Abstract
We investigated the mechanisms by which respiratory syncytial virus (RSV) infection decreases vectorial Na+ transport across respiratory epithelial cells. Mouse tracheal epithelial (MTE) cells from either BALB/c or C57BL/6 mice and human airway H441 cells were grown on semipermeable supports under an air–liquid interface. Cells were infected with RSV-A2 and mounted in Ussing chambers for measurements of short-circuit currents (Isc). Infection with RSV for 24 hours (multiplicity of infection = 1) resulted in positive immunofluorescence for RSV antigen in less than 10% of MTE or H441 cells. In spite of the limited number of cells infected, RSV reduced both basal and amiloride-sensitive Isc in both MTE and H441 cells by approximately 50%, without causing a concomitant reduction in transepithelial resistance. Agents that increased intracellular cAMP (forskolin, cpt-CAMP, and IBMX) increased mainly Cl− secretion in MTE cells and Na+ absorption in H441 cells. RSV infection for 24 hours blunted both variables. In contrast, ouabain sensitive Isc, measured across apically permeabilized H441 monolayers, remained unchanged. Western blot analysis of H441 cell lysates demonstrated reductions in α- but not γ-ENaC subunit protein levels at 24 hours after RSV infection. The reduction in amiloride-sensitive Isc in H441 cells was prevented by pretreatment with inhibitors of de novo pyrimidine or purine synthesis (A77-1726 and 6-MP, respectively, 50 μM). Our results suggest that infection of both murine and human respiratory epithelial cells with RSV inhibits vectorial Na+ transport via nucleotide release. These findings are consistent with our previous studies showing reduced alveolar fluid clearance after RSV infection of BALB/c mice.
Keywords: short circuit current, epithelial Na+ channels, H441 cells, uridine triphosphate, A77-1726
CLINICAL RELEVANCE
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract disease in infants and children worldwide. We have conducted in vitro studies to elucidate the mechanisms by which RSV causes fluid accumulation in the lungs.
Respiratory syncytial virus (RSV) is a member of the pneumovirus genus of the paramyxoviridae, and has a negative-sense, nonsegmented, single-stranded RNA genome. It is the most common cause of lower respiratory tract disease in infants and children worldwide (1), a frequent initiator of acute asthma exacerbations in young children, and has a disease impact comparable to that of nonpandemic influenza A in the elderly (2). In the United States, 50 to 70% of all infants are infected with RSV in the first year of life, and approximately 2 to 3% of all cases of RSV bronchiolitis result in severe hypoxemia, requiring hospitalization (3). RSV infections are responsible for a significant fraction of community-acquired lower respiratory track infections among adults (4) and cause significant morbidity and mortality in immunocompromised adults (5). Murata and Falsey (6) reported that approximately 170,000 hospitalizations and 10,000 deaths associated with RSV occur annually in persons over 65 years old in the United States alone. Importantly, repeated infections are common in all age groups, and previous infection is not protective.
There is no safe and effective RSV vaccine currently available, and specific antiviral pharmacotherapy for RSV is of questionable efficacy (7). In some cases, RSV infections are known to progress to acute respiratory distress syndrome (8). Thus, understanding the basic mechanisms responsible for RSV lung pathology may lead to new modes of treatment.
Respiratory epithelial cells are the primary replication site for RSV (9). Active, vectorial transport of sodium (Na+) and chloride (Cl−) ions across the airway lumen are seminal functions of bronchoalveolar epithelial cells, and is vital for clearance of fluid from airspaces as well as the proper hydration of mucus (10, 11). Previously we have shown that infection of BALB/c mice with RSV impaired alveolar fluid clearance (AFC, an in vivo measure of the ability of the bronchoalveolar epithelium to actively transport Na+ ions) at 48 to 96 hours after infection, which results in increased lung water and hypoxemia (12, 13). Nasal potential differences (NPD) in RSV-infected mice also became more positive, reflecting a decreases of either Cl− secretion or Na+ absorption across nasal epithelial cells (12). Concomitantly, RSV infection also increases levels of uridine and adenosine-5′-triphosphate (UTP and ATP, respectively) in bronchoalveolar lavage fluid of BALB/c mice (12).
Although our in vivo studies have provided much useful information regarding the effects of RSV infection on bronchoalveolar epithelial cell Na+ transport and lung fluid clearance, measurements of AFC and NPD (and even their amiloride-sensitive components) provide only limited information as to mechanisms by which RSV decreases active epithelial Na+ transport. Therefore, we have been unable to fully elucidate whether this effect is due to damage of apical epithelial Na+ channels transporters (mainly ENaC) or the basolaterally located Na+/K+ ATPase. Furthermore, it remains unclear from these in vivo studies whether altered AFC after RSV infection is a consequence of viral replication per se or results from the inflammatory response to the virus (12).
Herein, we isolated mouse tracheal epithelial cells from either C57BL/6 or BALB/c mice using two different methods based on the original report of Clarke and coworkers (14). We then infected both MTE and H441 cells, a human Clara cell line that expresses both ENaC and CFTR (15) with RSV strain A2, and measured both basal and forskolin-stimulated short circuit currents (Isc)and levels of α- and γ-ENaC by immunofluorescence and Western blotting. These measurements were then repeated in H441 cells after inhibition of purine and pyrimidine synthesis. Our findings indicate that replicating RSV virus per se decreases vectorial amiloride-sensitive Na+ transport by inhibiting ENaC and not Na,K-ATPase via UTP-related mechanisms, in spite of infecting a small fraction of epithelial cells. Furthermore, agents that increase intracellular cAMP increase vectorial Na+ and Cl− transport across RSV-infected monolayers, but their effect is considerably blunted compared with mock-infected monolayers. These findings provide new insights as to the mechanisms by which RSV damages vectorial Na+ transport in vivo.
MATERIALS AND METHODS
Preparation of Viral Inocula
Viral stocks were grown in monolayers of HEp-2 cells. Cells and media were harvested when 80 to 90% cytopathic effect was observed, and centrifuged to remove cell debris. Media was further clarified by ultracentrifugation through 35% sucrose (16). Virions were resuspended in fresh media, aliquoted, and rapidly frozen at −80°C. Viral titers were determined by serial dilution and plaque assay in Vero cells under agar (17). Plaque-forming units (PFU/ml of original sample) were calculated. Virus preparations were checked for mycoplasmal contamination by PCR using the Mycoplasma Plus PCR primer set (Stratagene, La Jolla, CA) and HotStarTaq DNA polymerase (Qiagen, Valencia, CA), in accordance with manufacturer's instructions. Endotoxin content of viral stocks was determined by a standard Limulus amebocyte assay. Stocks in which mycoplasmal or endotoxin contamination were detected were discarded. A mock-infected HEp-2 media stock, prepared in an identical fashion, served as a control to account for possible effects of cellular components in the viral inoculum.
Preparation of Mouse Tracheal Epithelial Cell Monolayers
We used two different protocols to isolate mouse tracheal epithelial cells from both BALB/c and C57Bl/6 mice. Both protocols are based on the original methodology of Clarke and colleagues (14) with important modifications. As described below and in Results, MTE cells isolated with method A have low baseline Isc that were partially inhibited by amiloride, while those isolated by method B have higher Isc that were almost completely inhibited by amiloride. These differences are due entirely to composition of the culture media, since similar results were obtained with method B from either species. Furthermore, BALB/c and C57BL/c mice have very similar levels of AFC and NDP values (13, 18). These two methods are described in detail below:
Method A.
