Abstract
Genetic hybrids of the genus Xiphophorus have historically been useful models for study of the genetic aspects of tumor formation. In the most studied Xiphophorus tumor model, two gene loci, XMRK and DIFF, are implicated as critical both to UV-induced and spontaneous melanoma formation in BC1 hybrids of crosses between X. maculatus and X. helleri, with X. helleri as the recurrent backcross parent. In addition to UV, the direct acting carcinogen N-methyl-N-nitrosourea (MNU) has been used to induce tumors in Xiphophorus BC1 hybrids from several cross types. In the present study, we address the hypothesis that excess melanomas in MNU-treated BC1 hybrids may have been generated by direct mutation of CDKN2AB, a candidate gene for DIFF. MNU treatment of F1 and BC1 hybrid fish significantly increased tumor incidence at 6 months; however, no association was found between MNU-induced tumor formation and zygosity of the candidate tumor suppressor CDKN2AB in BC1 hybrids, consistent with previously reported results. Sequence analysis of the X. maculatus CDKN2AB locus of heterozygous individuals (both BC1 and F1 hybrids) did not reveal any mutations caused by MNU, suggesting that the mechanism of MNU-induced melanoma formation in this Xiphophorus model does not involve direct mutation of CDKN2AB but may result from mutation of other critical genes.
Keywords: Xiphophorus, MNU, melanoma, CDKN2AB
Introduction
Fishes of the genus Xiphophorus have historically been useful models for the study of genetic aspects of melanoma formation. Interspecies Xiphophorus hybrids are often employed in these studies due to the increased susceptibility of hybrid offspring to various tumors (Gordon, 1931; Anders et al., 1979; Schartl, 1995; Nairn et al., 1996a). Studies of hybrids from these genetic crosses have yielded important information about both spontaneous and induced melanoma development (Vielkind et al., 1989; A. Schartl et al., 1997). The sex-linked pigmentation patterns in Xiphophorus fishes consist of the precursor melanocytes and large melanin-containing cells termed macromelanophores that are either enhanced or suppressed depending on the cross performed (Humm and Young, 1956). One such cross between X. maculatus Jp 163 B with a spotted-side (Sp) pigmentation pattern and X. helleri sarabia strains generates pigmented backcross hybrids that have been well studied for UV induction of melanoma. In this instance, backcross progeny develop melanomas if treated with UV shortly after birth (Setlow, et al., 1989; Nairn, et al., 1996b). The inheritance of melanoma susceptibility is linked to the segregation of a single autosomal gene (termed DIFF) with inheritance of the sex-linked pigment pattern locus (XMRK-Sp) (Anders, 1991; Schartl, 1995; Kazianis et al., 1998). Progeny of the initial cross (F1 hybrids) are heterozygous for both loci and show an enhancement of the X. maculatus Sp phenotype. When these F1 progeny are backcrossed to X. helleri, half do not inherit the Sp locus and consequently do not develop a pigmentation pattern. The remaining half are heterozygous for Sp and develop either a heavy or light pigmentation pattern depending on the zygosity of their inherited DIFF allele (Nairn et al., 2001). Thus the susceptibility of hybrid offspring to melanoma formation can be described by this two-gene model involving XMRK and DIFF.
The XMRK locus encodes an oncogenic receptor tyrosine kinase-related gene, which acquired a different promoter during a gene duplication event, resulting in altered transcriptional control specific for pigment cells (Adam et al., 1993). Two amino acid changes in the XMRK protein occurring after gene duplication have resulted in ligand independent and constitutively active signaling (Gomez et al., 2004) leading to induction of both Ras-Raf-MAPK-dependent and independent pathways, resulting in unregulated cellular proliferation (Meierjohann and Schartl, 2006).
