Abstract
Cell-based therapy in adult lung injury models is associated with highly variable donor cell engraftment and epithelial reconstitution. The role of marrow-derived cell therapy in neonatal lung injury is largely unknown. In this study, we determined the fate and effects of adult bone marrow cells in a model of neonatal lung injury. Wild-type mice placed in a normoxic or hyperoxic (95% O2) environment received bone marrow cells from animals expressing green fluorescent protein (GFP) at Postnatal Day (P)5. Controls received vehicle buffer. Lungs were analyzed between Post-Transplantation (TPX) Day 2 and Week 8. The volume of GFP-immunoreactive donor cells, monitored by stereologic volumetry, remained constant between Post-TPX Weeks 1 and 8 and was similar in normoxic and hyperoxia-exposed recipients. Virtually all marrow-derived cells showed colocalization of GFP and the pan-macrophage marker, F4/80, by double immunofluorescence studies. Epithelial transdifferentiation was not seen. Marrow cell administration had adverse effects on somatic growth and alveolarization in normoxic mice, while no effects were discerned in hyperoxia-exposed recipients. Reexposure of marrow-treated animals to hyperoxia at P66 resulted in significant expansion of the donor-derived macrophage population. In conclusion, intranasal administration of unfractionated bone marrow cells to newborn mice does not achieve epithelial reconstitution, but establishes persistent alveolar macrophage chimerism. The predominantly adverse effects of marrow treatment in newborn lungs are likely due to macrophage-associated paracrine effects. While this model and route of cell therapy may not achieve epithelial reconstitution, the role of selected stem cell populations and/or alternate routes of administration for cell-based therapy in injured newborn lungs deserve further investigation.
Keywords: stem cells, cell therapy, lung injury, newborns, BPD
CLINICAL RELEVANCE
The role of marrow-derived cell therapy in neonatal lung injury is largely unknown. We demonstrate that unfractionated adult marrow cells, administered intratracheally to newborn mice, result in a persistent pulmonary macrophage chimerism, without evidence of epithelial or mesenchymal transdifferentiation. Other cell-based therapy models remain to be investigated.
Premature infants with structurally immature lungs born between 23 and 28 weeks of gestation are at risk for development of bronchopulmonary dysplasia (BPD) or chronic lung disease of the newborn, a condition associated with high perinatal morbidity and mortality (1). An estimated 30% of infants with a birth weight between 500 and 1,500 g will develop BPD. Many of these infants require long-term ventilation and/or supplemental oxygen (2, 3). The main pathological hallmark of BPD is an arrest of alveolar development, characterized by large and simplified distal airspaces (4, 5). In addition, several recent reports have shown that the lungs of ventilated preterm infants with early BPD show markedly increased levels of alveolar epithelial cell death (6–8). We recently demonstrated that increased alveolar epithelial apoptosis in newborn mice is sufficient to disrupt alveolar remodeling (9), supporting our central hypothesis that loss of alveolar epithelial cells may play a critical role in the arrested alveolar development seen in BPD. The potential for stem cell–based therapy aimed at restoring or protecting the alveolar epithelium in newborn lungs is therefore very attractive.
Adult stem cell transplantation has recently emerged as a new alternative to stimulate lung repair. In the past decade, studies in animals and humans have documented the ability of adult bone marrow–derived stem cells to differentiate into an expanding repertoire of nonhematopoietic cell types, including brain, skeletal muscle, chondrocytes, liver, endothelium, and heart (10–32). Several lines of evidence in humans and mice suggest that adult bone marrow–derived stem cells can reconstitute injured or defective alveolar epithelium with functional new cells. In humans, lungs from bone marrow or lung transplant recipients demonstrate chimerism of epithelial and endothelial cells (33–37). In lethally irradiated mice, transplantation of a single donor marrow–derived stem cell resulted in engraftment and differentiation to nonhematopoietic tissues, including lung (38). At 11 months after transplantation, 20% of the alveolar epithelium was found to be donor-derived. This exciting study was followed by several similar studies reiterating the potential of adult bone marrow to generate lung epithelium. After bleomycin-induced injury in mice, mesenchymal stem cells were found to colonize the lung and differentiate into alveolar type I or type II cells (39, 40). In a study by Theise and coworkers (41), the proportion of donor-derived alveolar type II cells in lethally irradiated mice treated with whole bone marrow increased from 0.7% at Day 5 to as much as 14% at 6 months. Abe and colleagues (42) determined that 45% (range, 4–70%) of lung cells, including alveolar type I cells, were donor-derived 30 to 140 days after transplantation of whole bone marrow to irradiated mice. Recently, Aliotta and coworkers (43) achieved substantial donor engraftment (up to 18.9% of epithelial cells) in lungs of animals treated with irradiation combined with cardiotoxin and granulocyte-colony stimulating factor (G-CSF).
Ortiz and colleagues (40) addressed the functional effects of lung engraftment by marrow cells. In spite of relatively low levels of engraftment (donor DNA accounting for only 5.2 × 10−4% of total lung DNA), mesenchymal stem cell administration immediately after bleomycin exposure attenuated inflammation and fibrosis. This suggests that adequately timed systemic administration of marrow-derived cells may be beneficial in the treatment of lung disease, even if engraftment levels are comparatively low. The data further suggest that the benefits may be due less to bulk reconstitution/regeneration and more to paracrine effects on resident lung cells or the local milieu. Rojas and coworkers (44) similarly observed increased survival in bleomycin-treated and busulfan-myelosuppressed mice after intravenous delivery of mesenchymal stem cells. Two weeks after bleomycin insult, up to 29% of lung cells were donor-derived, with characteristics of alveolar type I and type II cells, fibroblasts, and endothelial cells. More recently, Gupta and colleagues (45) reported improved survival and attenuated endotoxin-induced lung injury in mice treated with bone marrow–derived mesenchymal stem cells.
Despite ample evidence supporting the ability of bone marrow–derived stem cells to give rise to alveolar lung epithelium, other laboratories have been unable to detect significant regeneration of lung tissue with bone marrow cells (46–48). This wide discrepancy in results can be attributed in part to the large variety of experimental methods used by different groups, including different lung injury models (irradiation, bleomycin, elastase, cardiotoxin); different donor stem cell subpopulations used (whole bone marrow, hematopoietic stem cells, side population cells, mesenchymal stem cells); different study lengths (days to months); different donor cell tracking methods; and different route of stem cell delivery (intravenous, intraperitoneal).
The vast majority of studies reporting successful engraftment in alveolar epithelium have used the intravenous route for delivery of marrow-derived stem cells, either with (25, 38, 41–43, 49) or without (39) irradiation-induced myeloablation and bone marrow reconstitution. Compared with this indirect systemic approach, direct intrapulmonary delivery of marrow-derived stem cells may represent a biologically and clinically more relevant approach. Indeed, intravenously administered stem cells must first be filtered and trapped in the lungs and, in addition, must cross multiple barriers (endothelium, interstitium, and one or two basement membranes) to reach the alveolar epithelium. In contrast, intrapulmonary delivered cells have immediate access to the alveolar compartment. Furthermore, the intrapulmonary route is clinically extremely relevant: whereas irradiation and myeloablation are clearly out of the question, intrapulmonary delivery of cells in intubated premature infants at risk for BPD is within the scope of the current practice of intrapulmonary delivery of exogenous surfactant and antioxidants.
The aim of the present study was to determine the fate and effects of adult bone marrow cells administered intranasally to newborn mice exposed to room air or hyperoxia (95% O2). As described by others (50), this oxygen regimen results in marked enlargement and diminished septation of the airspaces and thus replicates the alveolar disruption typical of preterm infants with BPD.
MATERIALS AND METHODS
Animals and Tissue Processing
Breeding colonies were established from homozygous matings of inbred C57BL/6J wild-type mice (Jackson Laboratories, Bar Harbor, ME) or heterozygous matings of mice transgenic for enhanced green fluorescent protein (eGFP) bred onto the same genetic background (C57BL/6-Tg(ACTB-EGFP)1Osb/J; Jackson Laboratories). The latter transgenic mouse line carries eGFP cDNA under the control of a chicken β-actin promoter and cytomegalovirus enhancer. Newborn C57BL/6J mice were exposed to room air or hyperoxia (> 95% O2) for 7 days. For hyperoxia experiments, mice were placed in an airtight Plexiglas chamber. Oxygen concentrations were continuously monitored and controlled with an in-line oxygen analyzer and controller system (ProOx 110; BioSpherix, Redfield, NY). Nursing dams were rotated daily between air- and oxygen-exposed litters to minimize maternal oxygen toxicity. Mice were killed between Post-Transplantation (TPX) Day 2 and Week 8 by pentobarbital overdose (Postnatal Day [P]1 = day of birth). To determine the fate of engrafted marrow cells in lungs exposed to injury long after marrow transplantation, marrow-treated mice previously exposed to hyperoxia during the newborn period were reexposed to 95% O2 from P66 to P68, and were killed at P78.