C57BL/6 mice (male, 8–12 wk old; 20–25 g body weight [BW]) were killed with intraperitoneal injections of ketamine (8.7 mg/100 g BW; Phoenix Scientific, St. Joseph, MO) and xylazine (1.3 mg/100 g BW; Vedco, St. Joseph, MO). The trachea proximal to the bronchial bifurcation was isolated and removed. The tracheae were then dissected and placed into 50-ml conical tubes and washed twice with 5 ml of Ca2+- and Mg2+-free phosphate-buffered saline (PBS) containing 500 U/ml penicillin, 500 μM streptomycin, 500 ng/ml fungizone, and 25 μg/ml gentamycin. Subsequently they were washed three times with 5 ml of Ca2+- and Mg2+-free Dulbecco's modified Eagle's medium (DMEM) containing the same antibiotics. While immersed in the second medium, tracheae were dissected further to remove trachealis muscle and extraneous connective tissue and were filleted open.
The tracheae were then immersed in a digestion medium (on ice) consisting of Ca2+- and Mg2+-free DMEM, containing the aforementioned antibiotics and 1% Protease XIV and 0.05% DNase I and incubated without agitation overnight at 4°C. Fetal bovine serum (FBS) (10%) was added subsequently to quell enzyme activity. Tracheae-containing conical tubes were inverted 22 times to dislodge cells, and the supernatant was collected. An equal volume of DMEM/F12 culture medium (containing in addition to the above reagents, 12% FBS, 5 ml of 100X L-glutamine stock, 200 U/ml penicillin/200 μM streptomycin, 250 ng/ml fungizone and 12.5 μg/ml gentamycin, 2 ml of an insulin-transferrin-selenium stock, 10 μg/ml hydrocortisone, 3.5 μg/ml bovine pituitary extract, and 3.5 μg/ml endothelial cell growth supplement) with 10% FBS was added and the tracheae were washed by inversion 22 times. The supernatant was collected and placed on ice. Fresh and warmed digestion medium (5 ml per trachea) was placed into the conical tubes containing the tracheae. The suspension was incubated for 1 hour at 37°C. FBS (10%) was added subsequently to quell enzyme activity. Tracheae-containing conical tubes were inverted 22 times to dislodge cells and the supernatant was collected. Equal volume of DMEM/F12 culture medium was added and the tracheae were washed again by inversion 22 times. The supernatants from all four stages (two digestions, two washes) were combined, as they all had visible cell pellets.
The resultant pooled cell pellet was resuspended in the residual medium in the bottom of the tube. Additional medium was added to seed approximately 100 μl of MTE cell suspension per 6.5-mm-diameter Transwell polyester clear filter support (Corning-Costar, Corning, NY). A day before seeding, the filter supports were coated with a proprietary extracellular matrix solution containing collagens, fibronectin, laminin, and bovine serum albumin (BSA). Each trachea generated enough cells to seed three-four filters.
Seeded MTE cells were undisturbed for 3 days to allow attachment and initial growth. After 72 hours, the apical medium was removed and monolayers were cultured with an air–liquid interface. Early indicators of monolayer formation included reduced basolateral to apical fluid leak along with the development of three-dimensional structures, including ridges and domes, by visual inspection. At this time, measurements of transepithelial resistance (RTE) and transepithelial voltage (VTE) were also conducted. Monolayers that developed ridges and domes, and RTE and VTE values higher than 1,000 Ohms and 5 mV, respectively (usually within 5–10 d after seeding), were used for Ussing chamber experiments described below.
Method B.
BALB/c and C57BL/7 mice were killed with intraperitoneal injections of ketamine (8.7 mg/100 g BW; Phoenix Scientific) and xylazine (1.3 mg/100 g BW; Vedco). The trachea proximal to the bronchial bifurcation was isolated and removed. The resected section was immediately placed in PBS. Under a dissecting microscope, esophageal remnants and adherent adipose tissue were removed by sharp dissection, and the tracheal sections were opened longitudinally. The tracheal sections were rinsed three times in PBS and then placed in fresh MEM containing 0.1% protease XIV (Sigma, St. Louis, MO), 0.01% DNase (Sigma), and 1% FBS at 4°C for 24 hours. FBS (0.5 vol) was added to the isolation to inhibit the protease, and cells were concentrated by centrifugation (500 × g, 5 min). The cells were washed twice in fresh MEM containing 5% FBS, then seeded onto 6.5-mm-diameter Transwell filters (3413; Corning-Costar) in a density of 0.8–1.5 × 105 cells/cm2 (∼ 3–6 filter per mouse). Before seeding, the inserts were collagen-coated by incubating them with a solution containing 0.1 g/L type VI collagen (C-7521; Sigma) and 2 ml/L glacial acetic acid at 37°C for 24 hours. MTE cells were grown in a 1:1 mixture of 3T3 fibroblast preconditioned DMEM (containing 10% FBS, 1% penicillin/streptomycin) and Ham's F-12 medium, supplemented with 10 μg/ml insulin, 1 μM hydrocortisone, 3.75 μg/ml endothelial cell growth supplement, 25 ng/ml epidermal growth factor, 30 nM triiodothyronine, 5 μg/ml iron saturated transferrin, and 10 ng/ml cholera toxin. The culture media on the basolateral side of the filters was replaced every 48 hours. Starting 4 days after seeding, fluid was removed from the apical side every time the basolateral culture medium was changed, and cells were cultured for 3 to 5 additional days at an air–liquid interface to enhance proper differentiation and polarization (19). Monolayers that remained leaky and did not prevent basolateral culture medium from seeping through to their apical surfaces were discarded.
Preparation of H441 Cell Monolayers
The NCI-H441 cell line (HTB-174; ATCC, Manassas, VA) is derived from a papillary adenocarcinoma of human lung Clara cells. Cells between passages 82 and 97 were grown at 37°C in a humidified atmosphere of 5% CO2 in air in RPMI 1640 medium with 2 mM L-glutamine, 1 mM sodium pyruvate, 2.0 g/L glucose, 10% FBS, penicillin (100 units/ml), and streptomycin (100 μg/ml), as previously described (15). Confluent cells were isolated from T-75 tissue culture flasks (Costar, Cambridge, MA) using 0.05% trypsin and 0.53 mM EDTA, and were seeded at a density of 1 × 106 cells/cm2 on 12-mm Millicell PCF filters (Millipore, Bedford, MA), which had been pretreated with human placental collagen. H441 cells were grown at an air–liquid interface for 8 to 16 days in RPMI 1640 medium containing 8.5% FBS and 100 nM dexamethasone, supplemented with 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenium sulfides (20).
Monolayer Infection Protocol
Confluent MTE or H441 cell monolayers on filters at an air–liquid interface were infected with 106 PFU of RSV strain A2, in 100 μl of media (approximate multiplicity of infection of 1, as assessed by cell counting). RSV was allowed to adhere to cells for 60 minutes, at which time the apical solution was aspirated and discarded. Basolateral media was also replaced at that time. In some cases, the UTP and ATP synthesis inhibitors A77-1726 and 6-MP (50 μM) were added into the basolateral media of H441 cells, 24 hours before infection with RSV. In this case, the basolateral media was replaced and fresh RPMI medium containing A77-1726 was added at the time of infection with RSV. Control wells were pretreated with an equal volume of DMSO (pH adjusted to 7.9 with 2 M Na2HPO4, respectively).
Ussing Chamber Measurements of Monolayer Isc and RTE
MTE or H441 cell monolayers with RTE exceeding 0.3 kΩ x cm2 were mounted in Ussing chambers (Jim's Instruments, Iowa City, IA) and bathed on both sides with solutions containing: 120 mM NaCl, 25 mM NaHCO3, 3.3 mM KH2PO4, 0.83 mM K2HPO4, 1.2 mM CaCl2, 1.2 mM MgCl2, and 10 mM HEPES (Na+-free), with 10 mM mannitol (apical compartment) or 10 mM glucose (basolateral compartment), as previously described (21, 22). Osmolarity of all solutions, as measured by a vapor pressure osmometer (Wescor, Inc., Logan, UT) was between 290 and 300 mOsm. Bath solutions were stirred vigorously by bubbling them continuously with 95% O2, 5% CO2 at 37°C (pH 7.4). Monolayers were short-circuited to 0 mV and basal and amiloride-sensitive Isc were continuously measured with an epithelial voltage clamp (VCC-600; Physiologic Instruments, San Diego, CA). A 10-mV pulse of 1-second duration was imposed every 10 seconds to monitor RTE, which was calculated using Ohm's law. Data were collected using the Acquire and Analyze program, version 1.45 (Physiologic Instruments). ΔIsc was calculated from the difference between the initial Isc value (baseline) and the value after adding a drug (amiloride, forskolin, UTP).
