Abstract
Secretoglobin (SCGB) 3A1 and 3A2 are members of the small molecular weight secretoglobin gene superfamily. SCGB3A1 is a tumor suppressor gene, whereas SCGB3A2 has anti-inflammatory properties. Both genes are mainly expressed in the lung and trachea in mice. Whether the expression and/or function of these two genes are related is not known. Here we show that the expression of SCGB3A1 and SCGB3A2 are bidirectionally regulated by oncostatin M (OSM) when examined in a mouse transformed Clara cell line (mtCC); SCGB3A1 is up-regulated by OSM, while SCGB3A2 is down-regulated in a time- and dose-dependent manner. OSM-activated STAT3/5, through binding to the STAT-binding element located at −201 to −209 bp in the mouse Scgb3a1 gene promoter, and the extracellular signal–regulated kinase (ERK)- and p38–mitogen-activated protein kinase (MAPK) pathways are responsible for the OSM-induced up-regulation of SCGB3A1 expression. On the other hand, the −113 to −273 bp region in the Scgb3a2 promoter appears to be responsible for the OSM induced down-regulation of the gene. No significant differences in the levels or patterns of specific DNA-binding proteins were found in the −113 to −273 bp region as determined by electrophoretic mobility shift assays. Neither the ERK- nor p38-MAPK pathways were involved in the OSM-induced reduction of Scgb3a2 promoter activity. These results suggest that OSM-induced suppression of SCGB3A2 expression is an indirect effect of OSM. Expression of the Clara cell marker, CYP2F2, was markedly decreased upon OSM treatment in parallel with the decrease of SCGB3A2 expression in mtCC cells. The differential regulation of Scgb3a1 and Scgb3a2 gene expression by OSM may explain the unique functions of these genes in the lung.
Keywords: oncostatin M, gene regulation, secretoglobin 3A1, secretoglobin 3A2, lung
CLINICAL RELEVANCE
This research shows the physiologic importance of two lung epithelial cell–specific genes, secretoglobin 3A1 and 3A2, in normal as well as in diseased lungs, in particular where oncostatin M level is temporarily raised. The oncostatin M–induced increase/decrease of these two genes may contribute to the transition in phenotypes between proximal and distal airway epithelial cells, which are found in many chronic lung diseases.
Secretoglobin (SCGB) 3A1 (also called UGRP2: uteroglobin-related protein 2 [1, 2] or HIN-1: high in normal 1 [3]) and SCGB3A2 (also called UGRP1 [2]) belong to the SCGB gene superfamily of secreted proteins of small molecular weight (4). Mouse SCGB3A1 and SCGB3A2 have 33% amino acid sequence identity (2). Mouse SCGB3A1 is primarily expressed in the trachea and lung, and weakly expressed in the heart, stomach, and small intestine (1, 5), while human SCGB3A1 is highly expressed in the trachea, lung, salivary gland, prostate, and mammary gland (1, 3). In contrast, SCGB3A2 is predominantly expressed in the epithelial cells of trachea, bronchus, and bronchioles (2). The SCGB3A1 and SCGB3A2 genes are localized on human chromosome 5q31-q35 and their homologous mouse chromosomes (1, 2). Human chromosome 5q31-q35 contains an asthma susceptibility locus, with a number of genes associated with inflammation such as IL-3, IL-4, IL-5, IL-13, and colony-stimulating factor-2 (1, 6).
SCGB3A1 (HIN-1) is a tumor suppressor and its expression is silenced by hypermethylation of the gene's promoter region in the majority of carcinomas, including those from breast, prostate, lung, and pancreas (3, 7–10). SCGB3A1 expression is up-regulated by retinoic acid–induced differentiation of human bronchial epithelial cells, suggesting a role for SCGB3A1 in mucinous epithelial cell differentiation (5). A recent report describes SCGB3A1 as a potent inhibitor of cell growth, cell migration, and invasion, and these activities may be mediated through the AKT signaling pathway (7). Involvement of the AKT pathway is also implicated in epidermal growth factor (EGF) and transforming growth factor (TGF)-α–induced SCGB3A1 expression (11). Further, the Th2 cytokines IL-4 and IL-13 induce SCGB3A1 expression, suggesting that this gene may potentially play a role in the pathogenesis of inflammatory lung diseases (12). In this respect, the involvement of SCGB3A2, a homologous gene to SCGB3A1, in lung inflammation has been described; proinflammatory cytokines IL-5 and IL-9 reduce SCGB3A2 expression (13, 14), whereas anti-inflammatory cytokine IL-10 enhances expression (15). Further, SCGB3A2 suppresses lung inflammation in mice when examined using an allergic airway inflammation mouse model in which recombinant adenovirus-expressing SCGB3A2 is intranasally administered (16). Thus, it seems that SCGB3A1 and SCGB3A2 possess both overlapping and distinct modes of expression and function. Whether and how these two genes are affecting each other's expression and/or function has not been studied.
Oncostatin M (OSM), an IL-6/leukemia inhibitory factor (LIF) family cytokine (also called gp130 cytokine), has been identified as an immediate early gene whose expression is up-regulated in response to IL-2, IL-3, and erythropoietin (17). Mouse and human OSM signaling differs; mouse OSM binds to a specific receptor complex (type II) composed of OSM receptor (OSMR) and gp130, whereas human OSM can bind both type I and type II receptor complex where the former is composed of LIF receptor and gp130 (18, 19). OSM's binding to a receptor complex activates JAK/STAT and mitogen-activated protein kinase (MAPK) signaling pathways, leading to a variety of activities involved in inflammation, remodeling of extracellular matrix, hematopoiesis, and modulation of cell growth and differentiation (17–20). It regulates gonadocyte and astrocyte differentiation, up-regulates liver acute phase protein synthesis by hepatocytes, and can induce tissue inhibitor of metalloproteinase 1 (TIMP-1), IL-6, and monocyte chemoattractant protein-1 (MCP-1) expression by mouse fibroblasts (18, 19, 21–23). OSM also induces eotaxin-1 expression in NIH 3T3 fibroblast and marked eosinophil infiltration in mouse lungs in vivo (24).
In this study, we demonstrate that OSM regulates expression of SCGB3A1 and SCGB3A2 in an opposite manner when examined in mouse lung Clara cell tumor–derived mtCC cells; OSM up-regulates SCGB3A1 expression while OSM down-regulates SCGB3A2 expression, thus indicating a dual role for OSM in regulation of SCGB3A1 and SCGB3A2 expression. These results should help further define the role of SCGB3A1 and SCGB3A2 in lung.
MATERIALS AND METHODS
Cell Culture
Mouse transformed Clara cells (mtCC) (25) and NIH3T3 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Biosource, Camarillo, CA) supplemented with 10% fetal bovine serum (FBS) (GEMINI Bio-Products, Woodland, CA) and Antibiotic-Antimycotic containing 100 units/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, and 250 ng/ml amphotericin B (Invitrogen Life Technologies, Carlsbad, CA). mtCC cells used were not a clonal population, and all the experiments were performed using cells within several passages. Recombinant murine OSM was purchased from R&D systems (Minneapolis, MN).
Animal Study
Two- to three-month-old C57BL/6 mice were intratracheally administered OSM (1 μg/20 g body weight) under anesthesia. Twenty hours after OSM administration, mice were killed and their lungs collected; the trachea and the bronchus were collected separately from the lung below the bronchus, which were considered as those containing the upper and lower airways, respectively. They were separately subjected to RNA preparation. The animal study was approved by the NCI Animal Care and Use Committee.