Body weights were recorded. For morphologic and morphometric studies, the lungs were formalin-fixed by standardized tracheal instillation at a constant pressure of 20 cm H2O. All lungs were equally inflated on the same apparatus. Immediately after inflation, the trachea was ligated and the lungs were immersed in formalin for overnight fixation. The following day, the lungs were dehydrated in graded ethanol solutions, embedded in paraffin, and stained with hematoxylin and eosin. Protocols were approved through the Institutional Animal Care and Use Committee.
Marrow Cell Harvesting and Administration
At P5, the pups received 10 × 106 GFP-positive whole bone marrow–derived cells by intranasal administration. Bone marrow cells were harvested from femur, tibia, and pelvis of adult mice transgenic for eGFP, yielding between 90 and 120 × 106 cells/animal. The cells were centrifuged (350 × g, 10 min) and resuspended in phosphate-buffered saline (PBS) at a concentration of 2 × 105 cells/μl. The bone marrow cells were administered by placing 25 μl of cell suspension, containing 5 × 106 cells, over the nasal orifices, thus ensuring aspiration of marrow cells into the lungs. This procedure was repeated 45 minutes later, resulting in a total delivery of 10 × 106 bone marrow cells per pup. Sham normoxic and hyperoxia-exposed controls received equal-volume (2 × 25 μl) vehicle buffer. Intranasal delivery was well tolerated by both normoxic and hyperoxia-exposed pups.
Analysis of Donor Cell Engraftment
The distribution and localization of donor cells was assessed by immunohistochemical analysis using a polyclonal rabbit anti-GFP antibody (Abcam, Inc., Cambridge, MA). Immunoreactivity was detected by streptavidin-biotin immunoperoxidase method followed by 3,3′-diaminobenzidine tetrachloride (DAB). Specificity of staining was demonstrated by omission of primary antibody, which abolished all reactivity. The pulmonary distribution of marrow cells after intranasal delivery was assessed by determining the percentage of microscope fields (×40 magnification) containing at least one GFP-positive cell at Post-TPX Day 2.
The population kinetics of engrafted donor cells between Post-TPX Weeks 1 and 8 were monitored by computer-assisted stereologic determination of the volume of GFP-immunoreactive cells, V(gfp), according to methods previously described in detail for determination of the total pulmonary volume of surfactant protein (SP)-C–immunoreactive alveolar type II cells (51, 52). The inflated lung volume, V(lu), was determined according to the Archimedes principle (53). The areal density of air-exchanging parenchyma, AA(ae/lu), was determined by point-counting based on computer-assisted image analysis. The number of points falling on air-exchanging parenchyma (peripheral lung parenchyma excluding airspace) in random lung fields was divided by the number of points falling on the entire field (tissue and airspace). AA(ae/lu) represents the tissue fraction of the lung and as such is the complement of the airspace fraction AA(air/lu). The total volume of air-exchanging parenchyma, V(ae), was calculated by multiplying AA(ae/lu) by V(lu). The areal density of GFP-immunoreactive cells, AA(gfp/ae), was determined by dividing the number of points falling of GFP-positive cells by the number of points falling on air-exchanging parenchyma. V(gfp) was calculated by multiplying AA(gfp/ae) by V(ae).
The phenotype of engrafted donor cells was determined by double immunofluorescence studies using anti-GFP antibody in combination with cell-specific antibodies directed against pro–SP-C (alveolar type II cell marker) (Abcam), mouse T1-α (39, 54) (alveolar type I cell marker) (clone 8.1.1; Developmental Studies Hybridoma Bank, Iowa City, IA), Clara Cell Secretory Protein (CCSP, CC-10, bronchial epithelial Clara cell marker) (Upstate Technologies, Lake Placid, NY), and F4/80 (pan-macrophage marker) (AbD Serotec, Raleigh, NC).
In addition to standard epifluorescence microscopy, the sections were viewed by confocal microscopy to ascertain the veracity of co-localization phenomena. Images were acquired with a Nikon C1 si laser scanning confocal microscope (Nikon, Mellville, NY) using 488- and 561-nm diode lasers. Serial optical sections were acquired separately for each channel with EZ-C1-imaging computer software (Nikon). Each acquisition was collected with a ×60 Plan Apo lens and a scan zoom of ×1.78. All images were collected at the same photomultiplier tube settings. Autoquant Deconvolution software was used before the assembly of the projections. Projection views, which ranged from 50 to 70 consecutive single optical sections, were taken at 0.1-μm intervals. NIS Elements AR 3.0 (Nikon) was used in slice or three-dimensional volume reconstruction and projections.
Analysis of Lung Growth and Alveolarization
Morphometric assessment of growth of peripheral air-exchanging lung parenchyma and contribution of the various lung compartments (airspace versus parenchyma) to the total lung volume was performed using standard stereological volumetric techniques, as described above. In addition, pulmonary cell proliferation was studied by immunohistochemical detection of the proliferation marker Ki-67 in lung sections (55). Bound antibody was detected using the ABC immunoperoxidase system with an anti-mouse Ki-67 antibody (BD Biosciences, San Jose, CA), followed by DAB treatment and hematoxylin counterstain. The Ki-67 labeling index of air-exchanging parenchyma was determined by manually counting the number of Ki-67–positive nuclei per total number of nuclei, expressed as a percentage. For each lung, at least 10 randomly selected fields per slide were evaluated (magnification ×400).
Alveolarization was quantified by computer-assisted histomorphometric analysis of the mean cord length (MCL). MCLs were determined by superimposing randomly oriented parallel arrays of lines across randomly selected microscope fields of air-exchanging lung parenchyma (at least 25 random fields per lung) and determining the distance between airspace walls (including alveoli, alveolar sacs, and ducts). All morphometric assessments were made on coded slides by a single observer who was unaware of the experimental condition of the animal analyzed.
Data Analysis
Values are expressed as mean ± SD or, where appropriate, as mean ± SEM. The significance of differences between groups was determined with ANOVA with post hoc Scheffe test. The significance level was set at P < 0.05. Statview software (Abacus, Berkeley, CA) was used for all statistical work.
RESULTS
Analysis of Engraftment of Marrow-Derived Donor Cells in Lungs of Normoxic and Hyperoxic Newborn Mice
Distribution and localization of donor cells.
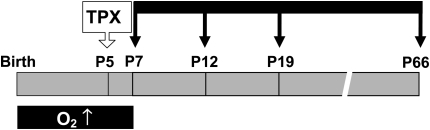
The lungs of marrow-treated and control animals were studied at selected time intervals between Post-TPX Day 2 (P7) and Post-TPX Week 8 (P66) (Figure 1). At Post-TPX Day 2, abundant cellular aggregates were present in the alveoli and airways of marrow recipients (Figure 2A). GFP staining highlighted the variable size and shape of the donor-derived cells, reflecting the heterogeneous composition of bone marrow (Figure 2B). From Post-TPX Week 1 on, marrow-derived cells were no longer identifiable by routine hematoxylin-eosin staining (Figure 2C). However, immunohistochemical analysis revealed scattered GFP-immunoreactive cells along the alveolar wall and, occasionally, overlying bronchial epithelium (Figure 2D). There was no evidence of inflammation, edema, or hemorrhage in normoxic marrow-treated animals at any time point. In hyperoxic marrow-treated and control animals, focal hemorrhages were noted at P12 and P19. Omission of the primary anti-GFP antibody quenched all immunoreactivity (Figure 2E). Lungs of GFP-expressing transgenic mice, included as positive control, revealed diffuse GFP immunoreactivity that was particularly intense in bronchial and alveolar epithelium (Figure 2F).
Figure 1.
Study design. Newborn mice were exposed to room air or hyperoxia (O2, 95% O2) from birth until Postnatal Day (P)7. At P5, mice underwent intranasal transplantation (TPX) of whole bone marrow–derived cells from green fluorescent protein (GFP)-transgenic adult mice. Sham controls received equal volume vehicle buffer. Lungs were studied at various intervals between P7 and P66 (black arrows).
Figure 2.