RT-PCR for RSV
Total RNA was isolated from H441 cell monolayers using the RNeasy Mini kit (Qiagen, Valencia, CA), according to manufacturer's instructions. cDNAs were generated by reverse transcription, using the Cells-to-cDNA kit (Ambion, Austin, TX). Negative control reactions (for genomic DNA contamination) were performed in the absence of reverse transcriptase. PCR was performed using the HotStarTaq Master Mix PCR kit (Qiagen) in accordance with manufacturer's instructions. After a hot start (3 min at 95°C), 2.5 μl of each cDNA was subjected to 40 cycles of PCR (30 s each at Tm 95°C, Ta 55°C, Te 72°C, per cycle) in 25 μl reaction volume on an Eppendorf Mastercycler (Eppendorf, Westbury, NY), using 100 pmol of primers specific for a 1,200-nt segment of the RSV L gene (a gift from Dr. Tara Cartee, Dept. of Microbiology, UAB) (23). The forward primer, GATTATATAG ATATCACATGGGTGGCATCG, corresponded to bases 10,800 to 10,829 and the reverse primer, CCTCATCATCTCAGTGGCTCTATCAATATCTG, corresponded to the reverse complement of bases 11,976 to 12,007 in the A2 virus genome. Reactions containing RNase-free water in place of cDNA were also performed, as negative controls for contamination with junk DNA. PCR products were visualized by electrophoresis by a standard protocol.
Measurement of Lactate Dehydrogenase Release
Two hundred microliters of normal saline was overlaid onto dry confluent MTE cell monolayers on Transwell filters. After incubation for 2 hours, this overlay fluid was aspirated and stored at −20°C for subsequent analysis. Lactate dehydrogenase (LDH) content of overlay fluid was measured using an LDH colorimetric end-point assay kit (Sigma).
Western Blotting Protocol
Control and RSV-infected cells were lysed in 500 μl of lysis buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 1% Triton X-100) supplemented with protease inhibitor cocktail (Complete Mini, EDTA-free; Roche Diagnostics, Mannheim, Germany), then cleared by centrifugation at 16,000 × g for 10 minutes at 4°C. The supernatant was then carefully removed and the protein concentration in all preparations was measured by the BCA method using BSA as a standard. All protein samples were stored at −80°C before use. H441 cell proteins were separated by SDS-PAGE (8%), transferred to PVDF membranes, and probed overnight at 4°C with 1:1,000 dilutions of rabbit primary antibodies to α-ENaC (PA1–920; Affinity Bioreagents, Golden, CO) or γ-ENaC (ab3468; Abcam, Cambridge, MA), stripped, and re-probed for β-actin (Cell Signaling Technology, Danvers, MA). Bound primary antibodies were detected with HRP-conjugated goat anti-rabbit secondary antibodies and enhanced chemiluminescence (GE Healthcare Life Sciences, Piscataway, NJ), followed by exposure to X-ray film. Band intensity was measured using AlphaEaseFC on a FluorChem imager (Alpha Innotech, San Leandro, CA) and normalized to β-actin levels.
Immunofluorescence
H441 and MTE cell monolayers on filters were rinsed in cold PBS three times, fixed in 3% formaldehyde in PBS for 45 min at room temperature, then post-fixed and permeabilized by addition of 0.5% triton X-100 at room temperature for 3 to 5 minutes. Nonspecific binding was minimized by incubating MTE or H441 cells with blocking buffer (5%BSA in PBS and 1:20 normal goat serum in PBS) for 60 minutes at room temperature. RSV was detected using a mouse monoclonal anti-RSV antibody (MTE cells: 1:50 dilution, for 60 min at 37°C; H441 cells: 1:100 dilution overnight at 4°C; Fitzgerald Industries, Concord, MA:), while α-ENaC was detected with a rabbit polyclonal antibody (Affinity Bioreagents, Golden, CO), diluted 1:100 and incubated overnight at 4°C. Nonimmune mouse or rabbit IgG (both 10 μg/ml; Sigma) in blocking buffer served as nonimmune controls. Bound primary antibodies were detected with secondary goat anti-mouse IgG conjugated to AlexaFluor 488 (1:500; Molecular Probes, Eugene, OR) and goat anti-rabbit IgG conjugated to AlexaFluor 594 (1:100; Molecular Probes) for 2 hours at room temperature in the dark. Slides were washed and counterstained with Hoechst 33258 (20 μg/ml in PBS) for 5 minutes at room temperature to identify nuclei. Filter membranes bearing the cell monolayer were either mounted and sealed on glass slides upside down for en face optical sections (XY plane) imaging through the Z axis or folded for side-view (sagittal) sections (XZ plane) imaging through Y axis. Images were taken using an Olympus IX 70 inverted microscope with epifluorescence optics (Olympus, Hamamatsu City, Japan) equipped with a Retiga 1,300 cooled CCD, fire wire, high resolution, monochromatic camera (QImaging, Houston, TX). The fluorescent filters used for visualization of green, red and blue fluorochromes were 83,000 Pinkel filter set (Chroma Technology Corp., Rockingham, VT).
Statistical Analyses
Descriptive statistics were calculated using GraphPad Instat software (Version 3.5; GraphPad Software, San Diego, CA). Differences between multiple group means were analyzed by ANOVA, followed by the with Tukey-Kramer multiple comparison post hoc test and considered significant if P < 0.05. All data values are presented as mean ± SEM.
RESULTS
Effects of RSV Infection on Isc in Mouse Tracheal Epithelial Cell Monolayer Cultures
Figure 1A shows a characteristic Ussing tracing from a monolayer of MTE cells isolated with Method A. As described in Materials and Methods, this isolation procedure is based in the protocol of Clarke and coworkers (14), but employs much lower concentrations of hormones and growth factors during the culture period. As shown in Figure 1A, baseline currents are considerably lower than previously reported (14, 24, 25). Addition of 100 μM amiloride into the apical compartment resulted in a 45% decrease of Isc. Slightly lower levels of Isc inhibition were achieved with lower levels of amiloride (10 μM; data not shown). The remaining current was totally abolished by addition of a specific inhibitor of CFTR (CFTRinh-172). Addition of 10 μM forskolin (a stimulator of adenylate cyclase) into the apical bath in the presence of 100 μM amiloride resulted in significant stimulation of Isc which was inhibited by progressive amounts of CFTR inhibitor added into the apical compartment (Figure 1). Addition of a mixture containing a cell permeable analog of cAMP (CPT-cAMP; 100 μM) and IBMX (3-lsobutyl-1-methylxanthine, a phosphodiesterase inhibitor; 100 μM), known to maximally increase intracellular cAMP resulted in a small, additional incrementally increase of Isc. Forskolin also stimulated Isc when added into the apical compartment before amiloride (Figure 2). The majority of the forskolin stimulated Isc was inhibited by CFTRinh-172. These data indicate that MTE cells isolated with this method exhibited both Na+ absorption (most likely through ENaC) and Cl− secretion (though CFTR) and that agents that increase cAMP stimulate mainly Cl− secretion.
Figure 1.