Luciferase Reporter Assay
mtCC cells were seeded in 24-well tissue culture plates, grown to 90% confluence, and transfected with pGL4 reporter plasmids containing −59, −189, −598, −1,336, and −1,957 bp of the 5′ flanking sequence of the mouse Scgb3a1 genomic DNA or −113, −273, −387, −506, −907 bp of the 5′ flanking sequence of the mouse Scgb3a2 genomic DNA using Fugene 6 (Roche Applied Science, Indianapolis, IN), together with plasmid pGL4.74 [hRluc/TK] containing the Renilla luciferase gene (Promega, Madison, WI) as an internal control. Cells were cultured in 0.5% FBS-containing medium 12 hours before transfection, and throughout the transfection experiments for 48 hours. Cells were lysed using passive lysis buffer (Promega), and luciferase activity measured according to the technical manual for the Dual-Luciferase Reporter Assay System (Promega).
RT-PCR
To prevent genomic DNA contamination, total RNAs were treated with RNase-free DNase I (Ambion, Austin, TX). For cDNA synthesis, total RNAs were first incubated at 65°C for 5 minutes and then chilled on ice. The cDNA synthesis reactions were performed in a final volume of 20 μl containing RNA, 4 μl of 5× first-strand synthesis buffer, 1 μl of mixture of four deoxynucleotide triphosphates (10 mM each), 2 μl of 0.1 M dithiothreitol (DTT), and 1 μl of 500 ng/μl N6 random hexamer. After incubation at 37°C for 2 minutes, 200 U of Superscript II reverse transcriptase (Invitrogen Life Technologies) was added, and the incubation was continued at 42°C for 60 minutes, which was then subjected to PCR using Advantage 2 Taq DNA polymerase (BD Biosciences, San Jose, CA) under the following conditions: denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds, 20 cycles for 18S and 30 cycles for OSM receptor; and denaturation at 95°C for 30 seconds, annealing at 56°C for 40 seconds, and extension at 72°C for 30 seconds, 30 cycles for gp130. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and 18S were used as control. Oligonucleotide primers used for RT-PCR were as follows: mouse OSM receptor, 5′-ATCCAAAGGCTCCGCAGGAC-3′ and 5′-GTAAGGTTGCAGGTCAAGGC-3′; gp130, 5′-ACATCGTGTGGAAGACCAAC-3′ and 5′-ACTCTGATTTCAAAGTGTAG-3′; GAPDH, 5′-GAAGGTCGGTGTGAACGGATTTGGC-3′ and 5′-CATGTAGGCCATGAGGTCCACCAC-3′; and mouse 18S, 5′-CGGCTACCACATCCAAGGAA-3′ and 5′-ATTGGAGCTGGAATTACCGC-3′.
Quantitative RT-PCR (qRT-PCR) was used to determine the expression levels of genes. Analysis was performed using SYBR Green master mixture and ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA). Primers used were as follows; SCGB3A1, 5′-GATGGCCAAGTGGCTTAATG-3′ and 5′-TCTGTGTGGCTCTGCTCAGT-3′, SCGB3A2, 5′-ACAGGGAGACGGTTGATGAG-3′ and 5′-GTTGGGCTTTCTGACTGCAT-3′, OSM receptor, 5′-AGGAGGAAGGCTGGATGAA-3′ and 5′-TCCACACGCCTGGACAGA-3′, gp130, 5′-TCATGTTCCTTCTATCGGGTC-3′ and 5′-CTGAGGGACCGGTGGTGT-3′, CYP2F2, 5′-TAATGTTGGACACAGAGCGG-3′ and 5′-ATCCTGGAAGAAGGCAGCTT-3′, Lysozyme, 5′-CGGTTTTGACATTGTGTTCG-3′ and 5′-AAGAATGCCTGTGGGATCAA-3′, MUC5AC, 5′-ATGGCTCCAGTCAGACCTTCA-3′ and 5′-CAGGCAGCCACACTTCTCAA-3′, and SPLUNC1, 5′-GCTGCGGATCAGTGATTTTT-3′ and 5′-GGCAATTCTAATGGCCTTGT-3′. Primer sequences for 18S as control were the same as that used for RT-PCR. PCR condition used was 95°C for 10 minutes, followed by 95°C for 3 seconds, 60°C for 30 seconds for 40 cycles. All data were calculated from Ct values and were normalized to 18S.
Electrophoretic Mobility Shift Analysis
Nuclear protein extracts were prepared from cytokine-treated mtCC or NIH3T3 cells using a modification of the original method described by Schreiber and coworkers (26). A double-stranded oligonucleotide (21 nt) probe was prepared based on the STAT-binding DNA sequence present in the mouse Ugrp2 gene promoter (12), and used as a probe in the gel-shift assays. Binding reactions were performed at room temperature for 30 minutes by incubating radiolabeled probe DNA (> 105 cpm) with 5 μg of nuclear extracts prepared from cytokine-stimulated cells (30 min at 37°C) in the presence of 2 μg poly dI-dC (Amersham Biosciences, Piscataway, NJ), 10 mM DTT, 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, and 50% glycerol in a final volume of 20 μl. For supershift analysis, 1 μl of antibody was added. After incubation, 8 μl of each mixture was electrophoresed on nondenaturing 6% polyacrylamide gels (Novex;, Invitrogen) using 0.25× TBE electrophoresis buffer containing 22 mM Tris-HCl (pH 8.0), 22 mM borate, and 0.5 mM EDTA. Gels were then dried and visualized by autoradiography. Several rabbit polyclonal anti-STAT antibodies were used to determine the composition of the STAT protein complexes that were detectable by electrophoretic mobility shift analysis (EMSA). The anti-STAT1 (sc-417), anti-STAT3 (sc-482), and anti-STAT5 (sc-835) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). An anti-actin antibody (sc-10731; Santa Cruz Biotechnology) was used as a nonspecific antibody control.
Northern Blot Analysis
Total RNAs from mtCC cells (5 μg) was electrophoresed on 1% agarose gel containing 0.22 M formaldehyde and blotted onto GeneScreen Plus nylon membranes (Perkin Elmer Life Sciences, Boston, MA). Filters were serially hybridized with mouse SCGB3A1, SCGB3A2, and ribosomal protein B36 (loading control) as a probe. Hybridization was performed in Perfect Hybridization solution (Amersham Biosciences) at 68°C overnight. The membrane was washed twice with 2× SSC containing 0.1% SDS at 68°C for 30 minutes, once with 2× SSC at 68°C for 30 minutes, followed by exposure to storm phosphoimager screen (Molecular Dynamics, Sunnyvale, CA). The signals were visualized and quantitated with ImageQuant software (Molecular Dynamics), and the band intensity for SCGB3A1 or SCGB3A2 was divided by that of ribosomal protein B36 for normalization.
Western Blot Analysis
mtCC cells were washed with phosphate-buffered saline (PBS) and lysed in RIPA buffer (20 mM Tris-HCl, 150 mM NaCl, 0.1% SDS, 0.1% sodium deoxycholate, 1% TritonX-100, 2 mM EDTA, 10 mM NaF, 1 mM Sodium Orthovanadate) with Protease Inhibitor Cocktail Tablets (Complete Mini; Roche Applied Science). The protein lysates were mixed with SDS sample loading buffer containing β-mercaptoethanol, electrophoresed on 10% SDS-polyacrylamide gel (PAGE), which were then electrotransferred to a PDVF membrane (Polyscreen; Perkin Elmer, Waltham, MA). Membranes were blocked with PBST (PBS with 0.2% Tween 20) containing 5% skim milk, and were incubated with primary antibody. For p-MAPKAPK2, TBST (TBS-Tween; 20 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20) were used instead of PBST. Membranes were washed with PBST or TBST, and incubated with a horseradish peroxidase–conjugated secondary antibody (GE Healthcare [Piscataway, NJ], Cell Signaling Technology [Danvers, MA] for p-MAPKAPK2). Protein bands were detected using chemiluminescence reagent (NEL 104001EA; Perkin Elmer) and exposing to a Scientific Imaging Film (Kodak, Rochester, NY). Antibodies used for Western blotting were as follows: STAT1 (sc-417) and STAT5 (sc-835X) from Santa Cruz Biotechnology; pSTAT1 (#9171), STAT3 (#9132), p-STAT3 (#9131), ERK (#9102), p-ERK (#9106), p38 (#9212), p-p38 (#9211), MAPKAPK2 (#3042), and p-MAPKAPK2 (#3044) from Cell Signaling Technology; and phospho-STAT5 (ab13593) from Abcam (Cambridge, MA).
