the total amount of STAT6 was comparable in the lung tissue of WT and mev mice. Phosphorylated STAT6 was barely seen in the lung of WT mice. In contrast, significantly increased levels of phosphorylated STAT6 were found in the lung tissue of mev mice, indicating activation of the Th2 signaling pathway in the lung of mev mice (Figure 5C). Furthermore, we examined the chemokine response in the lung. Th2 inflammation–related chemokines, such as eotaxin and monocyte chemotactic protein -1, -2, -3, and -5 (23), were up-regulated in the lung of mev mice at the mRNA level, and some were significantly increased at the protein level in the BAL fluid of mev mice. However, the expression of IFN-γ–responsive gene, interferon-inducible protein (IP)-10, did not change (Figures 6A and 6B).
Figure 5.
Cytokine expression and signal transducer and activator of transcription (STAT) 6 activation in the lung. (A) RT-PCR analysis of mRNA expression of T helper (Th) 2 and Th1 cytokines in the lung and densitometry of cytokine mRNA with β-actin mRNA as reference (n = 6 each group; *P < 0.05). (B) Protein levels of cytokines in the BAL fluids from WT and mev mice (n = 6 each) determined by ELISA (*P < 0.05). (C) Western blot analysis of total and phosphorylated STAT6 in the lungs of WT and mev mice. Data represent mean and SEM values.
Figure 6.
Chemokine expression and production in the lung. (A) Total lung RNA was obtained from WT and mev mice, and gene-specific primers were used in the RT-PCR analysis of chemokine expression, with β-actin as an internal control. (B) Protein levels of selected chemokines in BAL from WT and mev mice (n = 6 each group) measured by ELISA (*P < 0.05). Data represent mean and SEM values.
Role of Th2 Cytokines and Signaling Pathway
The pulmonary phenotype and cytokine expression profiles indicate that Th2 cytokines and the Th2 signaling pathway are involved in the lung inflammation of mev mice. To determine if specific molecules in the Th2 signaling pathway plays a role in the pulmonary inflammation of mev mice, we selectively deleted IL-4, IL-13, or STAT6 genes by crossbreeding with the respective KO mice, and examined the phenotypic changes in these mice.
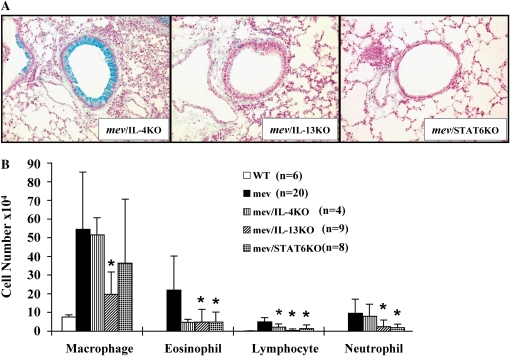
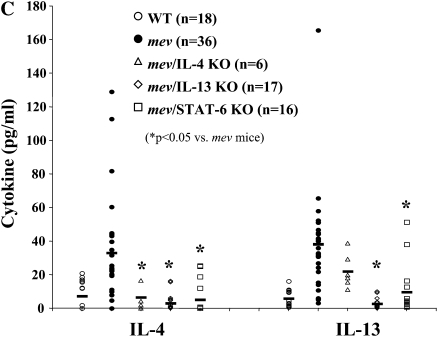
Examination of the lung inflammation and mucous metaplasia demonstrated that ablation of IL-4 gene only slightly reduced the tissue inflammation and BAL cell counts (Figure 7). No significant difference was seen in mucus staining between the lungs of mev mice and mev/IL-4KO mice (Figures 7A and 7B). The percentage of cell types in the BAL was comparable in these mice. As a result of IL-4 gene deletion, the IL-4 cytokine levels in the BAL of mev/IL-4KO mice were similar to that of the WT mice, and significantly lower than that of the mev mice (Figure 7C). However, the BAL IL-13 levels in these mice did not decrease significantly (Figure 7C).
Figure 7.