Effect of Respiratory syncytial virus (RSV) infection on mouse tracheal epithelial cells, isolated from C57BL/6 mice and cultured using Method A. (A) Representative Isc records from uninfected (solid black line) and 24 hours after RSV infection (gray line) mouse tracheal epithelial (MTE) monolayers. MTE cells were isolated and cultured using Method A (see Materials and Methods for details). Amiloride (Amil, 100 μM), forskolin (FSK, 10 μM), a mixture containing cpt-cAMP (100 μM) plus IBMX (100 μM), and CFTRinh-172 (CFTRinh, 100 μM) were added into the apical compartment of the Ussing chambers at the times indicated by arrows. (B) Mean values ± 1 SEM of Isc for the indicated conditions (n = 9 for control and n = 8 for RSV). (C) Mean changes of Isc after amiloride, cAMP+IBMX and CFTRinh as compared with the immediately preceding condition; *P < 0.05 compared with control.
Figure 2.
Effect of RSV infection on forskolin-induced Isc in mouse tracheal epithelial cells, isolated from C57BL/6 mice and cultured using Method A. (A) Representative Isc records from uninfected (solid black line) and 24 hours after RSV infection (gray line) MTE monolayers. MTE cells were isolated and cultured using Method A (see Materials and Methods for details). Forskolin (FSK, 10 μM), a mixture containing cpt-cAMP (100 μM) plus IBMX (100 μM), amil (10 μM), and CFTRinh-172 (CFTRinh, 100 μM) were added into the apical compartment of the Ussing chambers at the times indicated by arrows. (B) Mean values ± 1 SEM of Isc for the indicated conditions (n = 3 for control and n = 5 for RSV). (C) Mean changes of Isc after forskolin, amiloride, cAMP+IBMX, and CFTRinh as compared with the immediately preceding condition; *P < 0.05 compared with control.
MTE cells were productively infected with RSV strain A2, as confirmed by direct immunofluorescence, although levels of infection appeared low (< 5%) 24 hours post infection (Figure 3). No effects on basal and amiloride-sensitive Isc were seen in MTE cells 8 hours after infection (data not shown). However, infection of MTE cells with RSV A2 for 24 hours caused a significant reduction of both the baseline and of the amiloride-sensitive component of Isc (Figures 1 and 2). Significantly lower values of cAMP stimulated Isc were seen in RSV-infected MTE cells. As seen in Figures 1 and 2, addition of CPT-cAMP+IBMX after forskolin resulted in a marginal and nonsignificant increase of Isc above and beyond what observed with forskolin alone in both mock- and RSV-infected MTE cells. Infection with RSV did not alter transepithelial resistance (data not shown). Moreover, no LDH release into overlaid media was detectable in either mock-infected or RSV-infected MTE cell monolayers at either 24 or 28 hours after infection (data not shown). These findings indicate that RSV, in spite of its low infectivity, inhibits active ion transport in MTE cells through both ENaC and CFTR without causing cell death or overt cellular injury. Immunofluorescent studies (shown in Figure 3) using anti-RSV and anti-αENaC antibodies indicate that RSV infection significantly decreased αENaC levels in MTE cells both at the levels of the cytoplasm and apical plasma membranes. This impression was based on visual examination of a large number of monolayers. Quantitative studies were not attempted.
Figure 3.
Immunofluorescent images of mock (A, C, and E)- or RSV (B, D, and F)-infected MTE cells. (A, B) Confocal images of apical plasma membranes of MTE cells, 24 hours after (A) mock and (B) RSV infection stained with anti-RSV antibody followed by AlexaFluor 488–conjugated secondary antiboby. Green fluorescence indicates the presence of RSV virus. No staining is seen in mock-infected cells. (C, D) Confocal microscopy of (C) mock- and (D) RSV-infected MTE cells, stained with an antibody to αENaC, followed by a secondary antibody conjugated to AlexaFluor 594. Cell nuclei are counterstained with Hoechst 33258. Significantly lower levels of αENaC-associated fluorescence are seen in RSV-infected cells. (E, F) Side-view (saggital) sections as in C and D showing the apical plasma membranes. Similar results were obtained from three different MTE monolayers.
MTE cells isolated from either BABL/c (Figures 4 and 5) or C57BL/6 mice (data not shown) using Method B (which uses high concentrations of various hormones and growth factors known to stimulate Na+ transport) exhibited much higher levels of baseline Isc that were almost completely inhibited by amiloride. Infection of these cells with RSV resulted in significant inhibition of both the baseline and amiloride-sensitive Isc (Figures 4 and 5). Addition of forskolin, or of CPT-cAMP and IBMX, either after (Figure 4) or in the absence of amiloride (Figure 5), resulted in a large increase of Isc that was inhibited by CFTRinh in a dose-dependent fashion, indicating that it was mainly due to the secretion of anions (Cl− or HCO3−). Data shown in Figures 4 and 5 indicate that infection of MTE with RSV resulted in significant decrease of the cAMP-stimulated Isc.
Figure 4.
Effect of RSV infection on mouse tracheal epithelial cells, isolated from BALB/c mice and cultured using Method B. (A) Representative Isc records from uninfected (solid black line) and 24 hours after RSV infection (gray line) MTE monolayers. MTE cells were isolated and cultured using Method B (see Materials and Methods for details). Amiloride (Amil, 100 μM), forskolin (FSK, 10 μM), a mixture containing cpt-cAMP (100 μM) plus IBMX (100 μM) and CFTRinh-172 (CFTRinh, 100 μM) were added into the apical compartment of the Ussing chambers at the times indicated by arrows. (B) Mean values ± 1 SEM of Isc for the indicated conditions (n = 5 for control and n = 6 for RSV). (C) Mean changes of Isc after amiloride, cAMP+IBMX, and CFTRinh as compared with the preceding condition; *P < 0.05 compared with control.
Figure 5.
Effect of RSV infection on forskolin-induced Isc in mouse tracheal epithelial cells, isolated from BALB/c mice and cultured using Method B. (A) Representative Isc records from uninfected (solid black line) and 24 hours after RSV infection (gray line) MTE monolayers. MTE cells were isolated and cultured using Method B (see Materials and Methods for details). Forskolin (FSK, 10 μM), a mixture containing cpt-cAMP (100 μM) plus IBMX (100 μM), CFTRinh-172 (CFTRinh, 100 μM), glybenclamide (0.3 mM), and amil (100 μM) were added into the apical compartment of the Ussing chambers at the times indicated by arrows. (B) Mean values ± 1 SEM of Isc for the indicated conditions (n = 6 for control and n = 6 for RSV: [1] Basal, [2] FSK, [3] cAMPandIBMX, [4] CFTRinh, [5] glyb, [6] amil.). (C) Mean changes of Isc after forskolin, amiloride, cAMP+IBMX and CFTRinh as compared with the immediately preceding condition; *P < 0.05 compared with control.
H441 Cells Exhibit Vectorial Active Na+ Transport
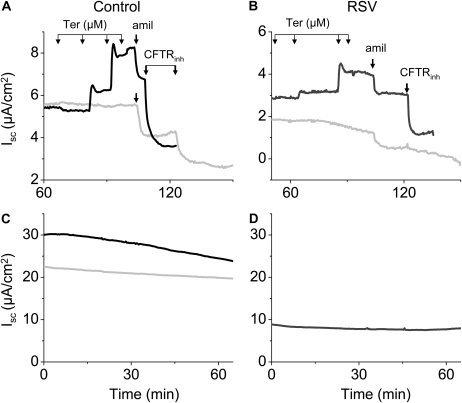
Because of the difficulty and expense of isolating MTE cells in sufficient numbers, additional studies assessing the effects of RSV on vectorial Na+ transport were performed on H441 cells. We first characterized the transport properties of these cells when grown to confluent monolayers and mounted in Ussing chambers. Addition of 1 to 100 μM amiloride into the apical compartment of the Ussing chamber inhibited more than 95% of Isc, with an IC50 of 0.50 ± 0.50 μM (n = 12, Figure 6). These findings indicate that the majority of Isc in H441 cell monolayers is due to the movement of Na+ ions through amiloride-sensitive channels (ENaC). These data indicate that in contrast to MTE cells isolated by Method A, the majority of the Isc in H441 cells is due to Na+ reabsorption, as we recently reported for ATII cells. In the presence of 100 μM amiloride, apical addition of 10 μM forskolin induced a small increase in Isc (a fraction of what was seen in MTE cells) which was inhibited either by ouabain (Figure 6A), by CFTRinh-172 (200 μM, Figure 6C) or glibenclamide (data not shown). These data indicate that the resulting currents is caused by anion secretion.