Flow Cytometry Analysis
After trypsinization, mtCC cells (1 million) were incubated with 100 ng of anti-mouse OSM receptor antibody (MBL international, Woburn, MA) or rat IgG2a isotype as a control in 1 ml of staining buffer (PBS containing 1% BSA) at 4°C for 30 minutes, followed by incubation with 100 ng of anti-rat IgG2a-phycoerythrin (PE) (eBioscience, San Diego, CA) in staining buffer for 30 minutes. Cells were washed twice with 1 ml of staining buffer after each antibody treatment. Cellular fluorescence was measured with a BD Biosciences FACSCalibur flow cytometer (Mountain View, CA).
RESULTS
Bidirectional Regulation of SCGB3A1 and SCGB3A2 mRNA Expression by OSM
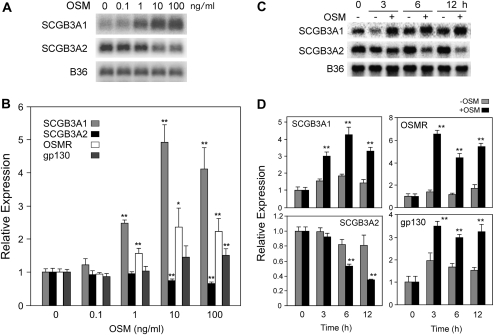
The effect of OSM on the expression of mouse SCGB3A1 and SCGB3A2 mRNAs was examined in mouse transformed Clara cells (mtCC) that are derived from tumor tissues of lungs obtained from transgenic mice expressing the simian virus 40 large T antigen gene under the control of the uteroglobin (UG)/Clara cell secretory protein (CCSP) promoter (25). This cell line constitutively expresses modest and high levels of SCGB3A1 and SCGB3A2 mRNAs, respectively (Figure 1) (11, 12). Treatment with 1 ng/ml OSM for 6 hours robustly induced SCGB3A1 expression, which reached the maximum levels at 10 ng/ml, as demonstrated by Northern blotting and quantitative RT-PCR analysis (Figures 1A and 1B, respectively). In contrast, a significant decrease of SCGB3A2 mRNA levels was observed at 10 ng/ml and higher concentrations of OSM, demonstrating an inverse dose response between these two genes. Time course experiments on the SCGB3A1 and SCGB3A2 mRNA levels in the presence and absence of 100 ng/ml OSM demonstrated that the OSM-induced enhancement of SCGB3A1 expression was statistically significant at 3 hours, while the reduction of SCGB3A2 expression was statistically significant after 6 hours of OSM treatment and thereafter up to 12 hours (Figures 1C and 1D). These results demonstrate that OSM increases SCGB3A1 and reduces SCGB3A2 expression in a time- and dose-dependent manner. The OSM-induced reduction of SCGB3A2 expression appears to be delayed compared with that of SCGB3A1.
Figure 1.
Regulation of secretoglobin (SCGB)3A1 and SCGB3A2 mRNA expression by oncostatin M (OSM). (A) Mouse transformed Clara cells (mtCC) were treated with indicated amount of OSM for 6 hours, followed by Northern blot analysis for the expression of SCGB3A1 and SCGB3A2. (B) The same experiments were performed as in A, but were analyzed for the expression of SCGB3A1, SCGB3A2, OSM receptor (OSMR), and gp130 by qRT-PCR. (C) mtCC cells were treated with 100 ng/ml of OSM for the indicated period of time, followed by Northern blot analysis for the expression of SCGB3A1 and SCGB3A2. (D) The same experiments were performed as in C, but were analyzed for the expression of SCGB3A1, SCGB3A2, OSMR, and gp130 by qRT-PCR. B36 was used as a loading control in the Northern blot. qRT-PCR results are shown as the mean ± SD from triplicate samples based on the level at 0 ng/ml OSM (in B) or time 0 (in D) as 1. *P < 0.05, **P < 0.01 by Student's t test relative to the level at 0 ng/ml OSM in each gene (B) or with and without OSM in each time point (D).
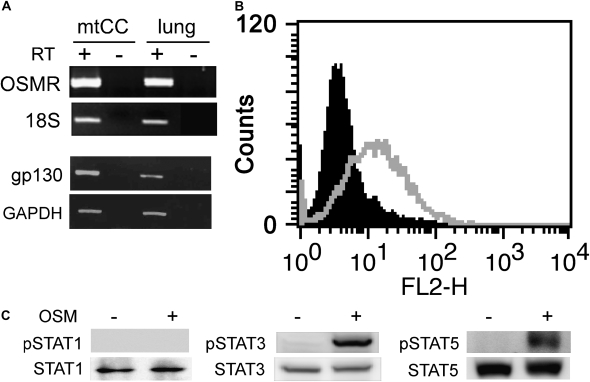
Since cytokine signaling starts with the binding of cytokines to their specific cell surface receptor complexes, the presence of receptors that mediate OSM signaling was analyzed by RT-PCR (Figure 2A) and fluorescence-activated cell sorter analysis (Figure 2B). The results demonstrated that both OSM receptor and gp130 are expressed in mtCC cells as determined by RT-PCR, and an OSM receptor is located on the surface of mtCC cells. This is in good agreement with a previous report in which the presence of the OSM receptor was demonstrated in the alveolar and bronchiolar epithelia of mouse neonate lung (27). When the effect of OSM on the expression of OSM receptor and gp130 was examined, their expression increased in a fashion similar to that of SCGB3A1. Maximum expression was obtained at 10 ng/ml OSM or higher, and 3 hours after OSM treatment (Figures 1B and 1D). Since OSM signaling is known to involve STAT1, STAT3, and STAT5, activation of these three STATs upon OSM stimulation in mtCC cells was next examined (17–19) (Figure 2C). A marked increase in phosphorylated STAT3 and STAT5 was observed upon OSM treatment, whereas no phosphorylation of STAT1 was observed by OSM treatment. These results suggest that STAT3 and STAT5 may be mainly involved in the OSM signaling pathway in mtCC cells.
Figure 2.
Analysis of OSM signaling molecules. (A) RT-PCR analysis for the presence of OSM (OSMR) and gp130 receptors in mtCC cells. The size of PCR product is 511 bp for OSMR and 325 bp for gp130 with the primers used. RT(−) is a negative control. RNAs prepared from fetal lungs were used as a positive control. PCR product for OSMR using lung RNA without RT reaction (lung, RT−) was run on a separate gel, and the image was attached to those showing other PCR products. (B) mtCC cells were subjected to flow cytometry analysis for the presence of OSM receptor on the cell surface. The solid area was obtained with mtCC cells alone. The gray line represents mtCC cells incubated with anti-OSMR and anti-goat IgG-PE. Note that all cells are positive for OSM receptor. (C) Activation of STATs in mtCC cells by OSM. mtCC cells were treated with 100 ng/ml OSM for 5 minutes, and whole cell lysates were subjected to Western blot analysis using antibody against STAT1, phospho (p)STAT1, STAT3, pSTAT3, STAT5, and pSTAT5 with (+) and without (−) OSM.