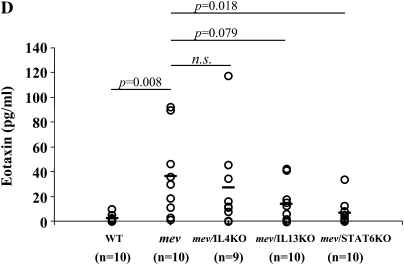
Effects of cytokine gene disruption on pulmonary inflammation and mucous metaplasia in mev mice. (A) AB staining of lung sections from mev, mev/IL-4 knockout (KO), mev/IL-13KO, and mev/STAT6KO mice. Representatives of multiple samples are shown. (B) Cellularity analysis of BAL samples from WT, mev, mev/IL-4KO, mev/IL-13KO, and mev/STAT6KO mice (*P < 0.05). Data represent mean and SEM values. (C and D) Effects of gene disruption on IL-4, IL-13, and eotaxin expression; IL-4 and IL-13 (C) and eotaxin (D) levels in the BAL fluids of WT, mev, mev/IL-4KO, mev/IL-13KO, and mev/STAT6KO mice were determined by ELISA.
Deletion of IL-13 gene in mev mice dramatically decreased the inflammatory infiltration in the lung, although some residual inflammation was still present (Figures 7A and 7B). In contrast to mev/IL-4KO mice, mev/IL-13KO mice showed significantly reduced mucous metaplasia, with very few AB-positive cells in the airways of these mice (Figure 7A). The numbers of all cell types were significantly decreased, but the percentages of the cell types did not change (Figure 7B). Disruption of IL-13 gene significantly reduced not only the levels of IL-13, but also that of IL-4 in the BAL fluid of mev mice (Figure 7C).
Deletion of the STAT6 gene in mev mice also significantly dereased the lung tissue inflammatory cell infiltration and BAL cellularity (Figures 7A and 7B), whereas no difference was seen in the percentage of inflammatory cells. AB-positive cells were hardly seen in the airways of mev/STAT6KO mice (Figure 7A). Lack of STAT6 gene resulted in significant reduction of both IL-4 and IL-13 in the BAL fluids of mev mice (Figure 7C).
The levels of IL-5 in the BAL fluid of these mice were close to or below the detection limit of the ELISA kit. Thus, no reliable data could be obtained.
Furthermore, we measured the levels of eotaxin in the BAL of different genotypes of mice (Figure 7D). Deletion of the IL-4 gene had little effect on the BAL eotaxin levels. Disruption of the IL-13 gene caused an obvious drop in eotaxin, but the difference between mev mice and mev/IL-13KO mice did not reach statistical significance. Finally, deletion of STAT6 gene caused a significant reduction in the eotaxin levels in the BAL (Figure 7D).
DISCUSSION
In several studies, SHP-1 has been implicated as a negative regulator of the Th2 signaling pathway. A glutamine-to-arginine substitution mutation (R576) in the cytoplasmic domain of the human IL-4Rα chain has been associated with atopy (24). Because the mutation is right next to a tyrosine residue, it was postulated that the R576 mutation may affect the binding of SHP-1. Indeed, phosphopeptides derived from the allele with the R576 mutation showed decreased binding of SHP-1. This also correlated with increased IL-4 signaling in peripheral blood mononuclear cells isolated from homozygous patients (24). In cultured cells, SHP-1 can dephosphorylate STAT6 after binding to the immunoreceptor tyrosine–based inhibitory domain of activated IL-4Rα (5–7). Reduced SHP-1 enzyme activity in the heterozygous me mice has been shown to lead to exaggerated responses to allergen stimulation in the lung (8). However, a functional role of SHP-1 in regulating Th2 activation pathway, and in controlling allergic inflammatory response in the lung, has not been established, particularly in the absence of obvious allergen stimulation.
Both me and mev mice are deficient in SHP-1 and have autoimmune diseases and immunodeficiency. These mice develop spontaneous inflammation in multiple organs, including the lung (10, 13–15, 25). However, the nature of the inflammation in the lung has not been defined, and the underlying mechanisms have not been explored. In this study, we investigated the pulmonary phenotype of mev mice, the molecular mechanisms, and the signaling pathways that are involved.