Figure 6.
Characterization of H441 Isc. (A) Representative Ussing chamber Isc traces from a H441 cell monolayer. Amiloride was added in the apical chamber in incremental amounts at times indicated by arrows to achieve steady-state concentrations of 0.01, 0.1, 1, 10, and 100 μM. Subsequent addition of forskolin (10 μM) caused a small increase of Isc (presumably due to anion secretion) that was inhibited by ouabain (1 mM), added into the basolateral compartment. (B) Dose–response inhibition of Isc by apical amiloride. Values are means ± 1 SEM; n = 12 monolayers for each point. Values are expressed as % of Isc before the addition of amiloride. (C) Representative Isc trace of H441 cell monolayer showing the effects of amiloride (amil. 100 μM), forskolin (FSK; 10 μM), and CFTRinh-172 (CFTRinh; 200 μM). As shown in A, in the presence of amiloride, forskolin caused a small increase of Isc that was inhibited by apical addition of CFTRinh. Measurements were reproduced using eight different monolayers with identical results.
H441 Cells Can Be Infected with RSV Strain A2
Productive infection of H441 cell monolayers with RSV was detected by RT-PCR for RSV L (polymerase) gene mRNA (Figure 7), and confirmed by immunofluorescence using a mouse monoclonal anti-RSV antibody (Figure 7). Approximately 5 to 10% of H441 cells were RSV-positive at 24 hours after infection, similar to what we observed from MTE cells (Figure 3).
Figure 7.
Infection of H441 cells with RSV strain A2. (A) Demonstration of RSV replication in H441 cells at 24 hours after infection by RT-PCR using primers specific for an 1,100-nt segment of the RSV L gene (indicated by arrow) in RNA isolated from three RSV-infected H441 cell monolayers (V1–V3). Note absence of PCR product in RNA isolated from three mock-infected monolayers (M1–M3), or in reverse transcriptase control (RT-), and cDNA control monolayer RNA preparations. (B and C) Confirmation of productive RSV infection of H441 cell monolayers (1 × 106 PFU per monolayer) at 24 hours by immunofluorescence using a mouse monoclonal anti-RSV antibody followed by a secondary goat anti-mouse IgG conjugated to AlexaFluor 488. Cell nuclei are counterstained blue with Hoechst 33258. No fluorescence was seen in mock-infected monolayers (data not shown). Representative immunofluorescence images from three repeats per condition are shown.
Effects of RSV Infection on Isc in H441 Cell Monolayer Cultures
RSV infection caused a significant (50%) reduction in basal and amiloride-sensitive Isc in H441 cell monolayers at 24 hours (Figures 8A and 8B), indicating that, as in MTE cells, RSV inhibits active, amiloride-sensitive Na+ transport. We did notice a decrease of transepithelial resistance after RSV infection; however, the difference was not significant (259 ± 174 Ω·cm2 in RSV infected monolayers, n = 23, versus 347 ± 170 Ω·cm2 in uninfected monolayers, n = 21, P = 0.7) and unlikely to contribute to the observed decrease of amiloride-sensitive Isc.
Figure 8.
Effects of RSV infection on Isc of H441 monolayers. (A) Typical Isc traces of control (uninfected; black line) and RSV-infected (1 × 106 PFU/monolayer; gray line) monolayers 24 hours after infection. Amiloride (100 μM) were added into the apical compartment at the indicated time. (D) Mean values ± 1 SEM of base and amiloride-sensitive Isc (n = 21 for control and n = 23 for RSV). (B) Representative Isc responses to forskolin (10 μM) of mock (black line)- and RSV (gray line)-infected monolayers followed by addition of 100 μM amiloride. Subsequently, the apical membrane was permeabilized by the addition of amphotericin-B (20 μM) in the apical compartment, followed by the addition of ouabain (1 mM). The difference current before and after addition of ouabain represents the maximum activity of the Na,K-ATPase. (E) Mean values ± 1 SEM of baseline, forskolin, and amiloride-sensitive (ΔAmil) Isc (n = 3 for control and n = 5 for RSV). (C, F) Same as in C and D for a group of monolayers with lower baseline Isc values. Notice that in this case, addition of forskolin (10 μM) returned Isc to the control baseline (n = 4 for both control and RSV). *P < 0.05 as compared with the corresponding control uninfected monolayers.
RSV infection for 24 hours also significantly reduced (by 32%) the forskolin (10 μM)-stimulated Isc (Figures 8C and 8D). Subsequent addition of 10 μM amiloride completely abrogated this forskolin-induced increase in Isc in both uninfected and infected monolayers, indicating that forskolin stimulates mainly active Na+ transport in H441 cells, and that this stimulation is impaired by RSV infection. Measurement of Isc in RSV-infected H441 cell monolayers after apical membrane permeabilization with 20 μM amphotericin B (thereby loading the cytosol with Na+ to elicit maximal Na+ transport by the basolateral Na+/K+ ATPase), followed by addition of 1 mM ouabain to inhibit Na+/K+ ATPase activity, indicated that RSV infection has no effect on the ouabain-inhibitable Na+/K+ ATPase Isc (mean ΔIsc after amphotericin B followed by ouabain was 4.98 ± 1.1 μA/cm2 in RSV-infected monolayers, n = 11, versus 5.04 ± 0.6 μA/cm2 in uninfected monolayers, n = 8; P > 0.1).
Previously we reported that RSV infection decreases Na+-dependent alveolar fluid clearance (AFC) by approximately 25 to 30% and that intratracheal instillation of forskolin restores AFC to its normal levels (12, 26, 27). In contrast, as shown in Figure 8B RSV infection of H441 cells decreased their Isc by more than 50% and although forskolin increased Isc, it did not restore it to its control, noninfected value. However, in another group of H441 cells that were cultured for shorter periods of time and had lower baseline Isc values, infection with RSV decreased their baseline values by only about 25%, and addition of forskolin returned the current to the noninfected baseline levels (Figures 8E and 8F). Subsequent addition of 8-Br-cGMP and IBMX (which greatly increase cAMP levels) had only a minor effect on Isc (indicating that forskolin generated the maximum response). Similar results were obtained with MTE cells cultured with Method A, which results in smaller baseline Isc values as compared with Method B. Terbutaline (up to 10 μM) as well as a number of other β-adrenergic receptor agonists (data not shown) increased Isc across mock- and (to a lesser extent) RSV-infected MTE cells (Figures 9A and 9B; Mean ± 1 SEM for 0, 1, 2.5, 5, and 10 μM terbutaline: 8 ± 1.4, 8.6 ± 2.0, 9.2 ± 1.9, 9.9 ± 2.0, and 10.2 ± 2.0 μA/cm2 [n = 6] while for RSV-infected monolayers: 5.3 ± 2.1, 6.3 ± 3.0, 7.0 ± 3.5, 7.6 ± 3.7, and 8.6 ± 4.6 μA/cm2 [n = 5]). In both cases, pretreatment with propranolol (10 μM) totally abolished the effects of terbutaline. In contrast, no effect of terbutaline was seen on Isc of either mock- or RSV-infected H441 cells (Figures 9C and 9D). These results suggest that H441 cells, cultured under our experimental conditions, lack either β2-adrenergic receptors at their plasma membrane or Gs proteins, which transduct signals from these receptors, resulting in increases of cAMP. In vivo findings indicate while forskolin increased AFC in RSV-infected mice, short- and long-term β2-agonists had no effect on this variable (27).