Identification of OSM-Responsive Elements in Mouse Scgb3a1 Gene Promoter
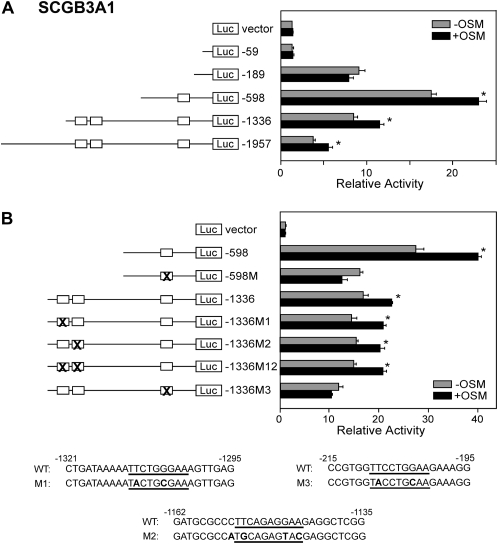
To determine whether STAT3/5 are involved in SCGB3A1 expression, six mouse Scgb3a1 gene promoter–luciferase constructs (pGL4, −59, −189, −598, −1,336, and −1,957) were prepared and subjected to transfection analysis into mtCC cells (Figure 3A). Basal luciferase activity markedly increased with the construct -189 as compared with −59, while OSM treatment did not affect the promoter activity in either construct. The reporter activity was significantly increased upon OSM treatment with constructs −598 and up to −1,957 bp. Within −1,336 bp of the Scgb3a1 gene promoter region, there were three putative STAT-binding elements (SBEs) (located −201 to −209, −1,144 to −1,153, and −1,302 to −1,310 bp relative to the transcription start site). Mutations were introduced in each binding site using reporter constructs −598 and −1,336, and transfection analysis was again performed (Figure 3B). All mutated constructs (−1336M1, M2, M12) showed similar luciferase activities to each other and to that of construct −1336 with and without OSM. Introduction of mutation into the −201 to −209 SBE (M3) completely abolished OSM-induced increase of luciferase reporter activities for both construct −598 and −1,336, suggesting that the −201 to −209 SBE is critical for the OSM-induced mouse Scgb3a1 gene promoter activity, and that the other two binding sites may be dispensable.
Figure 3.
Mouse Scgb3a1 gene promoter analysis. (A) A series of Scgb3a1 promoter–luciferase constructs as indicated were transiently transfected into mtCC cells for measurement of promoter activity in the presence and absence of 50 ng/ml OSM. (B) Luciferase promoter activities in mtCC cells using various STAT-binding element (SBE) mutants derived from the constructs −598 and −1,336 in the presence and absence of 50 ng/ml OSM. Relative luciferase activity is shown as the mean ± SD based on that of vector only as 1. Experiments were repeated three times, each performed in duplicate. Open boxes indicate a putative SBE. X shows SBE to which the mutation was introduced. *P < 0.05 by Student's t test between presence and absence of OSM in each construct. Mutated sequences are shown at the bottom. Three putative SBEs are underlined and the mutated nucleotides are boldfaced.
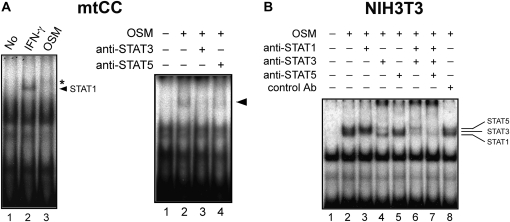
To determine whether STAT3/5 bind to the −201 to −209 SBE upon OSM stimulation, EMSA was performed using a 32P-labeled double-stranded oligonucleotide covering −195 to −215 bp of the mouse Scgb3a1 gene promoter (Figure 4). Nuclear extracts prepared from OSM-treated mtCC cells produced a very faint band(s), which moved more slowly than IFN-γ–activated STAT1 (Figure 4A, left panel). No band corresponding to STAT1 was observed upon OSM treatment in mtCC cells, supporting the Western blotting results shown in Figure 2C. The band intensity markedly decreased by the addition of anti-STAT3 antibody and slightly decreased by anti-STAT5 antibody (Figure 4A, right panel). The results suggest that the faint OSM-shifted band is in fact composed of two bands, which correspond to STAT3 and STAT5. To confirm the binding of STAT3 and STAT5 to the −201 to −209 SBE of the Scgb3a1 gene promoter, nuclear extracts prepared from NIH3T3 cells were subjected to EMSA (Figure 4B). In this cell line, three shifted bands were obtained upon OSM stimulation that corresponded to STAT1, 3, and 5. Each band was completely supershifted by the addition of each corresponding antibody, demonstrating that STAT1, 3, and 5 can all bind to the proximal SBE in the Scgb3a1 gene promoter. EMSA using oligonucleotides containing the −1,144 to −1,153 and −1,302 to −1,310 SBEs, did not produce any specific shifted band (data not shown), further suggesting that these two putative SBEs are not involved in Scgb3a1 gene regulation. These results altogether demonstrate that OSM-activated STAT3 and STAT5 can bind to the proximal −201 to −209 SBE in the Scgb3a1 promoter, which may lead to the activation of Scgb3a2 transcription in mtCC cells.
Figure 4.
Binding of STATs to the proximal SBE of Scgb3a1 promoter. Electrophoretic mobility shift analysis was performed using a probe containing the proximal SBE and nuclear extracts prepared from mtCC (A) and NIH3T3 cells (B) that had been treated with 100 ng/ml of OSM for 30 minutes with and without addition of various anti-STAT antibodies as indicated. IFN-γ–treated nuclear extract was used to demonstrate the position of STAT1-specific band (A, left panel, lane 2, indicated by an arrow). The band(s) produced by OSM treatment are indicated by an asterisk in the left panel and an arrow in the right panel in A. In B, the bands corresponding to STAT1, STAT3, and STAT5 are indicated on the right.
Analysis on OSM-Responsive Elements in Mouse Scgb3a2 Gene Promoter
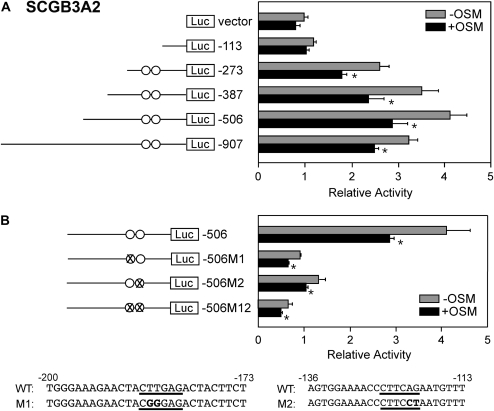
While SCGB3A1 is up-regulated upon OSM treatment, SCGB3A2 expression is down-regulated by OSM, as shown in Figure 1. To examine whether OSM transcriptionally represses SCGB3A2 expression, transfection analysis was performed with and without OSM treatment using six different mouse Scgb3a2 promoter luciferase-reporter constructs (pGL4, −113, −273, −387, −506, −907) (Figure 5). Transcriptional repression was clearly demonstrated with the constructs having upstream sequences longer than -273, suggesting that the area between −113 and −273 bp in relative to the transcription start site of the gene is responsible for the transcriptional repression (Figure 5A). Note that there was no STAT-binding site in this region, nor any other areas within −907 bp of the mouse Scgb3a2 promoter. In the −113 and −273 bp region, two NKX2-1–binding sites were found that were previously identified as responsible for transcription of the Scgb3a2 gene (2). When mutations were introduced into one of the NKX2-1–binding sites, reporter activity was down to 25 to 30% of wild-type, while the extent of OSM-induced reduction of promoter activity was similar to that observed with the parent construct (Figure 5B). Further, the double mutations significantly affected the relative luciferase activity, yet the OSM response was retained. These results suggest that NKX2-1 may not be involved in OSM repression of Scgb3a2 promoter activity.
Figure 5.