Previous work identified the gene CDKN2AB, a Xiphophorus homologue to the mammalian cyclin dependent kinase inhibitor (CDKN2) gene family, as a candidate gene for DIFF based on linkage and QTL analysis (Nairn, et al., 1996b; Kazianis et al., 1998). Sequence analysis of CDKN2AB alleles from X. maculatus and X. helleri revealed only two amino acid differences suggesting that there were no functional differences between the proteins encoded by these two alleles; however differential levels of transcription from X. maculatus and X. helleri alleles were detected, with the X. maculatus allele overexpressed more than 11-fold in BC1 heterozygotes. Additionally, backcross progeny homozygous for the X. helleri DIFF allele develop the heavily pigmented phenotype and are more likely to develop melanomas than heterozygous individuals (Kazianis et al., 1999). In the two-gene model for inheritance of melanoma susceptibility, DIFF would act downstream from XMRK as a tumor-suppressor gene with the X. maculatus and X. helleri alleles expressing CDKN2AB (candidate for DIFF) at different levels. In heterozygous hybrids, the higher expression from the X. maculatus allele may be sufficient to compensate for the proliferation signals driven by XMRK. However in homozygous BC1 individuals, which inherit only the CDKN2AB from X. helleri, the reduced expression may not fully mitigate the oncogenic signal from XMRK, leading to an increased incidence of melanoma in these individuals (Nairn et al., 2001; Butler et al., 2007).
The direct acting carcinogen N-methyl-N-nitrosourea (MNU) has been used in addition to UV to induce melanomas in hybrid fish of the cross described above (X. maculatus Jp 163 B with X. helleri). Previous work using MNU to induce melanomas in BC1 hybrids indicated that XMRK was a critical genetic determinant of induced melanoma susceptibility. However, unlike the results of experiments with UV-induced tumors, no genetic association was found between CDKN2AB alleles and MNU-induced melanoma susceptibility. Tumor incidence in these experiments was about twice that of UV-induced melanomas in the same cross (Kazianis et al., 2001).
In heterozygous individuals, the X. maculatus allele would be expected to provide adequate CDKN2AB protein to compensate for the lower expression of the X. helleri allele, thereby counteracting the proliferation signals generated by increased expression of XMRK (Nairn et al., 2001). However, if MNU treatment causes mutations within the X. maculatus CDKN2AB allele that either inactivate it or result in a lowered expression level, the anti-proliferative effect may be eliminated. The work described here aims to confirm the previous result that melanomas are induced by MNU treatment in this genetic cross, and to test the hypothesis that the X. maculatus CDKN2AB allele in heterozygous BC1 individuals might be a target for MNU, causing direct mutations in CDKN2AB that may result in the observed increase in tumor incidence in MNU-treated hybrids. Such a direct mutational mechanism may be masking any genetic association that would otherwise be revealed by linkage analysis.
Materials and methods
Generation of hybrid offspring and MNU exposure
Hybrid offspring were generated by the following crossing scheme (Figure 1): Female X. maculatus of strain Jp 163 B bearing the “spotted side” (Sp) pigment pattern were artificially inseminated with sperm collected from X. helleri males. F1 hybrid offspring were collected and backcrossed to X. helleri to generate backcross (BC1) progeny. Five week old F1 and BC1 hybrid offspring were treated with 1 mM MNU (Sigma, St. Louis) for one hour as described previously (Kazianis et al., 2001). This exposure was repeated every other day for a total of 4 treatments. UVB tumors were induced as described by Nairn et al. (1996b), then excised and quick frozen until use.
Figure 1.
Diagram depicting the crossing scheme used to generate the interspecies backcross hybrids used in this study. An X. maculatus homozygote for a spot sided pigment pattern (Sp) is crossed with a X. helleri lacking this pigmentation pattern (+/+). The resulting F1 hybrid individuals exhibit an enhancement of the spot sided pigment pattern and are heterozygous for both the Sp and DIFF locus. Male F1 hybrids are further crossed with female X. helleri to generate first-generation backcross (BC1) hybrids, which exhibit a Mendelian distribution of pigmentation phenotypes. Individuals heterozygous for Sp are pigmented to different degrees (heavy or light) as regulated by the DIFF gene. Those progeny heterozygous for the DIFF gene (one allele X. helleri, one allele X. maculatus) have pigmentation patterns resembling the F1 hybrids while those homozygous for the X. helleri DIFF allele are heavily pigmented and are more susceptible to UV-induced melanomas.