Lung histology and anti-GFP immunohistochemistry. (A) Normoxic recipient, Post-TPX Day 2 (P7). Representative micrograph showing abundant cellular aggregates within the airspaces. (B) Hyperoxia-exposed recipient, Post-TPX Day 2 (P7). GFP immunostaining showing numerous pleomorphic GFP-immunoreactive donor-derived cells in the alveoli. (C) Normoxic recipient, Post-TPX Week 8 (P66), showing well alveolarized lung parenchyma without evidence of inflammation or hypercellularity. (D) Normoxic recipient, Post-TPX Week 8 (P66). GFP immunostaining of section sequential to C showing scattered GFP-positive cells along the alveolar wall (arrows) and overlying bronchial epithelium (arrowheads). (E) Negative control. Omission of primary anti-GFP antibody abolished all immunoreactivity. (F) Positive control. GFP immunostaining of lung of GFP-expressing transgenic mouse. A and C, hematoxylin-eosin staining; B, D–F, GFP immunohistochemistry; 3,3′-diaminobenzidine tetrachloride (DAB) with hematoxylin counterstain (original magnification: ×400).
To assess the efficacy of the intranasal route for pulmonary delivery of marrow-derived cells, we determined the pulmonary distribution of GFP-immunoreactive cells at Post-TPX Day 2. GFP-positive donor-derived cells were consistently present in both lungs and distributed over more than 90% of microscope fields, including peripheral subpleural airspaces. The pulmonary delivery of marrow cells appeared equally efficient in hyperoxic and normoxic recipients.
Nasal administration of marrow cells could, in theory, lead to spillage of transplanted cells in the gastrointestinal tract and dissemination to the systemic circulation. To assess the possible systemic engraftment of marrow-derived donor cells, GFP immunoreactivity was studied in relevant organs, including bone marrow, spleen, liver, stomach, and intestines. Small numbers of GFP-immunoreactive cells were occasionally detected in the lumen of the gastrointestinal tract on Post-TPX Day 2 (not shown). However, other organs, including bone marrow, were uniformly devoid of GFP immunoreactivity at all time points. This indicates that disseminated engraftment of marrow-derived cells after intranasal administration was negligible.
Population kinetics of donor cells.
The growth kinetics of the GFP-immunoreactive donor cell population after marrow administration were monitored by stereological volumetric determination of V(gfp) (51, 52). The critical data set acquired to calculate V(gfp) is summarized in Table 1. V(gfp) in normoxic recipients remained remarkably constant during the time period studied (1.12 ± 0.22 μl at Post-TPX Week 1 versus 0.94 ± 0.18 μl at Post-TPX Week 8), suggesting that marrow-derived donor cells engraft as a stable chimeric cell population and undergo little cell loss after the initial engraftment. V(gfp) tended to be lower in hyperoxia-exposed animals compared with normoxic recipients in the early post-transplantation period (Table 1); however, this difference was not statistically significant.
TABLE 1.
STEREOLOGICAL VOLUMETRY OF V(gfp)
| Post-TPX Interval:
|
1 wk
|
2 wk
|
8 wk
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age
|
P12
|
P19
|
P66
|
|||||||||
| Normoxia | Hyperoxia | Normoxia | Hyperoxia | Normoxia | Hyperoxia | |||||||
| Sham | TPX | Sham | TPX | Sham | TPX | Sham | TPX | Sham | TPX | Sham | TPX | |
| (3) | (4) | (3) | (3) | (4) | (4) | (4) | (3) | (6) | (8) | (6) | (5) | |
| V(lu) (μl) | 240.0 ± 10.1 | 204.0 ± 16.9 | 181.7 ± 34.6 | 170.0 ± 22.0 | 322.8 ± 43.0 | 332.0 ± 15.4 | 222.0 ± 4.9 | 241.0 ± 21.0* | 396.4 ± 27.9 | 351.9 ± 18.4 | 417.2 ± 15.9 | 334.0 ± 22.2† |
| AA(ae/lu) (%) | 40.0 ± 1.4 | 32.4 ± 1.8† | 35.6 ± 1.2 | 27.6 ± 1.4† | 37.7 ± 2.5 | 32.2 ± 1.5 | 34.0 ± 0.6 | 33.5 ± 0.4 | 36.9 ± 0.6 | 32.2 ± 1.3† | 31.7 ± 1.7‡ | 31.3 ± 1.6 |
| V(ae) (μl) | 95.7 ± 3.3 | 66.5 ± 8.0† | 63.7 ± 10.9 | 47.3 ± 8.3 | 118.3 ± 8.7 | 106.5 ± 4.6 | 76.0 ± 1.1§ | 81.0 ± 4.9* | 147.0 ± 12.5 | 116.3 ± 6.8† | 132.4 ± 9.7 | 105.2 ± 11.1 |
| AA(gfp/ae) (%) | 1.53 ± 0.47 | 0.47 ± 0.06 | 1.77 ± 0.66 | 1.18 ± 0.83 | 0.82 ± 0.15 | 1.54 ± 0.43 | ||||||
| V(gfp) (μl) | 1.12 ± 0.22 | 0.27 ± 0.04 | 1.83 ± 0.67 | 0.96 ± 0.18 | 0.94 ± 0.18 | 1.68 ± 0.51 | ||||||
| V(gfp)/V(lu) (%) | 0.50 ± 0.22 | 0.14 ± 0.05 | 0.54 ± 0.16 | 0.36 ± 0.18 | 0.27 ± 0.13 | 0.48 ± 0.31 | ||||||
Definition of abbreviations: AA(ae/lu), areal fraction of air-exchanging parenchyma relative to lung parenchyma; BW, body weight; post-TPX interval, number of weeks between marrow cell transplantation (Postnatal Day [P]5) and killing; TPX, marrow cell-transplanted V(ae), volume of air-exchanging parenchyma;; V(lu), inflated lung volume.
Values represent mean ± SEM of (n) animals per group. Normoxia, exposure to room air; hyperoxia, exposure to 95% O2 from birth to P7.
P < 0.05 versus normoxic TPX (ANOVA with post hoc Scheffe-test).
P < 0.05 versus sham controls exposed to same oxygen levels (ANOVA with post hoc Scheffe-test).
P < 0.05 versus normoxic sham control (ANOVA with post hoc Scheffe-test).
P < 0.01 versus normoxic sham control (ANOVA with post hoc Scheffe-test).
The volume of GFP-positive cells in normoxic recipients accounted for approximately 0.50% of the total lung volume, V(lu), at Post-TPX Week 1 (Table 1). As V(gfp) remained relatively constant, but V(lu) increased about 2-fold between P7 and P66, the V(gfp)/V(lu) ratio decreased to 0.27% by Post-TPX Week 8. Parenthetically, assessment of engraftment efficiency by density-based morphometry (such as measurement of the number of GFP-positive cells per lung field) might have led to the false impression that the mass of engrafted cells decreases with time. Analysis by stereological volumetry demonstrated that the pulmonary volume of GFP-immunoreactive cells, in fact, remains constant over time.
Phenotype of engrafted donor cells.
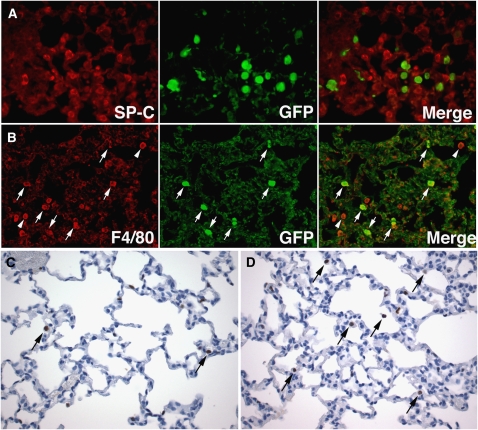
To determine the phenotype of the engrafted marrow-derived cells, we first investigated whether the donor cells showed evidence of epithelial transdifferentiation. Anti-GFP fluorescence labeling was combined with immunofluorescent identification of respiratory epithelial cells, using cell-specific antibodies against SP-C (alveolar type II cells), T1α (alveolar type I cells) (39, 54), or Clara Cell Secretory Protein/CC-10 (bronchial and bronchiolar Clara cells). As shown in Figure 3, SP-C immunohistochemical analysis identified alveolar type II cells as cuboidal to rounded cells of intermediate size, distributed at regular intervals along the alveolar walls. Compared with alveolar type II cells, GFP-immunoreactive cells generally appeared larger and showed greater variability in shape, ranging from elongated spindle-shaped to large-sized spherical cells (Figures 3A and 3B). A frequent association between GFP-positive cells and alveolar type II cells was noted, whereby the GFP-positive cells appeared to undergo a shape change to conform to the contours of the alveolar type II cells (Figure 3C). Occasionally, distinct cell projections were noted from GFP-positive donor cells to SP-C–positive type II cells (Figures 3C–3E). Partial overlap of GFP-positive cells and SP-C–positive cells was repeatedly noted, creating “overlay artifacts” (Figure 3D).
Figure 3.