Figure 9.
The effects of terbutaline on Isc of MTE and H441 monolayers. Typical Isc traces showing time courses of (A, C) mock- or (B, D) RSV (1 × 106 PFU)-treated MTE (A, B) and H441 (C, D) monolayers 24 hours after infection, after addition of terbutaline into both compartments of the Ussing chamber. In (A) control and (B) RSV-treated MTE cell monolayers, terbutaline (0, 1, 2.5, 5, and 10 μM) caused a dose-dependent increase of Isc that was significantly inhibited by amilorde and CFTRinh This effect was prevented by pretreatment with propranalol (10 μM), an inhibitor of β1- and β2-adrenergic receptors (gray lines). Terbutaline did not cause significant increase of Isc in either control or RSV-infected H441 cells (C, D). Typical records which were reproduced at least five times (see Results for mean values).
Inhibitors of De Novo Pyrimidine or Purine Synthesis Block RSV-Induced Inhibition of Isc in H441 Cells
We have reported that RSV infection inhibited AFC in BABL/c mice by increasing UTP levels in their BAL (12). Therefore, we tested whether RSV infection inhibits Isc of H441 cells through the same mechanism. First, H441 cells were tested for their response to UTP. As shown in Figure 10, addition of UTP decreased Isc in H441 cells in a dose-dependent manner. Second, when H441 cells were pretreated by de novo pyrimidine or purine synthesis inhibitor A77-1726 or 6-MP (50 μM), inhibition of amiloride-sensitive Isc at 24 hours after RSV infection was reversed (Figure 11). A77-1726 had no effect on basal Isc and amiloride sensitivity in uninfected H441 cell monolayers (Figure 11C). Thus, these data are consistent with our in vivo data and suggested that RSV infection inhibits active Na+ transport in respiratory epithelium by up-regulation of UTP.
Figure 10.
UTP decreases amiloride-sensitive Isc in H441 cells. (A) Representative Isc traces showing the effect of increasing concentrations of UTP (1–1,000 μM), or equal volumes of vehicle (water) added into the apical compartments of Ussing chambers containing monolayers of H441 cells followed by amiloride (100 μM). (B) UTP (or vehicle)-sensitive currents for the indicated concentrations. Mean values ± 1 SEM (n = 4 for vehicle-treated monolayers; n = 5 for UTP-treated monolayers). *P < 0.05, compared with vehicle-treated monolayers.
Figure 11.
Pretreatment of H441 cell monolayers with A77 or 6-MP reversed RSV-induced inhibition of amiloride-sensitive Isc. (A) Effect of pretreatment of monolayers with 50 μM of the de novo pyrimidine synthesis inhibitor A77-1726 on basal Isc 24 hours after infection (n = 24 for uninfected, A77-1726–treated monolayers; n = 25 for RSV-infected, untreated monolayers; n = 4 for RSV-infected, DMSO-treated monolayers ; n = 9 for RSV-infected, A77-1726–treated monolayers). (B) Effect of pretreatment of 50 μM A77-1726 on amiloride sensitive Isc (ΔAMIL) 24 h after infection for the groups mentions in the previous panel. (C). Effect on basal Isc of treatment of mock-infected monolayers for 24 h with 50 μM A77-1726 (n = 24 for untreated monolayers; n = 3 for DMSO-treated monolayers; n = 7 for A77-1726–treated monolayers). (D) Effect of pretreatment of monolayers with 50 μM of the de novo purine synthesis inhibitor 6-MP on Isc in mock-infected monolayers and at 24 hours after RSV infection (n = 24 for mock-infected, untreated monolayers; n = 3 for mock-infected, 6-MP–treated monolayers; n = 25 for RSV-infected, untreated monolayers; n = 7 for 6-MP–treated, RSV-infected monolayers),and A77 plus RSV monolayers .*P < 0.005, compared with mock-infected, untreated monolayers.
Infection with RSV Reduces H441 cell ENaC Subunit Protein Expression
Immunofluorescent analysis demonstrated that reduced amiloride-sensitive Isc after infection of H441 cells with RSV for 24 hours was accompanied by a reduction in α-ENaC subunit protein expression level (Figure 12). Likewise, Western blot analysis of whole-cell lysates also indicated that α- but not γ-ENaC protein level decreased in RSV-infected H441 cells after 24 hours (Figure 13).
Figure 12.
Immunofluorescent staining for αENaC in H441 cell monolayer. Mock (top panel)- or RSV (bottom panel)-infected H441 cells (1 × 106 PFU for 24 h) were immunostained with either anti-RSV antibody (A and B; see Figure 3 for details of RSV staining) or an antibody to α-ENaC (C and D, see Figure 3 for details of αENaC staining). The secondary antibodies for RSV and αENaC staining were goat anti-mouse IgG conjugated to AlexaFluor 488 (green fluoresecence) and goat anti-rabbit IgG conjugated to AlexaFluor 594 (red fluorescence), respectively. Only background immunofluorescence levels were seen in monolayers stained with an equivalent amount of nonimmune rabbit IgG, followed by secondary goat anti-rabbit IgG conjugated to AlexaFluor 594 (picture not shown). In all cases nuclei were counterstained blue with Hoechst 33258 dye. Representative immunofluorescence images from five repeats per condition are shown.
Figure 13.
RSV decreases α-ENaC levels in H441 cells. (A) Sample Western blots demonstrating immunodetection of α- and γ-ENaC subunit proteins in total cellular lysates of mock-infected H441 cells (V) or H441 cells infected with RSV strain A2 for 24 h (R). Representative blots from three repeats are shown. (B) Effect of infection of H441 cells with RSV for 24 hours on α- and γ-ENaC subunit protein expression level. Values are means ± 1 SEM (n = 3 per group; relative density normalized to β-actin). *P < 0.05 compared with the corresponding vehicle value.
DISCUSSION
Herein we show that infection of both mouse tracheal epithelial cells, derived from the lungs of two C57BL/6 and BALB/c mice, as well as H441 cells, a human Clara cell–like line, with RSV A2 decrease baseline amiloride-sensitive Isc and modulate the increase of Isc by cAMP. In contrast, Na,K-ATPase function was not affected. Importantly, despite the different baseline Isc values and proportion of amiloride-sensitive components within the cell systems, we observed similar extent of inhibition of amiloride-sensitive Na+ currents by RSV infection, which suggests that a common mechanism is involved. Moreover, our data indicate that the inhibition of ENaC requires active, replicating viruses and is independent of the RSV-induced inflammatory response.
We previously demonstrated that RSV-induced inhibition of AFC in BALB/c mice is mediated by the action of de novo synthesized UTP on bronchoalveolar epithelial P2Y purinergic receptors (12). In contrast, Kunzelmann and colleagues reported that nucleotide-mediated P2Y receptor activation plays no role in inhibition of active Na+ transport in mouse tracheal tissue after exposure to RSV for 60 minutes (28). Nevertheless, we found in the current study that the inhibitory effects of RSV infection for 24 hours on active Na+ transport (amiloride-sensitive Isc) in H441 cells appear to be nucleotide mediated: H441 cell basal Isc is inhibited by exogenous UTP (which suggests that these cells express functional P2Y receptors) and RSV-mediated inhibition of active Na+ transport in H441 cells can be reversed by inhibition of de novo pyrimidine or purine synthesis. This finding suggests that elevated de novo nucleotide synthesis and release is induced by RSV replication and gene expression within infected respiratory epithelial, but not by mere exposure to viral antigens.