Mouse Scgb3a2 gene promoter analysis. (A) A series of Scgb3a2 gene promoter–luciferase constructs were transfected into mtCC cells for measurement of promoter activity in the presence and absence of 50 ng/ml OSM. (B) Luciferase promoter activities in mtCC cells using NKX2-1–binding site mutants derived from the construct −506 in the presence and absence of 50 ng/ml OSM. Relative luciferase activity is shown as the mean ± SD based on that of vector only as 1. Experiments were repeated three times, each performed in duplicate. Circles indicate NKX2-1–binding sites (2). X indicates NKX2-1–binding site to which mutation is introduced. Mutated sequences for two NKX2-1–binding sites are shown at the bottom (underlined) and the mutated nucleotides are boldfaced. *P < 0.05 by Student's t test between presence and absence of OSM in each construct.
To determine the sequence between −113 and −273 bp of the Scgb3a2 gene to which a DNA-binding protein may bind upon OSM stimulation, EMSA was extensively performed using 11 oligonucleotides whose sequences are serially overlapping from −113 through −273 bp. No specific differences in band intensity and/or pattern were observed between OSM-treated and nontreated mtCC cells nuclear extracts (see Figure E1 in the online supplement). These results may suggest an indirect effect of OSM on OSM-induced decrease of SCGB3A2 expression.
Finally, to examine whether the sequence between −113 and −273 bp of the Scgb3a2 gene suppresses luciferase activity upon OSM treatment with a heterologous promoter, this region was inserted at both directions into −189 and −598 Scgb3a1 luciferase constructs, the latter containing the OSM-responsive proximal SBE. OSM treatment did not affect luciferase activity of either Scgb3a1 construct (data not shown), suggesting that OSM-induced inhibitory effect of the −113 to −273 bp region of Scgb3a2 gene on its promoter activity appears to be gene specific.
Involvement of MAPK Pathways in the Expression of SCGB3A1 and SCGB3A2
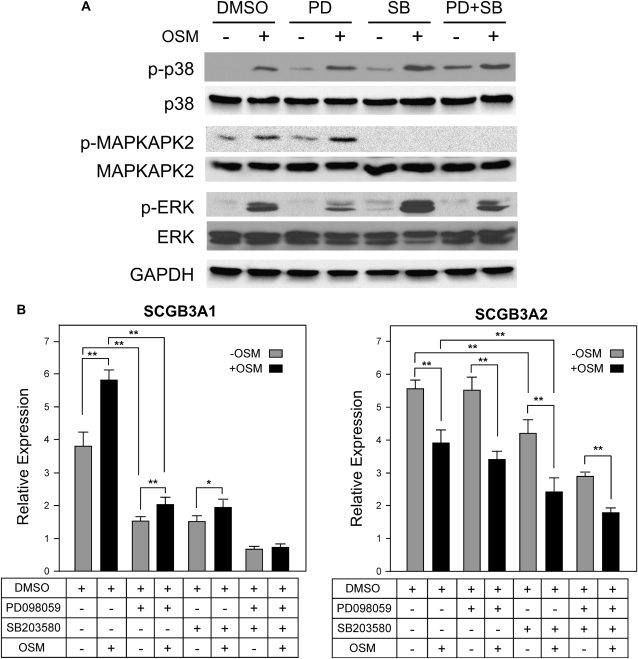
In addition to the JAK/STAT pathway, OSM is also known to activate MAPKs, in particular extracellular-regulated kinase (ERK)-1 and 2 (p42/p44), and P38 kinase (24). To determine if the ERK- and/or p38-MAPK pathways are activated in mtCC cells upon OSM stimulation, phosphorylation of ERK1/2, p38, and MAPKAPK2 was examined (Figure 6A). Treatment with the MAPK/ERK kinase (MEK) inhibitor PD098059 reduced OSM-induced ERK1/2 phosphorylation, whereas the p38 inhibitor SB203580 enhanced OSM-induced ERK1/2 phosphorylation. When both inhibitors were added together, the phosphorylation level of ERK was similar to the level in cells treated with OSM alone. SB202580 did not inhibit phosphorylation of p38 upon OSM treatment; however, the downstream MAPKAPK2 phosphorylation was completely inhibited. In contrast, the ERK and p38-MAPK pathways were endogenously slightly up-regulated by SB203580.
Figure 6.
Analysis of MAPK signaling pathways. (A) ERK1/2, p38, and MAPKAPK2 activation in mtCC cells by OSM stimulation was determined by Western blotting. mtCC cells were pretreated with either 10 μM of PD098059 (MEK inhibitor) or SB203580 (p38 kinase inhibitor) for 1 hour, followed by treatment of 20 ng/ml OSM in the presence of either or both inhibitors together for 30 minutes. GAPDH was used as a loading control. Photoshop curve adjustment was employed to increase the clarity of the bands. (B) Expression of SCGB3A1 and SCGB3A2 was detected by qRT-PCR analysis. DMSO used as a solvent for dissolving inhibitors was used as a control. *P < 0.05, **P < 0.01 by Student's t test between indicated groups.
In the absence of OSM stimulation, when mtCC cells were treated with PD098059, the endogenous level of SCGB3A1 expression decreased to less than half the DMSO control level (Figure 6B, left panel). A similar response was observed when mtCC cells were treated with SB203580. In both cases, OSM treatment increased SCGB3A1 expression, but to a smaller extent than control cells. With both inhibitors together, the level of SCGB3A1 expression further decreased, and the cells were no longer responsive to OSM stimulation. These results suggest that the ERK- and p38-MAPK pathways may be at least partly responsible for both endogenous and the OSM-induced increase of SCGB3A1expression.
In contrast, endogenous expression of SCGB3A2 was not affected by the addition of PD098059, but was reduced approximately 30% by SB203580 as compared with control cells (Figure 6B, right panel). Both inhibitors together decreased the endogenous SCGB3A2 expression level to approximately one-half of the control levels, suggesting a possible cooperative effect of the ERK- and p38-MAPK pathways on SCGB3A2 gene expression. OSM-induced decrease of SCGB3A2 expression was similar regardless of the presence or absence of either PD098059, SB203580, or both inhibitors together as compared with the control levels. These results suggest that the endogenous SCGB3A2 expression is partly regulated by the ERK- and p38-MAPK pathways. However, neither the ERK- nor p38-MAPK pathways are involved in the OSM-induced decrease of SCGB3A2 expression.
OSM-Induced Changes in Airway Phenotypes
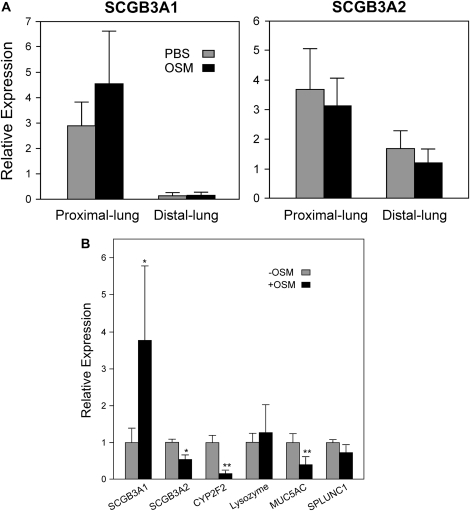
It was demonstrated that SCGB3A1 is a marker for the upper airway in mouse, while SCGB3A2 is expressed throughout the airway tree (28). To examine whether OSM may serve as a switch to the upper airway phenotype in the airway tree, OSM was intratracheally intubated to mice. The proximal and distal lungs were separately isolated, RNA prepared, and each subjected to qRT-PCR (Figure 7A). In control mice, the level of SCGB3A1 expression was high in proximal lung, while very low expression was found in the distal lung. In contrast, the level of SCGB3A2 expression was high in both proximal and distal lungs; distal lung expressed approximately half the level of SCGB3A2 in the proximal lung. These results validate the differential expression of SCGB3A1 and SCGB3A2 along the airway tree. Upon OSM treatment, a clear trend was obtained in which SCGB3A1 expression in proximal lung increased while SCGB3A2 expression decreased in both proximal and distal lungs. These results suggest that OSM treatment might shift airway cells toward those having proximal lung phenotypes.
Figure 7.