GFP (green) and SP-C (red) double immunofluorescence. (A) Normoxic recipient, Post-TPX Week 1 (P12). Multiple GFP-immunoreactive donor cells are present along the alveolar walls. Colocalization of SP-C and GFP immunoreactivity is not seen. Arrows point to GFP-positive cells localized close to alveolar type II cells. (B) Hyperoxia-exposed recipient, Post-TPX Week 8 (P66). GFP-positive cells are seen along alveolar septa and overlying bronchial epithelium. Partial overlay (left lower corner) of SP-C– and GFP-positive signals is seen. Br: bronchus. (C) Marrow recipients, Post-TPX Week 8 (P66), showing intimate cell–cell contacts between GFP-positive donor cells and SP-C–positive alveolar type II cells, associated with directed shape change of the GFP-positive cells (arrows). (D) Normoxic recipient, Post-TPX Week 8 (P66) showing cell extension from GFP-positive donor cell to alveolar type II cell. SP-C–positive material is present within the cytoplasm of the GFP-positive cell (arrow). A second GFP-positive cell is seen partially superimposed on a type II cell (right upper field), creating an overlay artifact. (E) Hyperoxia-exposed recipient, Post-TPX Week 8 (P66). A single GFP-positive cell is seen, showing a distinct cell projection toward a type II cell. SP-C–positive material is noted within the GFP-positive cell (arrow) (pro–SP-C [Cy3, red] and GFP [fluorescein isothiocyanate, green] double immunofluorescence; original magnification: ×400). A–C, Epifluorescence microscopy; D and E, Confocal fluorescence microscopy.
In extremely rare instances (< 0.1% of GFP-positive cells), unequivocal colocalization of GFP and SP-C immunoreactivity was noted, confirmed by confocal microscopy (Figures 3D, 3E, and 4). This colocalization phenomenon was observed in cells with the general size, shape, and location of SP-C–negative GFP-positive cells. Furthermore, the rare SP-C–positive donor cells often exhibited cell–cell contacts with native alveolar type II cells (Figures 3D and 3E). This suggests that colocalization of SP-C– and GFP-positive immunofluorescence signals resulted from transfer of SP-C–positive cellular material (surfactant protein-containing cellular components) from alveolar type II cells to adjacent GFP-positive donor cells, rather than bona fide conversion of donor cells to alveolar type II cells.
Figure 4.
Confocal fluorescence microscopy of lungs of mice transplanted with GFP-positive marrow cells and lungs of GFP-transgenic mice. (A) Hyperoxia-exposed recipient, Post-TPX Week 8 (P66). Several large-sized and irregularly-shaped GFP-positive cells are seen, contrasting with the smaller and more uniform SP-positive type II cells. Merging of the images shows co-localization of finely granular SP-positive material in the lower GFP-positive cell (arrow). SP-positive material in native type II cells is coarsely granular, consistent with intact lamellar bodies. Colocalization persisted at all three-dimensional volume angles analyzed. Volume slices along the xz (*) and yz (**) axes both show colocalization of green and red signals in the same cell, resulting in a yellow-orange composite signal and confirming the unequivocal presence of SP-positive material in this GFP-positive donor cell. (B) Hyperoxia-exposed recipient, Post-TPX Week 8 (P66). Apparent colocalization of an SP-positive granular structure is seen in a single GFP-positive cell (arrow). Three-dimensional volume analysis at various angles and x/y/z analysis of selected slices demonstrated that the SP-immunoreactive material is located outside of the GFP-positive cell, likely in an underlying type II cell (asterisks). This is an example of “overlay” artifact. (C) Age-matched GFP-transgenic mouse. Coarse granaular SP-positive structures, consistent with surfactant-containing lamellar bodies, are seen in GFP-positive (example shown by arrow) and GFP-negative (example shown by arrowhead) type II cells (pro–SP-C [Cy3, red] and GFP [fluorescein isothiocyanate, green] double immunofluorescence; original magnification: ×400, confocal fluorescence microscopy).
The morphologic appearance of SP-C–immunoreactive material within GFP-positive donor cells was compared with the appearance of type II cells of age-matched mice transgenic for GFP (Figure 4). As shown in Figure 4A, SP-C–positive material in GFP-positive donor cells was generally finely granular and localized to one side of the cell. Figure 4A also illustrates the uniform and relatively small size and spherical shape of native SP-C–positive type II cells. In contrast, the GFP-positive donor-derived cells were generally larger and more irregular, which, in association with their location and propensity to form cell projections, is highly suggestive of macrophages. A three-dimensional volume reconstruction and volume slice with x/y/z axis analysis confirmed location of the SP-C–positive material within the cells (Figure 4A). An example of “overlay” is shown in Figure 4B. Whereas routine epifluorescence microscopy might suggest localization of SP-positive granular material within the GFP-positive donor cell, three-dimensional volume analysis and x/y/z volume slice analysis demonstrated that the SP-positive material was, in fact, contained within a type II cell underlying the GFP-positive cell (Figure 4B). The appearance of SP-C immunoreactivity in alveolar type II cells of GFP-transgenic mice is shown in Figure 4C. In such cells, SP-C–positive structures (lamellar bodies) were coarsely granular, relatively large-sized, and usually evenly distributed over the cytoplasm. Occasionally, SP-C–positive granules were seen in association with the cell membrane, suggestive of secretion. Interestingly, not all type II cells in GFP-transgenic mice were GFP immunoreactive (Figure 4C).
To assess possible transdifferentiation of marrow-derived donor cells to alveolar type I cells, anti-GFP labeling was combined with anti-T1α (8.1.1.) antibody (39, 54) labeling. As shown in Figure 5A, GFP-positive cells were localized superficial to the type I cells, which were identified by membranous anti-T1α immunostaining. Similarly, GFP-positive cells associated with bronchial epithelium were located superficial to CCSP-positive bronchial epithelial Clara cells, as demonstrated by GFP/CCSP double labeling (Figure 5B). Definitive colocalization of CCSP or T1α and GFP immunoreactivity was not observed.
Figure 5.
GFP (green) immunofluorescence combined with T1α, CCSP, and F4/80 (red) labeling. (A) Hyperoxia-exposed recipient, Post-TPX Week 8 (P66). GFP-positive cells are seen superficial (adluminal) to alveolar type I cells, delineated by membranous T1α-staining. No colocalization of T1α and GFP-positive signals is seen. (B) Normoxic recipient, Post-TPX Week 8 (P66). A GFP-positive donor cell is seen overlying CCSP-positive bronchial epithelial (Clara) cells. (C) Hyperoxic recipient, Post-TPX Week 8 (P66). Three GFP-positive cells show complete colocalization of F4/80 and GFP immunofluorescence, identifying these donor-derived cells as macrophages (arrows). In addition, GFP-negative and F4/80-positive native macrophages are noted (arrowheads). T1α, CCSP, and F4/80 (Cy3, red) and GFP (fluorescein isothiocyanate, green) double immunofluorescence; original magnification: ×400).
The above double labeling studies failed to show unequivocal transdifferentiation of marrow cells to respiratory epithelial cells. The size, shape, capacity to change shape, and location of the GFP-immunoreactive cells, as well as their tendency to interact with alveolar and bronchial epithelial cells, were highly suggestive of alveolar macrophages. To assess that possibility, anti-GFP immunofluorescence was combined with immunostaining using an antibody against the pan-macrophage marker, F4/80. We determined that at Post-TPX Weeks 1, 2, and 8, over 95% of GFP-positive cells demonstrated uniform and unequivocal colocalization of GFP and F4/80 positivity, both in normoxic and hyperoxia-exposed recipients (Figure 5C). In addition to GFP-positive macrophages, variable numbers of GFP-negative native macrophages were present (Figure 5C). The total number of macrophages (GFP-positive and GFP-negative) appeared higher in lungs of animals exposed to hyperoxia than normoxia. Of note, the tendency of macrophages to interact with respiratory epithelial cells was not limited to GFP-positive donor-derived macrophages but was also seen with GFP-negative native macrophages.
From these studies, we conclude that intranasal administration of whole bone marrow to normoxic or hyperoxic newborn mice results in the creation of alveolar macrophage chimerism whereby virtually all (if not all) donor-derived GFP-immunoreactive cells in the lung persist as alveolar macrophages. The occasional presence of SP-C–positive material in the cytoplasm of GFP-positive macrophages was suggestive of uptake of SP-C–positive material from native type II cells by these notoriously highly phagocytic cells, likely occurring at the site of the observed intimate cell–cell contacts between macrophages and respiratory epithelial cells.
Analysis of Effects of Adult Marrow Cells in Lungs of Normoxic and Hyperoxic Newborn Mice
Effects on lung and body growth.