Interestingly, the inhibitory effect of Sendai virus on active Na+ transport reported by Kunzelmann and coworkers (28) is also not mediated by pyrimidines, but is instead induced by ATP, acting on P2Y receptors in a similar manner to that we have described here and elsewhere for RSV (12, 13, 26, 27). However, it is not clear from the above study whether this ATP is derived from de novo purine synthesis. Again, it should be noted that, in vivo, RSV induces elevated release of both ATP and UTP into the bronchoalveolar space, although only UTP appears to mediate impaired AFC after RSV infection (26). ATP may be inactive in vivo because RSV infection has been shown to enhance ecto-nucleotide pyrophosphatase phosphohydrolase-mediated extracellular ATP hydrolysis (29). It is possible that in our H441 cell culture system, this effect does not occur, and so both ATP and UTP can mediate inhibition of active Na+ transport. Alternatively, the comparable effects of A77-1726 and 6-MP may instead reflect the co-ordinate regulation of cellular de novo nucleotide synthesis, which we have found to result in concomitant suppression of both pathways by inhibitors. Finally, the findings reported herein indicate that the respiratory epithelium itself is at least one of the sites of elevated de novo nucleotide synthesis and release after RSV infection, since the in vivo effects of RSV on AFC and nucleotide synthesis/release could be recapitulated in vitro in the absence of any other cell type. Moreover, they demonstrate that RSV inhibition of Na+ transport can occur in the absence of immune cell activity.
We found that RSV reduced forskolin-stimulated Isc in both MTE and H441 cell monolayers at 24 hours after infection. Forskolin-stimulated Isc was reduced by RSV when forskolin was added in both the absence and presence of amiloride. In the former case, RSV reduced cAMP-mediated stimulation of amiloride-sensitive active Na+ transport, while in the latter case, RSV most likely blocked cAMP-mediated activation of anion secretion via CFTR. An inhibitory effect of RSV on anion secretion by respiratory epithelial cells has not previously been described, although it was suggested in a recent report by Tarran and colleagues (29). This group demonstrated that in well-differentiated, ciliated, airway epithelial cell cultures from normal donors, the majority (50%) of periciliary liquid (PCL) secretion under phasic motion occurs via CFTR (with a further 20% occurring via Ca2+-activated Cl− channels). However, they also showed that after RSV infection, PCL height falls by 44%, which is highly suggestive of a significant effect of RSV on anion secretion via CFTR (29). Similarly, Kunzelmann and coworkers (28) reported that, after an initial transient stimulatory effect on Ca2+-activated Cl− channels, acute exposure to Sendai virus caused a reduction in forskolin-stimulated Isc in mouse tracheal tissue. However, these studies were not performed in the presence of amiloride, so it is unclear whether this reduction in forskolin-stimulated Isc was due to a reduction in both Cl− secretion and Na+ absorption, or solely the latter (as was also observed in our studies). Interestingly, this group did not find any effect of RSV on either Ca2+-activated or forskolin-stimulated Isc responses after exposure of mouse tracheal tissue to virus for 1 hour (30), which clearly differs from our findings: again, this may reflect a difference between exposure to RSV viral antigen and viral infection and replication/gene expression. Activity of CFTR has been proposed as a necessary component of the normal AFC mechanism (31, 32). Moreover, RSV has been proposed as a candidate vector system for airway gene therapy in cystic fibrosis (9). This aspect of the effect of RSV on respiratory epithelial ion transport therefore deserves further study.
It should be noted that inhibition of active ion transport in RSV-infected MTE or H441 cell monolayers is not a consequence of cell death, in agreement with earlier in vitro studies that have demonstrated that RSV does not cause cytopathology or syncytium formation in well-differentiated, polarized, primary human airway epithelial cells (9, 33). In addition, the fact that Na+ transport inhibition by RSV in H441 cell monolayers could be prevented by pretreatment with inhibitors of de novo nucleotide synthesis also indicates that the defect in ion transport is functional (as we found in RSV-infected mice [26]), rather than being a consequence of cell death. Finally, the absence of effect of RSV on function of the basolateral Na+/K+ ATPase in H441 cells also indicates that the inhibitory effects of RSV on amiloride-sensitive and forskolin-stimulated ion transport are selective and not a result of nonspecific cytopathic effects on respiratory epithelial cells. This observation also extends our previous in vitro findings—while we found that the predominant inhibitory effect of RSV is on the amiloride-sensitive component of AFC (which suggests that RSV has little or no effect on bronchoalveolar epithelial cell Na+/K+ ATPase function), we have been unable to directly measure the effects of RSV on ouabain-sensitive AFC in vivo because of the extreme cardiotoxicity of this ATPase inhibitor (34).
The exact mechanisms by which the RSV mediated increased UTP levels decrease ENaC have not been elucidated. In a recent study, we infected H441 cells with RSV expressing GFP. Cells expressing GFP (indicating that they had been infected with RSV) had lower amiloride-sensitive currents and lower levels of αENaC mRNA as compared with uninfected cells (35). Incubation with A77 increased the values of the amiloride-sensitive currents but did not alter αENaC mRNA. In the present study we report that infection with RSV decreased αENaC protein levels both by immunofluorescence and Western blotting. However, the overall decrease of αENaC was small (abut 30%) and unlikely to account for the large decrease of amiloride-sensitive currents. However, it is still unclear as to how RSV infection up-regulates UTP production and how elevated UTP mediates the inhibition of Na+ channel currents. P2Y receptors (the main targets of UTP) are G protein–coupled and act via the inositol phosphate pathway to stimulate Ca2+ release from intracellular stores, but can also act via multiple secondary signal transduction pathways, including protein kinase C (PKC) (36). Activation of PKC has been shown to reduce ENaC activity and modify its subunit composition (37, 38). Our previous data indicate that infection of mice with RSV results in activation of PKCζ as indicated by its phosphorylation and translocation from the cytoplasm to the plasma membrane (27).