Effect of OSM on gene expression. (A) Mice were intratracheally administered 1 μg/20 g body weight of OSM, and 24 hours later, RNAs were separately prepared from the proximal and distal lungs. SCGB3A1 and SCGB3A2 expression levels were determined by qRT-PCR. The expression levels are shown as the mean ± SD from eight mice for OSM and four mice for PBS treatment as control. (B) mtCC cells were treated with or without 100 ng/ml OSM for 6 hours, and isolated RNAs were subjected to qRT-PCR for various genes indicated.
To examine whether OSM alters expression of other proteins characteristic of the airway epithelia, qRT-PCR was performed using RNAs isolated from mtCC cells for the expression of CYP2F2 (cytochrome P450 2F2) (28), SPLUNC1 (short palate, lung, and nasal epithelium clone 1) (29, 30), lysozyme (31), and MUC5AC (mucin 5AC) (32, 33) (Figure 7B). In parallel with the decrease of SCGB3A2 expression, CYP2F2 expression was markedly decreased upon OSM treatment. MUC5AC expression was also reduced to approximately half of that without OSM. Lysozyme and SPLUNC1 expression stayed at similar levels regardless of OSM treatment. Since CYP2F2 is expressed in Clara cells (28), from which mtCC cells are originally derived (25), these results suggest that OSM may mostly affect Clara cell physiology. SCGB3A2 was suggested to serve as a molecular marker for the lineage of Clara cells in the experiments using the naphthalene-treated injured mouse lung model (28, 34). Thus, the expression levels of SCGB3A1, SCGB3A2, OSM receptor, gp130, and CYP2F2 were determined after mtCC cells were subjected to transfection, the process that may be interpreted as an injury by mtCC cells. None of the mRNA levels were changed by transfection compared with sham transfection, as determined by qRT-PCR (data not shown).
DISCUSSION
In the present study, we demonstrated that OSM regulates the expression of SCGB3A1 and SCGB3A2 in an opposite manner; OSM up-regulates SCGB3A1 expression while it down-regulates SCGB3A2 expression. OSM is a multifunctional cytokine that belongs to the IL-6/LIF family and is mainly produced in activated T cells, monocytes, and macrophages (18, 19). Although OSM was originally recognized by its unique activity to inhibit the proliferation of tumor cells, accumulating evidence indicates that OSM exhibits many unique biological activities in inflammation, hematopoiesis, and development (18, 19). In the lung, OSM induces inflammation through eosinophil infiltration (24), and strongly elevates expression of TIMP-1, matrix metalloproteinase (MMP)-1, and MMP-9 in fibroblasts that are involved in remodeling of extracellular matrix (18, 35, 36), suggesting the importance of OSM in lung inflammation. Previously, SCGB3A1 and SCGB3A2 were suggested to be involved in lung inflammation (1, 2, 12–15). In fact, a suppressive role of SCGB3A2 in lung inflammation was demonstrated using recombinant adenovirus expressing SCGB3A2 that was intranasally administered to a mouse model for allergic airway inflammation (16).
While circulating levels of OSM are normally undetectable in vivo, levels rise over 100-fold to 45 pM (∼ 1 μg/ml) in patients with sepsis (37). Patients diagnosed with the acute respiratory distress syndrome exhibit even greater increases in circulating OSM levels in plasma (38). In culture, dendritic cells after stimulation with lipopolysaccharide produces OSM in the pg to ng/ml range (39), while treating Th1 cells with anti-CD3 produces OSM at the level of several hundreds pg/ml (40). Thus, it is possible that OSM locally produced under an inflammatory response may reach levels as high as μg/ml, within the concentration range (1–100 ng/ml) that was used in this study. Under these OSM levels, SCGB3A1 and SCGB3A2 expression are positively and negatively regulated. This further supports the notion that SCGB3A1 and SCGB3A2 play a role in lung inflammation.
OSM's bidirectional regulation of SCGB3A1 and SCGB3A2 expression in mtCC cells might imply that OSM shifts mtCC cells that have distal airway cell phenotypes toward those having a proximal airway cell phenotype. Our in vivo study may support this hypothesis; when mice were administered OSM intratracheally, a clear trend was obtained in which SCGB3A1 expression increased in the proximal lungs while SCGB3A2 expression decreased in both proximal and distal lungs. Molecular phenotype of this conversion was further studied by the expression of other epithelial proteins in mtCC cells. Among the genes examined, CYP2F2 expressed in Clara cells (28) demonstrated markedly decreased expression upon OSM treatment, which was in good accordance with SCGB3A2 expression. The parallel reduction of CYP2F2 and SCGB3A2 expression after OSM treatment suggest that OSM may mainly affect Clara cell physiology, resulting in apparent shift of airway cell phenotype.
Alternatively, bidirectional regulation of SCGB3A1 and SCGB3A2 expression by OSM might have a role in lung carcinogenesis. In recent years, the connection between inflammation and cancer has been clearly documented (41, 42). SCGB3A1 was established as a tumor suppressor and its expression is silenced in the majority of carcinomas, including those from breast, prostate, lung, and pancreas (3, 7–10). Promoter methylation plays a role in the expression of human SCGB3A1 in normal versus cancerous tissues (3, 7–10) and mouse SCGB3A1 tissue-specific expression (43). In contrast, we found that SCGB3A2 is highly expressed in tumor tissues from mammary gland and colon in humans in whom no expression is normally found (T. Kusakabe and S. Kimura, unpublished observation). While the mechanism for this ectopic expression is not known, methylation might also play a role in tissue-specific and/or ectopic expression of SCGB3A2 in tumor cells. It is interesting to speculate that at least in some tumor cells, OSM might contribute to inhibition of tumor growth through bidirectional regulation of SCGB3A1 as a tumor suppressor and SCGB3A2 as a gene whose expression is increased in tumors. The exact role for this bidirectional control of OSM in SCGB3A1 and 3A2 expression in the lung, however, requires further studies.
STAT3/5 appear to be responsible for up-regulation of SCGB3A1 expression by OSM. Promoter reporter analysis revealed that OSM-activated STAT3/5 bind to an SBE located at −201 to −209 bp upstream of the Scgb3a1 transcription start site, and activates transcription of the gene in mtCC cells. The proximal −201 to −209 bp SBE was previously described as the STAT6-binding site upon IL-4/IL-13 treatment, which resulted in increase of SCGB3A1 expression (12). It seems that the proximal SBE is critical for OSM-induced as well as IL-4/IL-13–induced enhancement of SCGB3A1 expression through the binding of OSM-activated STAT3/5 and IL-4/IL-13–activated STAT6 to the same proximal SBE. The experiments using MAPK inhibitors revealed that both ERK- and p38-MAPK pathways are at least partially responsible for endogenous and/or the OSM-induced increase in SCGB3A1 expression. A large decrease in endogenous SCGB3A1 activity found in the presence of PD098059 without OSM did not correlate well with the fact that this inhibitor barely inhibited endogenous basal ERK phsophorylation. We do not know the reason for this discrepancy. Nevertheless, OSM regulates SCGB3A1 expression through multiple OSM signaling pathways.
The reduction of Scgb3a2 gene expression appears to be transcriptionally regulated. The reporter assay data for Scgb3a2 was in good agreement with the mRNA analysis. The region containing NKX2-1–binding sites (−113 to −273 bp) may be responsible for the OSM-induced reduction of Scgb3a2 gene expression; however, NKX2-1 did not seem to be directly involved in this event. The −113 to −273 sequence did not have any inhibitory effect when connected to Scgb3a1 reporter construct, suggesting that it is a promoter-dependent event. Analysis with MAPK pathway inhibitors PD098059 and SB203580 suggests that the p38-MAPK pathway may be predominantly involved in the endogenous level of SCGB3A2 expression, but not OSM-induced reduction. Extensive EMSA analysis failed to identify the binding of a possible transcription factor(s) to the −113 to −273 sequence that might be involved in OSM down-regulation of Scgb3a2 transcription. Note that there is no STAT-binding site within −907 bp of the mouse Scgb3a2 promoter. These results suggest that OSM indirectly inhibits SCGB3A2 expression through unknown mechanism. This may be the reason for the delayed response of SCGB3A2 to OSM stimulation as compared with SCGB3A1 as seen in Figure 1.