At Post-TPX Weeks 1 and 2, the body weights of marrow-treated normoxic pups were 35 to 40% smaller than those of normoxic sham controls, indicating that marrow cell administration had significant adverse effects on somatic growth in the early post-transplantation period (Figure 6). As expected, hyperoxia exposure resulted in markedly lower body weights in hyperoxic sham controls compared with normoxic sham controls at P12 and P19. Contrary to its effects in normoxic animals, bone marrow administration had no obvious early effects on body weight in hyperoxia-exposed animals (Figure 6).
Figure 6.
Body weights. Values represent mean ± SEM of at least three animals per group. *P < 0.01 versus sham controls exposed to same oxygen levels; °P < 0.05 versus normoxic sham controls; °°P < 0.02 versus normoxic sham controls (ANOVA with post hoc Scheffe test).
The effects of marrow treatment on lung growth were assessed first by stereologic volumetry of the volume of air-exchanging parenchyma, V(ae) (52) (Table 1). At all time points studied, V(ae) tended to be smaller in normoxic marrow-treated animals compared with normoxic controls. V(ae) similarly tended to be lower in marrow-treated hyperoxic animals compared with controls. However, this difference was not statistically significant with the sample sizes studied.
The pulmonary growth kinetics of marrow-treated and control mice were further assayed by immunohistochemical analysis of expression of the proliferation marker, Ki-67. At P12, the Ki67 labeling index was significantly higher in hyperoxic sham controls than in normoxic controls (Figure 7). At this time point, the pulmonary proliferative activity was significantly lower in marrow-treated hyperoxia-exposed animals compared with corresponding sham controls. A similar trend was noted in normoxic animals. Ki-67–positive nuclei were distributed over epithelial and interstitial lung tissue. By P19, the pulmonary proliferative activity of marrow-treated hyperoxic animals was similar to that of hyperoxic sham controls, and lower than that of corresponding normoxic animals. By P66, the pulmonary proliferative activity was equally low in all groups (Figure 7).
Figure 7.
Proliferation analysis by Ki67-immunohistochemistry. Top: Immunohistochemical analysis of Ki-67 labeling in lungs of sham- and marrow-treated animals exposed to normoxic or hyperoxic conditions (P12 and P66). (A) Normoxic sham control, P12, showing early alveolarization associated with dramatic cell proliferation. (B) Normoxic marrow-treated animal, P12 (Post-TPX Week 1), showing larger and more simple airspaces and diminished cell proliferation compared with A. (C) Hyperoxia-exposed sham control, P12, showing deficient alveolarization but high levels of proliferative activity. (D) Hyperoxia-exposed marrow-treated animal, P12 (Post-TPX Week 1), showing markedly disrupted alveolar development, resulting in very large and primitive airspaces with little evidence of secondary crest formation. Cell proliferation is decreased compared with C. (E) Normoxic sham control, P19, showing increasing complexity of the alveolar structures. Proliferative activity is diminished compared with A. (F–H) Normoxic marrow-treated animal (F), hyperoxic sham control (G), and hyperoxic marrow-treated animal (H), at P19. Alveolar remodeling is impaired compared with normoxic sham control (E). Ki-67–positive nuclei are distributed over epithelial and interstitial compartments. Proliferative activity appears lower in hyperoxic conditions. (I) Normoxic sham control, P66, showing fully alveolarized lungs with rare scattered Ki-67–positive cells. The morphologic appearance of several Ki-67-positive cells is suggestive of alveolar macrophages (arrows). (J) Normoxic marrow-treated animal, P66 (Post-TPX Week 8), showing sparse Ki-67 reactivity. Focal Ki-67–positive macrophage-like cells are seen (arrows). (K) Hyperoxia-exposed sham control, P66, showing a persistent lack of alveolarization compared with normoxic animals. Ki-67–positive cells are present along the alveolar septa and in cells consistent with macrophages (arrows). (L) Hyperoxia-exposed marrow-treated animal, P66 (Post-TPX Week 8) showing similar alveolar simplification as hyperoxia-exposed controls, associated with rare Ki-67 reactivity. Arrows indicate macrophage-like proliferating cells (Ki-67 immunohistochemistry; 3,3′-diaminobenzidine tetrachloride [DAB] with hematoxylin counterstain; original magnification: ×400). Bottom: Ki-67 labeling index. Values represent mean ± SEM of at least three animals per group. *P < 0.01 versus normoxic sham controls. **P < 0.01 versus hyperoxic sham controls. °P < 0.05 versus corresponding normoxic animals (ANOVA with post hoc Scheffe test).
Effects on alveolar remodeling.
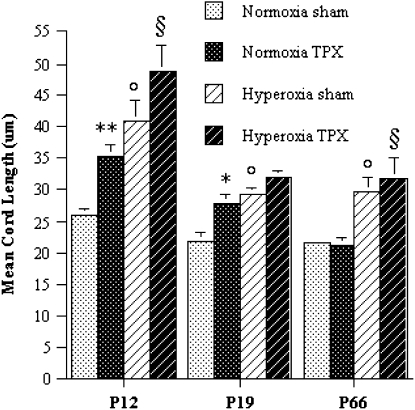
The effects of marrow cell administration on alveolarization were quantified by computer-assisted morphometric assessment of the MCL. At Post-TPX Weeks 1 and 2, the MCL was significantly (25–35%) higher in normoxic marrow-treated animals compared with normoxic controls, suggesting that marrow cell transplantation during the newborn period disrupts normal alveolar remodeling (Figure 8). By Post-TPX Week 8, the MCL was similar in sham- and marrow-treated normoxic animals. Consistent with the known adverse effects of hyperoxia on alveolar remodeling, the MCL of hyperoxic controls was markedly larger than that of normoxic controls at all time points. The MCL of hyperoxic marrow-treated animals tended to be larger than that of hyperoxic controls at all time points, but this difference did not reach statistical significance (Figure 8).
Figure 8.
Morphometric analysis of mean cord length (MCL). Values represent mean ± SEM of at least three animals per group. *P < 0.05 versus sham controls exposed to same oxygen levels. **P < 0.02 versus sham controls exposed to same oxygen levels. °P < 0.02 versus normoxic sham controls. §P < 0.05 versus normoxic marrow-treated animals. (ANOVA with post hoc Scheffe test).
The combined findings suggest that intranasal bone marrow treatment in normoxic conditions has adverse effects on somatic growth, lung growth, and alveolar remodeling during the early post-TPX period. In hyperoxia-exposed recipients, intranasal marrow cells similarly reduced pulmonary proliferation in the immediate post-TPX period, but had no other noticeable effects on somatic growth or alveolar remodeling. It is unclear whether this indicates relative preservation of growth and proliferation in hyperoxic conditions or whether any adverse effects are masked in these already severely growth-restricted lungs. Marrow cell treatment did not prevent or correct alveolarization defects in hyperoxia-exposed newborn mice.
Analysis of Effects of Subsequent Hyperoxic Injury in Previously Marrow-Treated Lungs
To determine the fate of chimeric macrophages at time of subsequent lung injury, animals that had been exposed to hyperoxia and treated with marrow cells in the newborn period were re-exposed to hyperoxia (95% O2) at P66 (Post-TPX Week 8). This study was focused on mice that were hyperoxic during the newborn period because the volume of engrafted cells, V(gfp), at P66 tended to be higher in hyperoxia-exposed animals compared with normoxic animals (Table 1) and, consequently, any effects from this “second-hit” hyperoxia were expected to be more pronounced in these animals. In view of the reported poor tolerance of hyperoxia in adult animals compared with newborn mice (56, 57), the duration of this second exposure to hyperoxia was limited to 48 hours. The animals were killed and the lungs were examined 10 days after hyperoxia (P78). Results were compared with marrow-treated animals exposed to hyperoxia as pups studied at P66 under the presumption that the size of the engrafted cell population undergoes no significant changes between P66 and P78.
Lungs of marrow-treated mice reexposed to “second-hit” hyperoxia at Post-TPX Week 8 displayed large numbers of GFP-positive cells within the airspaces (Figures 9A and 9B). V(gfp), determined by stereologic volumetry, was 65% higher in reexposed hyperoxic marrow recipients compared with marrow recipients exposed to hyperoxia in the newborn period only (Table 2) (2.78 ± 0.41 μl versus 1.68 ± 0.51 μl, P < 0.05). Double immunofluorescence studies using anti-GFP and anti-F4/80 antibodies identified virtually all GFP-positive cells as macrophages (Figure 9B). No unequivocal colocalization of GFP and SP-C labeling was seen (Figure 9A). Reexposure to hyperoxia induced a 40% increase in pulmonary proliferative activity (Table 2). Interestingly, pulmonary Ki-67 positivity in animals reexposed to hyperoxia was often noted in cells with the morphologic appearance of alveolar macrophages (Figure 9C). This suggests that the expansion of the donor-derived macrophage population after exposure to hyperoxia is caused, at least in part, by proliferation of these cells.