Analyses of the lungs of mev mice showed presence of large numbers of cells in the airways and in the lung parenchyma, including abundant eosinophils and lymphocytes. Lung histology showed prominent mucous metaplasia, epithelial hypertrophy, and subepithelial and parenchymal fibrosis. Pulmonary physiology assessment showed significantly increased baseline airway resistance and AHR in response to stimulation. Cytokine analyses revealed that Th2 cytokines IL-4 and IL-13 were highly up-regulated in the lung of mev mice, whereas the levels of IFN-γ and IP-10 did not change. Consistent with these results, IL-4/IL-13 downstream target genes, including chemokines, eotaxin and monocyte chemotactic protein-1, mucin, and TGF-β, were also up-regulated. Furthermore, studies using selective gene KO demonstrated that Th2 cytokine IL-13 and Th2 signaling molecule STAT6 were critical in mediating pulmonary inflammation and mucous metaplasia in mev mice, whereas IL-4 had some part in lung inflammation, but no effect on mucous production. Deletion of IL-13 gene significantly reduced IL-4 cytokine expression in the lung of mev mice, whereas deletion of IL-4 had no reciprocal effect on IL-13 expression. These studies reveal that mev mice develop a typical type 2 immune response in the lung, and the inflammation is largely dependent on Th2 cytokines, particularly IL-13, and on the Th2 signaling molecule, STAT6, suggesting that SHP-1 plays a critical role as a negative regulator in the Th2 signaling pathway and in maintaining lung homeostasis.
The observation that pulmonary inflammation in mev mice develops spontaneously in the absence of overt allergen stimulation suggests that innate immunity is involved. This is supported by findings in studies in which backcrossing me mice to strains deficient in myeloid progenitors showed that the me phenotype, including the lung pathology, was partially dependent on the myeloid cell population (26, 27). On the other hand, when mev mice were backcrossed to recombinant activating gene (RAG)-1−/− mice, which lack mature T and B cells, the pathology in these mice did not change, indicating that T and B cells, critical in adaptive immunity, are not required for the development of the me phenotype (28). We found that SHP-1 plays a role in host response to oxidant stress, and that deficiency in SHP-1 causes increased inflammation to aeroallergen, possibly through activation of innate immune cells (9). Together, these studies suggest that innate immunity may be critical for initiating the immune response seen in these mice, and that myeloid cells, particularly macrophages, monocytes, and mast cells, are acting as important initiating and effector cell types in the pathogenesis of pulmonary inflammation of these mice. Among these cell types, mast cells and basophils are capable of producing Th2 cytokines and other proinflammatory cytokines. The role of these cell types in generating the immune response in the lung of mev mice is under investigation.
It is interesting to note that disruption of IL-4 gene had no significant effect on the levels of IL-13 cytokine in the lung of mev mice, whereas disruption of IL-13 gene had significant inhibitory effects on the IL-4 levels in the lung (Figure 7C). Together with the effects of cytokine gene deletion on inflammation and mucous metaplasia, these observations indicate that IL-13 plays a major role in the development of the mev pulmonary phenotype, where IL-4 has a minor or no role. This is probably further evidence to support the view that the mev pulmonary phenotype is not mediated by adaptive immunity, as IL-4 is known to be critical in T cell differentiation in adaptive immune responses, whereas IL-13 is more of an effector cytokine that can be produced by T cells and by cell types of innate immunity.