This work was supported by PHS grants HL-31197, HL-51173, NIEHS-1U01ES015676, 1U54NS063739, and 1U54ES017218-01 to S.M. and RR-17626 to I.C.D.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0034OC on October 23, 2008
Conflict of Interest Statement: E.S.'s company DiscoveryBioMed did receive compensation under a “fee-for-service” agreement for primary mouse lung cells prepared and used by Dr. Matalon's group in this paper. I.C.D., W.M.S., and S.M. have been granted US Provisional Patent Application # 60/573558 (Filed May 21, 2004; Inventors: Dr. Ian C Davis, Dr. Wayne Sullender and Dr. Sadis Matalon), which converted to International PCT application (#PCT/US2005/017939); May 2005. I.C.D. has received $500 in consultancy fees from Inspire Pharmaceuticals for advising on licensing issues related to this patent. I.C.D. is the principal investigator for a grant from Inspire Pharmaceuticals (11/01/2007–10/31/2008; $130,000, indirect + direct costs). S.M. is the principal investigator for a grant from Inspire Pharmaceuticals, from 7/15/06–7/15/07 for $130,000 for direct costs; as a principal investigator for a grant from Talecris Biotherapeutics, Inc from 7/01/07–12/31/07 for $38,000 for direct costs; and as a principal investigator for a grant from Sepracor, Inc for $164,196 (date pending). W.M.S. served as a co-investigator for the grant from Inspire Pharmaceuticals, from 7/15/06–7/15/07 for $130,000 for direct costs. None of the experiments and results presented in this article were funded from these grants. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Smyth RL, Openshaw PJ. Bronchiolitis. Lancet 2006;368:312–322. [DOI] [PubMed] [Google Scholar]
- 2.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005;352:1749–1759. [DOI] [PubMed] [Google Scholar]
- 3.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986;140:543–546. [DOI] [PubMed] [Google Scholar]
- 4.Dowell SF, Anderson LJ, Gary HE Jr, Erdman DD, Plouffe JF, File TM Jr, Marston BJ, Breiman RF. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis 1996;174:456–462. [DOI] [PubMed] [Google Scholar]
- 5.Whimbey E, Ghosh S. Respiratory syncytial virus infections in immunocompromised adults. Curr Clin Top Infect Dis 2000;20:232–255. [PubMed] [Google Scholar]
- 6.Murata Y, Falsey AR. Respiratory syncytial virus infection in adults. Antivir Ther 2007;12:659–670. [PubMed] [Google Scholar]
- 7.Law BJ, Wang EE, MacDonald N, McDonald J, Dobson S, Boucher F, Langley J, Robinson J, Mitchell I, Stephens D. Does ribavirin impact on the hospital course of children with respiratory syncytial virus (RSV) infection? An analysis using the pediatric investigators collaborative network on infections in Canada (PICNIC) RSV database. Pediatrics 1997;99:E7. [DOI] [PubMed] [Google Scholar]
- 8.Hammer J, Numa A, Newth CJ. Acute respiratory distress syndrome caused by respiratory syncytial virus. Pediatr Pulmonol 1997;23:176–183. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol 2002;76:5654–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 2002;82:569–600. [DOI] [PubMed] [Google Scholar]
- 11.Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 1999;61:627–661. [DOI] [PubMed] [Google Scholar]
- 12.Davis IC, Lazarowski ER, Hickman-Davis JM, Fortenberry JA, Chen FP, Zhao X, Sorscher E, Graves LM, Sullender WM, Matalon S. Leflunomide prevents alveolar fluid clearance inhibition by respiratory syncytial virus. Am J Respir Crit Care Med 2006;173:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis IC, Lazarowski ER, Chen FP, Hickman-Davis JM, Sullender WM, Matalon S. Post-infection A77–1726. blocks pathophysiologic sequelae of respiratory syncytial virus infection. Am J Respir Cell Mol Biol 2007;37:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke LL, Burns KA, Bayle JY, Boucher RC, Van Scott MR. Sodium- and chloride-conductive pathways in cultured mouse tracheal epithelium. Am J Physiol Lung Cell Mol Physiol 1992;263:L519–L525. [DOI] [PubMed] [Google Scholar]
- 15.Lazrak A, Matalon S. cAMP-induced changes of apical membrane potentials of confluent H441 monolayers. Am J Physiol Lung Cell Mol Physiol 2003;285:L443–L450. [DOI] [PubMed] [Google Scholar]
- 16.Mbiguino A, Menezes J. Purification of human respiratory syncytial virus: superiority of sucrose gradient over percoll, renografin, and metrizamide gradients. J Virol Methods 1991;31:161–170. [DOI] [PubMed] [Google Scholar]
- 17.Sullender WM, Anderson K, Wertz GW. The respiratory syncytial virus subgroup B attachment glycoprotein: analysis of sequence, expression from a recombinant vector, and evaluation as an immunogen against homologous and heterologous subgroup virus challenge. Virology 1990;178:195–203. [DOI] [PubMed] [Google Scholar]
- 18.Hickman-Davis JM, Nicholas-Bevensee C, Davis IC, Ma HP, Davis GC, Bosworth CA, Matalon S. Reactive species mediate inhibition of alveolar type II sodium transport during mycoplasma infection. Am J Respir Crit Care Med 2006;173:334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitcutt MJ, Adler KB, Wu R. A biphasic chamber system for maintaining polarity of differentiation of cultured respiratory tract epithelial cells. In Vitro Cell Dev Biol 1988;24:420–428. [DOI] [PubMed] [Google Scholar]
- 20.Sayegh R, Auerbach SD, Li X, Loftus RW, Husted RF, Stokes JB, Thomas CP. Glucocorticoid induction of epithelial sodium channel expression in lung and renal epithelia occurs via trans-activation of a hormone response element in the 5′-flanking region of the human epithelial sodium channel alpha subunit gene. J Biol Chem 1999;274:12431–12437. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Patel RP, Teng X, Bosworth CA, Lancaster JR Jr, Matalon S. Mechanisms of cystic fibrosis transmembrane conductance regulator activation by s-nitrosoglutathione. J Biol Chem 2006;281:9190–9199. [DOI] [PubMed] [Google Scholar]
- 22.Lazrak A, Thome U, Myles C, Ware J, Chen L, Venglarik CJ, Matalon S. cAMP regulation of Cl(-) and HCO(-)(3) secretion across rat fetal distal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 2002;282:L650–L658. [DOI] [PubMed] [Google Scholar]
- 23.Cartee TL, Megaw AG, Oomens AG, Wertz GW. Identification of a single amino acid change in the human respiratory syncytial virus L protein that affects transcriptional termination. J Virol 2003;77:7352–7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thome U, Lazrak A, Chen L, Kirk MC, Thomas MJ, Forman HJ, Matalon S. Novel SIN-1 reactive intermediates modulate chloride secretion across murine airway cells. Free Radic Biol Med 2003;35:662–675. [DOI] [PubMed] [Google Scholar]
- 25.Bebok Z, Varga K, Hicks JK, Venglarik CJ, Kovacs T, Chen L, Hardiman KM, Collawn JF, Sorscher EJ, Matalon S. Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP-mediated Cl- secretion in airway epithelia. J Biol Chem 2002;277:43041–43049. [DOI] [PubMed] [Google Scholar]
- 26.Davis IC, Sullender WM, Hickman-Davis JM, Lindsey JR, Matalon S. Nucleotide-mediated inhibition of alveolar fluid clearance in BALB/c mice after respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol 2004;286:L112–L120. [DOI] [PubMed] [Google Scholar]
- 27.Davis IC, Xu A, Gao Z, Hickman-Davis JM, Factor P, Sullender WM, Matalon S. Respiratory syncytial virus induces insensitivity to beta-adrenergic agonists in mouse lung epithelium in vivo. Am J Physiol Lung Cell Mol Physiol 2007;293:L281–L289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunzelmann K, Konig J, Sun J, Markovich D, King NJ, Karupiah G, Young JA, Cook DI. Acute effects of parainfluenza virus on epithelial electrolyte transport. J Biol Chem 2004;279:48760–48766. [DOI] [PubMed] [Google Scholar]
- 29.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, et al. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem 2005;280:35751–35759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunzelmann K, Sun J, Meanger J, King NJ, Cook DI. Inhibition of airway Na+ transport by respiratory syncytial virus. J Virol 2007;81:3714–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol 2002;119:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 2006;290:L242–L249. [DOI] [PubMed] [Google Scholar]
- 33.Roberts SR, Compans RW, Wertz GW. Respiratory syncytial virus matures at the apical surfaces of polarized epithelial cells. J Virol 1995;69:2667–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardiman KM, Lindsey JR, Matalon S. Lack of amiloride-sensitive transport across alveolar and respiratory epithelium of iNOS(−/−) mice in vivo. Am J Physiol Lung Cell Mol Physiol 2001;281:L722–L731. [DOI] [PubMed] [Google Scholar]
- 35.Song W, Liu G, Bosworth CA, Walker JR, Megaw GA, Lazrak A, Abraham E, Sullender WM, Matalon S. Respiratory syncytial virus inhibits ENaC by upregulating iNos. J Biol Chem 2009;284:7294–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther 2000;295:862–869. [PubMed] [Google Scholar]
- 37.Ling BN, Eaton DC. Effects of luminal Na+ on single Na+ channels in A6 cells, a regulatory role for protein kinase C. Am J Physiol 1989;256:F1094–F1103. [DOI] [PubMed] [Google Scholar]
- 38.Stockand JD, Bao HF, Schenck J, Malik B, Middleton P, Schlanger LE, Eaton DC. Differential effects of protein kinase C on the levels of epithelial Na+ channel subunit proteins. J Biol Chem 2000;275:25760–25765. [DOI] [PubMed] [Google Scholar]