Figure 9.
Effects of re-exposure to hyperoxia. (A) SP-C (red) and GFP (green) double immunofluorescence of hyperoxia-exposed marrow recipient, reexposed to hyperoxia from P66 to P68. Abundant donor-derived GFP-positive cells are noted within the airspaces and along the alveolar walls. Occasional granular SP-C–positive material is noted in GFP-positive cells with macrophage morphology, suggestive of phagocytosis of surfactant protein-containing material from alveolar type II cells. (B) F4/80 (red) and GFP (green) double immunofluorescence of hyperoxia-reexposed marrow recipient. Complete colocalization of F4/80 and GFP-positivity is seen in all GFP-positive cells (arrows), identifying these donor-derived cells as macrophages. In addition, several GFP-negative, F4/80-positive native macrophages are seen (arrowheads) (pro–SP-C and F4/80 [Cy3, red] and GFP [fluorescein isothiocyanate, green] double immunofluorescence; original magnification: ×400). (C) Ki-67 labeling of marrow-treated animal, exposed to hyperoxia as newborn (P66). Cells with morphologic appearance of macrophages showing Ki-67 positivity are indicated by arrows. (D) Ki-67 labeling of marrow-treated animal exposed to hyperoxia as newborn and re-exposed to hyperoxia at P66. Increased numbers of proliferating macrophage-like cells are noted (arrows) (Ki-67 immunohistochemistry; DAB with hematoxylin counterstain; original magnification: ×400). The role of marrow-derived cell therapy in neonatal lung injury is largely unknown. We demonstrate that unfractionated adult marrow cells, administered intratracheally to newborn mice, result in a persistent pulmonary macrophage chimerism, without evidence of epithelial or mesenchymal transdifferentiation. Other cell based therapy models remain to be investigated.
TABLE 2.
BIOMETRY AND LUNG MORPHOMETRY OF ANIMALS RE-EXPOSED TO HYPEROXIA
| 10 wk
|
||
|---|---|---|
| Post-TPX interval:
|
P66
|
P78
|
| Age | Hyperoxia × 1 | Hyperoxia × 2 |
| TPX (5) | TPX (6) | |
| Body weight, g | 18.18 ± 0.59 | 20.81 ± 1.39 |
| V(lu), μl | 334.0 ± 22.2 | 379.3 ± 34.8 |
| AA(ae/lu), % | 31.3 ± 1.64 | 33.6 ± 0.88* |
| V(ae), μl | 105.2 ± 11.1 | 126.5 ± 9.2† |
| V(ae)/body weight, μl/g | 5.78 ± 0.56 | 6.64 ± 0.59 |
| AA(gfp/ae), % | 1.54 ± 0.43 | 2.26 ± 0.42 |
| V(gfp), μl | 1.68 ± 0.51 | 2.78 ± 0.41† |
| Ki-67 labeling index, #/HPF | 12.28 ± 2.49 | 16.56 ± 2.97* |
Definition of abbreviations: AA(ae/lu), areal fraction of air-exchanging parenchyma relative to lung parenchyma; AA(gfp/ae), areal fraction of GFP-immunoreactive parenchyma relative to air-exchanging parenchyma; HPF, high-power field; post-TPX interval, number of weeks between marrow cell transplantation (P5) and killing; TPX: marrow cell-transplanted; V(ae), volume of air-exchanging parenchyma; V(gfp), volume of GFP-immunoreactive cells; V(lu), inflated lung volume.
Values represent mean ± SEM of (n) animals per group. Hyperoxia × 1: marrow-treated animals exposed to hyperoxia as newborns only (P1-P7). Hyperoxia × 2: marrow-treated animals exposed to hyperoxia as newborns (P1-P7) and reexposed to hyperoxia at P66 (P66-P68).
P < 0.05 versus hyperoxia × 1 group.
P < 0.02 versus hyperoxia × 1 group.
DISCUSSION
We determined the fate and effects of whole bone marrow cells in lungs of newborn mice. Unfractionated bone marrow cells from GFP-transgenic mice were administered intranasally to normoxic or hyperoxic mice at P5, corresponding to the time point of maximal apoptotic cell death in hyperoxia-exposed newborn mice (personal observations, publication pending). The phenotype of marrow-derived donor cells was assessed by double immunofluorescence studies using anti-GFP antibodies in combination with various cell-specific antibodies. We determined that by Post-TPX Week 1, virtually all engrafted donor-derived cells had an F4/80-immunoreactive macrophage phenotype and appeared morphologically indistinguishable from native resident alveolar macrophages. While others had previously described the presence of donor-derived cells with morphologic features of macrophages/monocytes among other types of donor-derived cells after marrow or stem cell transplantation (42), macrophages appeared to be the major donor-derived cells in the present study. Evidence of transdifferentiation of donor cells to respiratory epithelial cells (alveolar type I or type II cells, bronchial epithelial Clara cells) was not seen at any time point.
Although in concordance with some recent reports (46, 48, 58, 59), the lack of epithelial differentiation in this study is in sharp contrast with earlier reports describing up to 20% donor-derived epithelial cells after marrow or stem cell transplantation (38–43). As has been suggested by others (48), we speculate that these discrepant results may be attributed, at least in part, to variations in interpretation of the fluorescence findings. Indeed, while straightforward in theory, the microscopic co-localization of fluorescent signals can be treacherous, especially in architecturally complex organs such as the lung. In this study, we observed a distinct tendency for alveolar macrophages, whether donor-derived or native, to be closely associated with alveolar and bronchial epithelial cells. Close juxtaposition or superposition of the GFP-positive and cell-specific fluorescent signals may easily be interpreted erroneously as originating from the same cell, a well-known phenomenon termed “overlay.”
In addition to overlay artifacts, we occasionally detected the unequivocal presence of SP-C–immunoreactive material in the cytoplasm of GFP-positive cells, confirmed by confocal microscopy. The colocalization of SP-C and GFP immunoreactivity within the same cell may be interpreted as evidence for alveolar epithelial transdifferentiation of donor-derived cells. However, the GFP-positive cells had typical features of macrophages: they were generally larger than alveolar type II cells, formed cell processes, and were located superficial to respiratory epithelial cells. In addition, cell–cell contacts were often seen between SP-C–containing donor cells and adjacent native alveolar type II cells. These findings suggest that the observed colocalization of SP-C and GFP staining reflects phagocytosis of SP-C–positive material from alveolar type II cells by donor-derived macrophages, presumably facilitated by the observed frequent cell contacts.
Some investigators have previously suggested that the “transdifferentiation” phenomenon is, at least in some cases, the result of cell fusion of marrow-derived cells with existing organ-specific cells (60–62), rather than true transdifferentiation. Fusion of donor bone marrow–derived cells with hepatocytes, Purkinje cells, and cardiomyocytes of the recipient has been documented (60, 61). In the lung, fusion of adult marrow-derived cells with epithelial cells may occur in vitro, but fusion was until now not believed to occur to significant extent in vivo (63, 64). Although we did not formally assess fusion at the nuclear or chromosome level, our findings are consistent with either phagocytosis or cytoplasmic fusion between marrow-derived macrophages and respiratory epithelial cells in vivo in lungs of marrow-treated animals.
We detected no contribution of donor-derived marrow cells to the lung mesenchyme in the present study. Recent studies described rare apparent engraftment of pulmonary interstitium and vasculature after total marrow transplant in a variety of injury models (65, 66). The many variables that may influence epithelial, interstitial, or pulmonary vascular engraftment with donor-derived cells remain to be explored (67). Different injury models, donor-derived cell types, and routes of administration may be associated with different engraftment tendencies (67). It is thus possible that differentiation into mesenchymal (vascular or interstitial) cell lines may be induced or facilitated by systemic, rather than intratracheal delivery of cells.
The source of the donor-derived alveolar macrophages identified in recipient lungs after whole marrow transplantation is unclear. Under normal circumstances most alveolar macrophages are derived from circulating monocytes (68). However, it is unlikely that the donor-derived alveolar macrophages in our study were recruited from the systemic circulation, as we did not find evidence of systemic engraftment of donor-derived marrow cells. Hematopoietic organs of marrow-treated animals, such as bone marrow, spleen, and liver, were consistently found to be devoid of GFP-immunoreactive cells. Macrophages are notoriously long-lived and have a survival time of several months (68, 69). It is therefore plausible that the donor-derived macrophages detected at 2 months after transplantation were introduced as monocytes/macrophages at the time of intranasal administration of whole bone marrow. Alternatively, the macrophages may have differentiated from undetected macrophage precursors/hematopoietic stem cells present within the bone marrow graft.