An inhibitory effect of signaling molecules on gene expression was documented for STAT6 (44) and TGF-β1 (45–47). STAT6 was demonstrated to bind to the Th1-specific IL-4 silencer element located in the 3′ untranslated region of the IL-4 gene and inhibit IL-4 silencer function in IL-4–producing cells, thus playing a permissive role in determining the commitment to the Th2 phenotype (44). In the case of TGF-β1, the mechanism for inhibitory effect of TGF-β1 varies; TGF-β1 down-regulates TNF-α–induced RANTES production through blocking the degradation of cytosolic IκB-α, thus decreasing TNF-α-induced NF-κB binding to the RANTES promoter (47). TGF-β1–mediated repression of lipoprotein lipase gene expression involves Sp1/3; however, Sp1/3′s binding to their specific binding sites in the promoter and their steady-state polypeptide levels are not affected by the presence of TGF-β1 as revealed by EMSA, suggesting a novel mechanism (45). Further, TGF-β1-inhibition of the rat prolactin promoter activity is due to the TGF-β1 inhibitory element located −116/−54 region in the promoter, in which TGF-β1 failed to alter significantly the levels of specific DNA–protein binding (46). The latter two results are somewhat similar to what we observed for the inhibitory effect of OSM on SCGB3A2 expression. The exact mechanism for the OSM down-regulation of Scgb3a2 gene expression without significantly affecting binding affinity of DNA binding proteins is not known. This might be accomplished by modulation of accessory proteins that are able to interact with DNA-binding proteins in a way that does not significantly affect their DNA binding.
In conclusion, OSM bidirectionally regulates SCGB3A1 and SCGBA2 expression transcriptionally in a time- and dose-dependent manner. STAT3/5 are involved in up-regulation of SCGB3A1 expression by OSM while both ERK- and p38-MAPK pathways are responsible for endogenous as well as OSM-induced increase of SCGB3A1 expression. In contrast, the p38-MAPK pathway is mainly responsible for endogenous expression of SCGB3A2, while OSM down-regulation of SCGB3A2 may be through an indirect mechanism. This bidirectional control of SCGB3A1 and SCGB3A2 expression by OSM may underlie their individual unique function in the lung.
Supplementary Material
Acknowledgments
We would like to thank Drs. Francesco DeMayo for mtCC cells and Frank Gonzalez for his critical review of the manuscript.
This research was supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2008-0062OC on October 31, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Niimi T, Copeland NG, Gilbert DJ, Jenkins NA, Srisodsai A, Zimonjic DB, Keck-Waggoner CL, Popescu NC, Kimura S. Cloning, expression, and chromosomal localization of the mouse gene (Scgb3a1, alias Ugrp2) that encodes a member of the novel uteroglobin-related protein gene family. Cytogenet Genome Res 2002;97:120–127. [DOI] [PubMed] [Google Scholar]
- 2.Niimi T, Keck-Waggoner CL, Popescu NC, Zhou Y, Levitt RC, Kimura S. UGRP1, a uteroglobin/clara cell secretory protein-related protein, is a novel lung-enriched downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor. Mol Endocrinol 2001;15:2021–2036. [DOI] [PubMed] [Google Scholar]
- 3.Krop IE, Sgroi D, Porter DA, Lunetta KL, LeVangie R, Seth P, Kaelin CM, Rhei E, Bosenberg M, Schnitt S, et al. HIN-1, a putative cytokine highly expressed in normal but not cancerous mammary epithelial cells. Proc Natl Acad Sci USA 2001;98:9796–9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klug J, Beier HM, Bernard A, Chilton BS, Fleming TP, Lehrer RI, Miele L, Pattabiraman N, Singh G. Uteroglobin/clara cell 10-kDa family of proteins: Nomenclature committee report. Ann N Y Acad Sci 2000;923:348–354. [DOI] [PubMed] [Google Scholar]
- 5.Porter D, Lahti-Domenici J, Torres-Arzayus M, Chin L, Polyak K. Expression of high in normal-1 (HIN-1) and uteroglobin related protein-1 (UGRP-1) in adult and developing tissues. Mech Dev 2002;114:201–204. [DOI] [PubMed] [Google Scholar]
- 6.Postma DS, Bleecker ER, Amelung PJ, Holroyd KJ, Xu J, Panhuysen CI, Meyers DA, Levitt RC. Genetic susceptibility to asthma–bronchial hyperresponsiveness coinherited with a major gene for atopy. N Engl J Med 1995;333:894–900. [DOI] [PubMed] [Google Scholar]
- 7.Krop I, Parker MT, Bloushtain-Qimron N, Porter D, Gelman R, Sasaki H, Maurer M, Terry MB, Parsons R, Polyak K. HIN-1, an inhibitor of cell growth, invasion, and AKT activation. Cancer Res 2005;65:9659–9669. [DOI] [PubMed] [Google Scholar]
- 8.Krop I, Player A, Tablante A, Taylor-Parker M, Lahti-Domenici J, Fukuoka J, Batra SK, Papadopoulos N, Richards WG, Sugarbaker DJ, et al. Frequent HIN-1 promoter methylation and lack of expression in multiple human tumor types. Mol Cancer Res 2004;2:489–494. [PubMed] [Google Scholar]
- 9.Wong TS, Kwong DL, Sham JS, Tsao SW, Wei WI, Kwong YL, Yuen AP. Promoter hypermethylation of high-in-normal 1 gene in primary nasopharyngeal carcinoma. Clin Cancer Res 2003;9:3042–3046. [PubMed] [Google Scholar]
- 10.Shigematsu H, Suzuki M, Takahashi T, Miyajima K, Toyooka S, Shivapurkar N, Tomlinson GE, Mastrangelo D, Pass HI, Brambilla E, et al. Aberrant methylation of HIN-1 (high in normal-1) is a frequent event in many human malignancies. Int J Cancer 2005;113:600–604. [DOI] [PubMed] [Google Scholar]
- 11.Yamada A, Kimura S. Induction of uteroglobin-related protein 2 (Ugrp2) expression by EGF and TGFα. FEBS Lett 2005;579:2221–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada A, Sheikh F, Niimi T, DeMayo FJ, Keegan AD, Donnelly RP, Kimura S. Induction of uteroglobin-related protein 2 (Ugrp2) gene expression by the Th2 cytokines IL-4 and IL-13. J Immunol 2005;175:5708–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiba Y, Kusakabe T, Kimura S. Decreased expression of uteroglobin-related protein 1 in inflamed mouse airways is mediated by IL-9. Am J Physiol Lung Cell Mol Physiol 2004;287:L1193–L1198. [DOI] [PubMed] [Google Scholar]
- 14.Chiba Y, Srisodsai A, Supavilai P, Kimura S. Interleukin-5 reduces the expression of uteroglobin-related protein (UGRP) 1 gene in allergic airway inflammation. Immunol Lett 2005;97:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srisodsai A, Kurotani R, Chiba Y, Sheikh F, Young HA, Donnelly RP, Kimura S. Interleukin-10 induces uteroglobin-related protein (UGRP) 1 gene expression in lung epithelial cells through homeodomain transcription factor T/EBP/NKX2.1. J Biol Chem 2004;279:54358–54368. [DOI] [PubMed] [Google Scholar]