Further down the signaling pathway, STAT6 is a major signaling molecule, transducing the signals of IL-4 and IL-13 to the downstream target genes (29). However, other mechanisms are also known to be involved in this pathway. Signaling molecules from the insulin receptor substrate (IRS) family can interact with IL-4Rα and activate the phosphatidylinositol 3-kinase (PI3K) pathway (1). Some recent studies have shown that activation of the PI3K pathway is a critical part of immune responses to allergen stimulation in mouse models of asthma (30, 31). It has been demonstrated that SHP-1 regulates the IRS–PI3K pathway downstream of the insulin receptor (32). However, it is not known whether the IRS–PI3K pathway downstream of IL-4Rα plays a role in the mev lung phenotype due to loss of SHP-1. Our findings suggest that STAT6 is largely responsible for the Th2 inflammatory phenotype in the mev lung, but other mechanisms are also involved, and should be investigated further.
Our understanding of Th2 inflammatory disorders has advanced rapidly in recent years. However, the vast majority of our research effort is focused on the up-regulation of Th2 cytokines and the activation of Th2 pathways. Little is known about the negative regulation of Th2 inflammation. Our findings establish a direct functional relationship between tyrosine phosphatase SHP-1 and pulmonary Th2 inflammation. This may add a new facet to our understanding of the critical role of SHP-1 in controlling Th2 immune response, and to our understanding of allergic and asthmatic disorders from the perspective of negative regulation. Aberrant expression of SHP-1, or a functional deficiency in SHP-1, in humans may have unrecognized effects on some of the allergic and asthmatic disorders. On the other hand, enhanced SHP-1 function may offer an alternative mechanism for controlling Th2 inflammation.
Acknowledgments
The authors thank Ms. Fan Wu for her technical assistance.
This work was supported by National Institutes of Health grants R01HL079349-01 (Z.Z.), K08 AI055064 (T.Z.), and R01AI075025-01.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0225OC on October 23, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol 1999;17:701–738. [DOI] [PubMed] [Google Scholar]
- 2.Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol 2000;105:1063–1070. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol 2000;12:361–378. [DOI] [PubMed] [Google Scholar]
- 4.Pao LI, Badour K, Siminovitch KA, Neel BG. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annu Rev Immunol 2007;25:473–523. [DOI] [PubMed] [Google Scholar]
- 5.Haque SJ, Harbor P, Tabrizi M, Yi T, Williams BR. Protein-tyrosine phosphatase Shp-1 is a negative regulator of IL-4– and IL-13–dependent signal transduction. J Biol Chem 1998;273:33893–33896. [DOI] [PubMed] [Google Scholar]
- 6.Kashiwada M, Giallourakis CC, Pan PY, Rothman PB. Immunoreceptor tyrosine-based inhibitory motif of the IL-4 receptor associates with SH2-containing phosphatases and regulates IL-4–induced proliferation. J Immunol 2001;167:6382–6387. [DOI] [PubMed] [Google Scholar]
- 7.Hanson EM, Dickensheets H, Qu CK, Donnelly RP, Keegan AD. Regulation of the dephosphorylation of STAT6: participation of Tyr-713 in the interleukin-4 receptor alpha, the tyrosine phosphatase SHP-1, and the proteasome. J Biol Chem 2003;278:3903–3911. [DOI] [PubMed] [Google Scholar]
- 8.Kamata T, Yamashita M, Kimura M, Murata K, Inami M, Shimizu C, Sugaya K, Wang CR, Taniguchi M, Nakayama T. Src homology 2 domain-containing tyrosine phosphatase SHP-1 controls the development of allergic airway inflammation. J Clin Invest 2003;111:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho YS, Oh SY, Zhu Z. Tyrosine phosphatase SHP-1 in oxidative stress and development of allergic airway inflammation. Am J Respir Cell Mol Biol 2008;39:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green MC, Shultz LD. Motheaten, an immunodeficient mutant of the mouse. I. Genetics and pathology. J Hered 1975;66:250–258. [DOI] [PubMed] [Google Scholar]