Previous studies describing the effects of bone marrow–derived stem cells on lung repair have used models of alveolar epithelial lung injury, including total body irradiation (25, 38, 41–43, 49, 70), bleomycin (39, 40), elastase (71, 72), and/or cardiotoxin (43). In noninjured control lungs, lung engraftment by donor cells had been either absent (42) or significantly diminished compared with injured lungs (39, 40). These studies all involved adult recipients, suggesting that successful transplantation in normally quiescent adult lungs may critically depend on the creation of an engraftable niche through injury, proliferation, and repair. In the present study, we determined that the volume of GFP-positive engrafted cells was similar in hyperoxic (injured) and normoxic (noninjured) mice. In fact, V(gfp) tended to be lower in hyperoxic marrow recipients than in normoxic recipients during the first post-transplantation weeks. This suggests that in newborn mice, in contrast with adult mice, injury is not a prerequisite for efficient engraftment. We speculate that the inherently higher cell turnover in developing lungs during the stage of alveolarization provides a milieu conducive to engraftment even without concomitant lung injury.
The ratio of engrafted donor cell volume over total lung volume was very low, ranging from 0.50% at Post-TPX Week 1 to 0.27% at Week 8. In spite of this relatively low ratio, marrow cell administration had distinct—and overall negative—functional effects. In the early post-transplantation period, marrow cell administration in normoxic newborn mice resulted in decreased somatic growth, decreased lung growth, and decreased alveolarization. In hyperoxic newborn animals, marrow cells had no obvious adverse effects, which may be because newborn mice exposed to hyperoxia are intrinsically extremely growth restricted and additional growth-inhibiting effects may therefore be less obvious. The overall adverse effects of marrow cell administration in the present study contradict previous reports describing at least partial protection against the effects of lung injury in animals treated with a range of bone marrow–derived cell populations (40, 44, 45, 71, 73, 74). The reasons for these discrepant results are unclear, but may include different marrow cell populations, different lung injury models, and a different temporal relationship between marrow cell administration and injury.
While our model does not provide structural epithelial reconstitution by marrow cells, the GFP-positive macrophage chimera may provide a powerful model to study the role and regulation of interactions between alveolar macrophages and alveolar or bronchial epithelial cells in injury and repair. In our study, the strong GFP immunoreactivity of the donor-derived alveolar macrophages allowed high resolution visualization of the close interactions between alveolar macrophages and respiratory epithelial cells. Adherence between alveolar epithelium and alveolar macrophages has been described in a variety of conditions (75–77) and is believed to be pivotal in the regulation of function of both cell types in injury and repair (78, 79).
Our observations raise interesting but speculative and unexplored therapeutic possibilities. While unlikely to result in direct tissue remodeling, the long-lasting macrophage chimera has potential indirect beneficial effects that may provide a viable therapeutic approach for treatment of lung diseases characterized by quantitative or qualitative alveolar macrophage deficiencies. The longevity of transplanted alveolar macrophages and their apparent capacity for replication makes use of these cells in human disorders characterized by defective or deficient pulmonary macrophages, such as alveolar proteinosis and pulmonary storage diseases, an attractive possibility, if only for temporary relief.
In conclusion, we determined that intranasal administration of adult bone marrow cells to newborn mice resulted in stable alveolar macrophage chimerism, without evidence of transdifferentiation of marrow-derived cells to respiratory epithelial cells. The engraftment efficiency was similar in normoxic and hyperoxia-exposed pups, suggesting that in newborn animals, in contrast to adult animals, lung injury is not required for effective engraftment of marrow cells. Marrow cell administration had adverse effects on somatic and lung growth during the early post-transplantation period, likely related to paracrine effects originating from the engrafting macrophages. “Second-hit” exposure to hyperoxia of adult mice engrafted as newborns resulted in expansion of donor-derived macrophage population, suggesting that these cells remain capable of activation and replication. While intranasal administration of whole marrow cells may not have a role in reconstructive therapy for neonatal lungs, its use for gene product delivery and alveolar macrophage reconstitution remains undetermined. Furthermore, the role of selected stem cell populations and/or alternate routes of administration for cell-based therapy for injured newborn lungs deserves further investigation.
Acknowledgments
The generous gift by an anonymous donor designated for stem cell research at Women and Infants Hospital is gratefully acknowledged. The 8.1.1 antibody developed by A. Farr was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
This work was supported in part by National Institutes of Health grants P20-RR18728 (J.F.P., M.E.D.P.), and K08 HL86868 (J.M.A.), and by a Stem Cell Research Grant to Women and Infants Hospital (M.E.D.P.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0176OC on November 6, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev 1998;53:81–94. [DOI] [PubMed] [Google Scholar]
- 2.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–1729. [DOI] [PubMed] [Google Scholar]
- 3.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet 2006;367:1421–1431. [DOI] [PubMed] [Google Scholar]
- 4.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 1998;29:710–717. [DOI] [PubMed] [Google Scholar]
- 5.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 1999;46:641–643. [DOI] [PubMed] [Google Scholar]
- 6.Hargitai B, Szabo V, Hajdu J, Harmath A, Pataki M, Farid P, Papp Z, Szende B. Apoptosis in various organs of preterm infants: histopathologic study of lung, kidney, liver, and brain of ventilated infants. Pediatr Res 2001;50:110–114. [DOI] [PubMed] [Google Scholar]
- 7.Lukkarinen HP, Laine J, Kaapa PO. Lung epithelial cells undergo apoptosis in neonatal respiratory distress syndrome. Pediatr Res 2003;53:254–259. [DOI] [PubMed] [Google Scholar]
- 8.May M, Strobel P, Preisshofen T, Seidenspinner S, Marx A, Speer CP. Apoptosis and proliferation in lungs of ventilated and oxygen-treated preterm infants. Eur Respir J 2004;23:113–121. [DOI] [PubMed] [Google Scholar]
- 9.De Paepe ME, Gundavarapu S, Tantravahi U, Pepperell JR, Haley SA, Luks FI, Mao Q. Fas-ligand-induced apoptosis of respiratory epithelial cells causes disruption of postcanalicular alveolar development. Am J Pathol 2008;173:42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci USA 1997;94:4080–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998;279:1528–1530. [DOI] [PubMed] [Google Scholar]
- 12.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science 1999;284:1168–1170. [DOI] [PubMed] [Google Scholar]
- 13.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 1999;100:II247–II256. [DOI] [PubMed] [Google Scholar]
- 14.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 2000;290:1779–1782. [DOI] [PubMed] [Google Scholar]
- 15.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 2000;6:1229–1234. [DOI] [PubMed] [Google Scholar]
- 16.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology 2000;31:235–240. [DOI] [PubMed] [Google Scholar]
- 17.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701–705. [DOI] [PubMed] [Google Scholar]
- 18.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg 2002;73:1919–1925. (discussion 1926). [DOI] [PubMed] [Google Scholar]
- 19.Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol 2002;174:11–20. [DOI] [PubMed] [Google Scholar]
- 20.Bailey AS, Jiang S, Afentoulis M, Baumann CI, Schroeder DA, Olson SB, Wong MH, Fleming WH. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood 2004;103:13–19. [DOI] [PubMed] [Google Scholar]
- 21.Almeida-Porada G, Porada CD, Chamberlain J, Torabi A, Zanjani ED. Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood 2004;104:2582–2590. [DOI] [PubMed] [Google Scholar]
- 22.Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, Hanley A, Scadova H, Qin G, Cha DH, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest 2005;115:326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawn B, Bolli R. Adult bone marrow-derived cells: regenerative potential, plasticity, and tissue commitment. Basic Res Cardiol 2005;100:494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira RF, Halford KW, O'Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci USA 1995;92:4857–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–49. [DOI] [PubMed] [Google Scholar]