- 16.Chiba Y, Kurotani R, Kusakabe T, Miura T, Link BW, Misawa M, Kimura S. Uteroglobin-related protein 1 expression suppresses allergic airway inflammation in mice. Am J Respir Crit Care Med 2006;173:958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimura A, Ichihara M, Kinjyo I, Moriyama M, Copeland NG, Gilbert DJ, Jenkins NA, Hara T, Miyajima A. Mouse oncostatin M: An immediate early gene induced by multiple cytokines through the JAK-STAT5 pathway. EMBO J 1996;15:1055–1063. [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka M, Miyajima A. Oncostatin M, a multifunctional cytokine. Rev Physiol Biochem Pharmacol 2003;149:39–52. [DOI] [PubMed] [Google Scholar]
- 19.Chen SH, Benveniste EN, Oncostatin M. A pleiotropic cytokine in the central nervous system. Cytokine Growth Factor Rev 2004;15:379–391. [DOI] [PubMed] [Google Scholar]
- 20.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 2003;374:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara T, Tamura K, de Miguel MP, Mukouyama Y, Kim H, Kogo H, Donovan PJ, Miyajima A. Distinct roles of oncostatin M and leukemia inhibitory factor in the development of primordial germ cells and sertoli cells in mice. Dev Biol 1998;201:144–153. [DOI] [PubMed] [Google Scholar]
- 22.Yanagisawa M, Nakashima K, Taga T. STAT3-mediated astrocyte differentiation from mouse fetal neuroepithelial cells by mouse oncostatin M. Neurosci Lett 1999;269:169–172. [DOI] [PubMed] [Google Scholar]
- 23.Richards CD, Kerr C, Tanaka M, Hara T, Miyajima A, Pennica D, Botelho F, Langdon CM. Regulation of tissue inhibitor of metalloproteinase-1 in fibroblasts and acute phase proteins in hepatocytes in vitro by mouse oncostatin M, cardiotrophin-1, and IL-6. J Immunol 1997;159:2431–2437. [PubMed] [Google Scholar]
- 24.Langdon C, Kerr C, Tong L, Richards CD. Oncostatin M regulates eotaxin expression in fibroblasts and eosinophilic inflammation in C57BL/6 mice. J Immunol 2003;170:548–555. [DOI] [PubMed] [Google Scholar]
- 25.Magdaleno SM, Wang G, Jackson KJ, Ray MK, Welty S, Costa RH, DeMayo FJ. Interferon-gamma regulation of clara cell gene expression: in vivo and in vitro. Am J Physiol Lung Cell Mol Physiol 1997;272:L1142–L1151. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res 1989;17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura S, Morikawa Y, Tanaka M, Miyajima A, Senba E. Developmental expression pattern of oncostatin M receptor β in mice. Mech Dev 2002;115:127–131. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds SD, Reynolds PR, Pryhuber GS, Finder JD, Stripp BR. Secretoglobins SCGB3A1 and SCGB3A2 define secretory cell subsets in mouse and human airways. Am J Respir Crit Care Med 2002;166:1498–1509. [DOI] [PubMed] [Google Scholar]
- 29.Chu HW, Thaikoottathil J, Rino JG, Zhang G, Wu Q, Moss T, Refaeli Y, Bowler R, Wenzel SE, Chen Z, et al. Function and regulation of SPLUNC1 protein in mycoplasma infection and allergic inflammation. J Immunol 2007;179:3995–4002. [DOI] [PubMed] [Google Scholar]
- 30.Weston WM, LeClair EE, Trzyna W, McHugh KM, Nugent P, Lafferty CM, Ma L, Tuan RS, Greene RM. Differential display identification of plunc, a novel gene expressed in embryonic palate, nasal epithelium, and adult lung. J Biol Chem 1999;274:13698–13703. [DOI] [PubMed] [Google Scholar]
- 31.Hiemstra PS. Epithelial antimicrobial peptides and proteins: their role in host defence and inflammation. Paediatr Respir Rev 2001;2:306–310. [DOI] [PubMed] [Google Scholar]
- 32.Gray T, Koo JS, Nettesheim P. Regulation of mucous differentiation and mucin gene expression in the tracheobronchial epithelium. Toxicology 2001;160:35–46. [DOI] [PubMed] [Google Scholar]
- 33.Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, Davis CW, Randell SH. Mucin gene expression during differentiation of human airway epithelia in vitro. MUC4 and MUC5B are strongly induced. Am J Respir Cell Mol Biol 1999;20:595–604. [DOI] [PubMed] [Google Scholar]
- 34.Watson TM, Reynolds SD, Mango GW, Boe IM, Lund J, Stripp BR. Altered lung gene expression in CCSP-null mice suggests immunoregulatory roles for Clara cells. Am J Physiol Lung Cell Mol Physiol 2001;281:L1523–L1530. [DOI] [PubMed] [Google Scholar]
- 35.Korzus E, Nagase H, Rydell R, Travis J. The mitogen-activated protein kinase and JAK-STAT signaling pathways are required for an oncostatin M-responsive element-mediated activation of matrix metalloproteinase 1 gene expression. J Biol Chem 1997;272:1188–1196. [DOI] [PubMed] [Google Scholar]
- 36.Richards CD, Shoyab M, Brown TJ, Gauldie J. Selective regulation of metalloproteinase inhibitor (TIMP-1) by oncostatin M in fibroblasts in culture. J Immunol 1993;150:5596–5603. [PubMed] [Google Scholar]
- 37.Guillet C, Fourcin M, Chevalier S, Pouplard A, Gascan H. ELISA detection of circulating levels of LIF, OSM, and CNTF in septic shock. Ann N Y Acad Sci 1995;762:407–409. [DOI] [PubMed] [Google Scholar]
- 38.Modur V, Feldhaus MJ, Weyrich AS, Jicha DL, Prescott SM, Zimmerman GA, McIntyre TM. Oncostatin M is a proinflammatory mediator. In vivo effects correlate with endothelial cell expression of inflammatory cytokines and adhesion molecules. J Clin Invest 1997;100:158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suda T, Chida K, Todate A, Ide K, Asada K, Nakamura Y, Suzuki K, Kuwata H, Nakamura H. Oncostatin M production by human dendritic cells in response to bacterial products. Cytokine 2002;17:335–340. [DOI] [PubMed] [Google Scholar]
- 40.Broxmeyer HE, Bruns HA, Zhang S, Cooper S, Hangoc G, McKenzie AN, Dent AL, Schindler U, Naeger LK, Hoey T, et al. Th1 cells regulate hematopoietic progenitor cell homeostasis by production of oncostatin M. Immunity 2002;16:815–825. [DOI] [PubMed] [Google Scholar]
- 41.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balkwill F, Mantovani A. Inflammation and cancer: back to virchow? Lancet 2001;357:539–545. [DOI] [PubMed] [Google Scholar]
- 43.Tomita T, Kimura S. Regulation of mouse Scgb3a1 gene expression by NF-Y and association of CpG methylation with its tissue-specific expression. BMC Mol Biol 2008;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kubo M, Ransom J, Webb D, Hashimoto Y, Tada T, Nakayama T. T-cell subset-specific expression of the IL-4 gene is regulated by a silencer element and STAT6. EMBO J 1997;16:4007–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irvine SA, Foka P, Rogers SA, Mead JR, Ramji DP. A critical role for the Sp1-binding sites in the transforming growth factor-beta-mediated inhibition of lipoprotein lipase gene expression in macrophages. Nucleic Acids Res 2005;33:1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farrow KN, Gutierrez-Hartmann A. Transforming growth factor-beta1 inhibits rat prolactin promoter activity in GH4 neuroendocrine cells. DNA Cell Biol 1999;18:863–873. [DOI] [PubMed] [Google Scholar]
- 47.Cho ML, Min SY, Chang SH, Kim KW, Heo SB, Lee SH, Park SH, Cho CS, Kim HY. Transforming growth factor beta 1(TGF-beta1) down-regulates TNFalpha-induced RANTES production in rheumatoid synovial fibroblasts through NF- kappaB-mediated transcriptional repression. Immunol Lett 2006;105:159–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.