- 11.Shultz LD, Coman DR, Bailey CL, Beamer WG, Sidman CL. “Viable motheaten,” a new allele at the motheaten locus. I. Pathology. Am J Pathol 1984;116:179–192. [PMC free article] [PubMed] [Google Scholar]
- 12.Shultz LD, Schweitzer PA, Rajan TV, Yi T, Ihle JN, Matthews RJ, Thomas ML, Beier DR. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell 1993;73:1445–1454. [DOI] [PubMed] [Google Scholar]
- 13.Shultz LD, Green MC. Motheaten, an immunodeficient mutant of the mouse. II. Depressed immune competence and elevated serum immunoglobulins. J Immunol 1976;116:936–943. [PubMed] [Google Scholar]
- 14.Clark EA, Shultz LD, Pollack SB. Mutations in mice that influence natural killer (NK) cell activity. Immunogenetics 1981;12:601–613. [DOI] [PubMed] [Google Scholar]
- 15.Ward JM. Pulmonary pathology of the motheaten mouse. Vet Pathol 1978;15:170–178. [DOI] [PubMed] [Google Scholar]
- 16.Rossi GA, Hunninghake GW, Kawanami O, Ferrans VJ, Hansen CT, Crystal RG. Motheaten mice—an animal model with an inherited form of interstitial lung disease. Am Rev Respir Dis 1985;131:150–158. [DOI] [PubMed] [Google Scholar]
- 17.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, Murray R, Grencis R, McKenzie AN. Impaired development of Th2 cells in IL-13–deficient mice. Immunity 1998;9:423–432. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999;103:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ Jr, Chapman HA Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase– and cathepsin-dependent emphysema. J Clin Invest 2000;106:1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda K, Haczku A, Lee JJ, Irvin CG, Gelfand EW. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol 2001;281:L394–L402. [DOI] [PubMed] [Google Scholar]
- 21.Homer RJ, Zhu Z, Cohn L, Lee CG, White WI, Chen S, Elias JA. Differential expression of chitinases identify subsets of murine airway epithelial cells in allergic inflammation. Am J Physiol Lung Cell Mol Physiol 2006;291:L502–L511. [DOI] [PubMed] [Google Scholar]
- 22.Guo L, Johnson RS, Schuh JC. Biochemical characterization of endogenously formed eosinophilic crystals in the lungs of mice. J Biol Chem 2000;275:8032–8037. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today 1998;19:568–574. [DOI] [PubMed] [Google Scholar]
- 24.Hershey GK, Friedrich MF, Esswein LA, Thomas ML, Chatila TA. The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor. N Engl J Med 1997;337:1720–1725. [DOI] [PubMed] [Google Scholar]
- 25.Lutzner MA, Hansen CT. Motheater: an immunodeficient mouse with markedly less ability to survive than the nude mouse in a germfree environment. J Immunol 1976;116:1496–1497. [PubMed] [Google Scholar]
- 26.Paulson RF, Vesely S, Siminovitch KA, Bernstein A. Signalling by the W/Kit receptor tyrosine kinase is negatively regulated in vivo by the protein tyrosine phosphatase SHP1. Nat Genet 1996;13:309–315. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz U, Bergemann AD, Steinberg HN, Flanagan JG, Li X, Galli SJ, Neel BG. Genetic analysis reveals cell type–specific regulation of receptor tyrosine kinase c-Kit by the protein tyrosine phosphatase SHP1. J Exp Med 1996;184:1111–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu CC, Tsui HW, Ngan BY, Shulman MJ, Wu GE, Tsui FW. B and T cells are not required for the viable motheaten phenotype. J Exp Med 1996;183:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pernis AB, Rothman PB. JAK-STAT signaling in asthma. J Clin Invest 2002;109:1279–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myou S, Leff AR, Myo S, Boetticher E, Tong J, Meliton AY, Liu J, Munoz NM, Zhu X. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med 2003;198:1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwak YG, Song CH, Yi HK, Hwang PH, Kim JS, Lee KS, Lee YC. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest 2003;111:1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubois MJ, Bergeron S, Kim HJ, Dombrowski L, Perreault M, Fournes B, Faure R, Olivier M, Beauchemin N, Shulman GI, et al. The SHP-1 protein tyrosine phosphatase negatively modulates glucose homeostasis. Nat Med 2006;12:549–556. [DOI] [PubMed] [Google Scholar]