- 26.Wagers AJ, Christensen JL, Weissman IL. Cell fate determination from stem cells. Gene Ther 2002;9:606–612. [DOI] [PubMed] [Google Scholar]
- 27.Korbling M, Estrov Z, Champlin R. Adult stem cells and tissue repair. Bone Marrow Transplant 2003;32:S23–S24. [DOI] [PubMed] [Google Scholar]
- 28.Prockop DJ. Further proof of the plasticity of adult stem cells and their role in tissue repair. J Cell Biol 2003;160:807–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood 2003;102:3483–3493. [DOI] [PubMed] [Google Scholar]
- 30.Anjos-Afonso F, Siapati EK, Bonnet D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J Cell Sci 2004;117:5655–5664. [DOI] [PubMed] [Google Scholar]
- 31.Neuringer IP, Randell SH. Stem cells and repair of lung injuries. Respir Res 2004;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tallheden T, Brittberg M, Peterson L, Lindahl A. Human articular chondrocytes–plasticity and differentiation potential. Cells Tissues Organs 2006;184:55–67. [DOI] [PubMed] [Google Scholar]
- 33.Suratt BT, Cool CD, Serls AE, Chen L, Varella-Garcia M, Shpall EJ, Brown KK, Worthen GS. Human pulmonary chimerism after hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003;168:318–322. [DOI] [PubMed] [Google Scholar]
- 34.Albera C, Polak JM, Janes S, Griffiths MJ, Alison MR, Wright NA, Navaratnarasah S, Poulsom R, Jeffery R, Fisher C, et al. Repopulation of human pulmonary epithelium by bone marrow cells: a potential means to promote repair. Tissue Eng 2005;11:1115–1121. [DOI] [PubMed] [Google Scholar]
- 35.Mattsson J, Jansson M, Wernerson A, Hassan M. Lung epithelial cells and type II pneumocytes of donor origin after allogeneic hematopoietic stem cell transplantation. Transplantation 2004;78:154–157. [DOI] [PubMed] [Google Scholar]
- 36.Kleeberger W, Versmold A, Rothamel T, Glockner S, Bredt M, Haverich A, Lehmann U, Kreipe H. Increased chimerism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. Am J Pathol 2003;162:1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spencer H, Rampling D, Aurora P, Bonnet D, Hart SL, Jaffe A. Transbronchial biopsies provide longitudinal evidence for epithelial chimerism in children following sex mismatched lung transplantation. Thorax 2005;60:60–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369–377. [DOI] [PubMed] [Google Scholar]
- 39.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 2001;128:5181–5188. [DOI] [PubMed] [Google Scholar]
- 40.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 2003;100:8407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, Badve S, Saxena R, Krause DS. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol 2002;30:1333–1338. [DOI] [PubMed] [Google Scholar]
- 42.Abe S, Lauby G, Boyer C, Rennard SI, Sharp JG. Transplanted BM and BM side population cells contribute progeny to the lung and liver in irradiated mice. Cytotherapy 2003;5:523–533. [DOI] [PubMed] [Google Scholar]
- 43.Aliotta JM, Keaney P, Passero M, Dooner MS, Pimentel J, Greer D, Demers D, Foster B, Peterson A, Dooner G, et al. Bone marrow production of lung cells: the impact of G-CSF, cardiotoxin, graded doses of irradiation, and subpopulation phenotype. Exp Hematol 2006;34:230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 2005;33:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 2007;179:1855–1863. [DOI] [PubMed] [Google Scholar]
- 46.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 2005;33:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 2002;297:2256–2259. [DOI] [PubMed] [Google Scholar]
- 48.Chang JC, Summer R, Sun X, Fitzsimmons K, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol 2005;33:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grove JE, Lutzko C, Priller J, Henegariu O, Theise ND, Kohn DB, Krause DS. Marrow-derived cells as vehicles for delivery of gene therapy to pulmonary epithelium. Am J Respir Cell Mol Biol 2002;27:645–651. [DOI] [PubMed] [Google Scholar]
- 50.McGrath-Morrow SA, Stahl J. Apoptosis in neonatal murine lung exposed to hyperoxia. Am J Respir Cell Mol Biol 2001;25:150–155. [DOI] [PubMed] [Google Scholar]
- 51.De Paepe ME, Johnson BD, Papadakis K, Luks FI. Lung growth response after tracheal occlusion in fetal rabbits is gestational age-dependent. Am J Respir Cell Mol Biol 1999;21:65–76. [DOI] [PubMed] [Google Scholar]
- 52.De Paepe ME, Johnson BD, Papadakis K, Sueishi K, Luks FI. Temporal pattern of accelerated lung growth after tracheal occlusion in the fetal rabbit. Am J Pathol 1998;152:179–190. [PMC free article] [PubMed] [Google Scholar]
- 53.Aherne WA, Dunnill MS. The estimation of whole organ volume. In: Aherne WA, Dunnill MS, editors. Morphometry. London: Edward Arnold Ltd.; 1982. pp. 10–18.
- 54.Farr AG, Berry ML, Kim A, Nelson AJ, Welch MP, Aruffo A. Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. J Exp Med 1992;176:1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000;182:311–322. [DOI] [PubMed] [Google Scholar]
- 56.Frank L, Bucher JR, Roberts RJ. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol 1978;45:699–704. [DOI] [PubMed] [Google Scholar]
- 57.Yam J, Frank L, Roberts RJ. Oxygen toxicity: comparison of lung biochemical responses in neonatal and adult rats. Pediatr Res 1978;12:115–119. [DOI] [PubMed] [Google Scholar]
- 58.Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med 2006;173:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacPherson H, Keir PA, Edwards CJ, Webb S, Dorin JR. Following damage, the majority of bone marrow-derived airway cells express an epithelial marker. Respir Res 2006;7:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 2003;422:897–901. [DOI] [PubMed] [Google Scholar]
- 61.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature 2003;422:901–904. [DOI] [PubMed] [Google Scholar]
- 62.Vassilopoulos G, Russell DW. Cell fusion: an alternative to stem cell plasticity and its therapeutic implications. Curr Opin Genet Dev 2003;13:480–485. [DOI] [PubMed] [Google Scholar]
- 63.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci USA 2003;100:2397–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science 2004;305:90–93. [DOI] [PubMed] [Google Scholar]
- 65.Spees JL, Pociask DA, Sullivan DE, Whitney MJ, Lasky JA, Prockop DJ, Brody AR. Engraftment of bone marrow progenitor cells in a rat model of asbestos-induced pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raoul W, Wagner-Ballon O, Saber G, Hulin A, Marcos E, Giraudier S, Vainchenker W, Adnot S, Eddahibi S, Maitre B. Effects of bone marrow-derived cells on monocrotaline- and hypoxia-induced pulmonary hypertension in mice. Respir Res 2007;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weiss DJ, Kolls JK, Ortiz LA, Panoskaltsis-Mortari A, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc 2008;5:637–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol 2001;70:163–170. [PubMed] [Google Scholar]
- 69.Murphy J, Summer R, Wilson AA, Kotton DN, Fine A. The prolonged life-span of alveolar macrophages. Am J Respir Cell Mol Biol 2008;38:380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004;113:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett 2004;556:249–252. [DOI] [PubMed] [Google Scholar]
- 72.Kuang PP, Lucey E, Rishikof DC, Humphries DE, Bronsnick D, Goldstein RH. Engraftment of neonatal lung fibroblasts into the normal and elastase-injured lung. Am J Respir Cell Mol Biol 2005;33:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol 2004;172:1266–1272. [DOI] [PubMed] [Google Scholar]
- 74.McCarter SD, Mei SH, Lai PF, Zhang QW, Parker CH, Suen RS, Hood RD, Zhao YD, Deng Y, Han RN, et al. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med 2007;175:1014–1026. [DOI] [PubMed] [Google Scholar]
- 75.Beck-Schimmer B, Schimmer RC, Madjdpour C, Bonvini JM, Pasch T, Ward PA. Hypoxia mediates increased neutrophil and macrophage adhesiveness to alveolar epithelial cells. Am J Respir Cell Mol Biol 2001;25:780–787. [DOI] [PubMed] [Google Scholar]
- 76.Beck-Schimmer B, Madjdpour C, Kneller S, Ziegler U, Pasch T, Wuthrich RP, Ward PA, Schimmer RC. Role of alveolar epithelial ICAM-1 in lipopolysaccharide-induced lung inflammation. Eur Respir J 2002;19:1142–1150. [DOI] [PubMed] [Google Scholar]
- 77.Hirano S. Interaction of rat alveolar macrophages with pulmonary epithelial cells following exposure to lipopolysaccharide. Arch Toxicol 1996;70:230–236. [DOI] [PubMed] [Google Scholar]
- 78.Striz I, Slavcev A, Kalanin J, Jaresova M, Rennard SI. Cell-cell contacts with epithelial cells modulate the phenotype of human macrophages. Inflammation 2001;25:241–246. [DOI] [PubMed] [Google Scholar]
- 79.Hjort MR, Brenyo AJ, Finkelstein JN, Frampton MW, LoMonaco MB, Stewart JC, Johnston CJ, D'Angio CT. Alveolar epithelial cell-macrophage interactions affect oxygen-stimulated interleukin-8 release. Inflammation 2003;27:137–145. [DOI] [PubMed] [Google Scholar]