Tumors from both treated and untreated fish were scored at 6 months or earlier and were allowed to progress until they were large enough to yield sufficient tissue for analysis. Tissues were removed and immediately frozen on liquid nitrogen then kept at −80°C. Selected tumor tissues were fixed in 10% buffered formalin and embedded in paraffin for histological examination.
DNA isolation, genotyping and genetic analysis of CDKN2AB allele
DNA was isolated from frozen gill samples taken from tumor-bearing hybrid fish using the Puregene kit (Gentra Systems, Minneapolis, MN) and the genotype of each BC1 fish was determined at the CDKN2AB locus using a previously described PCR-based assay (Kazianis et al., 1998). DNA was then isolated from tumors of heterozygous BC1 individuals. Two regions encompassing the two exons of the X. maculatus CDKN2AB allele were amplified by PCR using the primer sets p16S3 (TGTCGCCGACAATGACCG) + p16AS1 (TGCCTTGTCTGCCAGTTATTACC) with a 68°C annealing temperature yielding a 331 bp product and p16S2 (GTAGTAGTGCAATAGCGAGACATAAA) + p16AS2 (GCCTTATTCACGGTTCTCAATCAG) with a 66°C annealing temperature yielding a 542 bp (Figure 2). Each PCR product was purified (Promega SV PCR cleanup kit) and cloned using the Invitrogen TA cloning system. Six independent clones were picked from each reaction, grown up and DNA was prepared using the Promega Wizard Plus Miniprep system. After confirming that all clones contained the appropriately sized insert, each was sequenced with the T7 primer contained within the TA cloning vector using an ABI Prism 3100 DNA Sequencer and the BigDye Terminator kit (ABI). All sequences were aligned and analyzed using MacVector software (MacVector, Inc. Cary, NC).
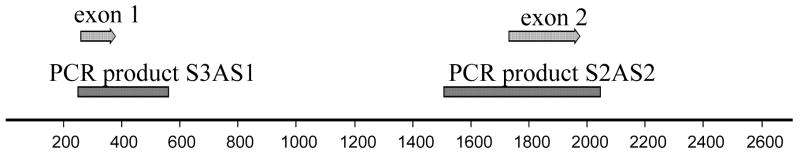
Figure 2.
Diagram of X. maculatus CDKN2AB gene locus. Shown is the complete gene (without the 5′ UTR) with the locations of the two exons depicted as light gray arrows. The two PCR products used for sequence analysis are shown as dark gray boxes below the two exons. See Materials and Methods for primer sequences.
Results
The present work was performed for the purpose of validating previously reported results regarding the induction of melanomas by MNU in offspring of a specific Xiphophorus cross (Kazianis et al., 2001), as well as to test the hypothesis that the X. maculatus CDKN2AB allele in heterozygous BC1 individuals might be a target for MNU. During the course of this study 176 F1 hybrid and 401 BC1 hybrid offspring of the Xiphophorus cross depicted in Figure 1 were treated with MNU yielding 14 and 87 cases of melanoma respectively (Table 1). Melanomas were only observed in offspring inheriting the Sp pigmentation pattern, consequently incidence was only calculated for Sp-bearing fish. Untreated control F1 (186 individuals) and BC1 (230 individuals) fish were also maintained and monitored for melanoma development. A subset of melanomas was sampled for histology and representative examples are shown in Figure 3 where the infiltration of the fish musculature by melanoma cells is clearly evident. The incidence of melanoma formation was significantly elevated in both groups of hybrids treated with MNU when compared to control groups (Table 1). However, the difference in incidence was more dramatic in BC1 hybrid individuals (7.95% in F1 hybrids compared to 21.7% in BC1 hybrids).
Table 1.
BC1 hybrid melanoma incidence†
| Treatment group | Total fish | Melanoma (% incidence) | |
|---|---|---|---|
| F1 hybrid | Control | 186 | 2 (1.07%) |
| MNU | 176 | 14 (7.95%)* | |
|
| |||
| BC1 hybrid | Control | 230 | 17 (6.1%) |
| MNU | 401 | 87 (21.7%)** | |
Incidence was calculated for Sp-bearing fish only
Significant difference using Chi square test p<0.005
Significant difference using Chi square test p<0.001
Figure 3.
Histopathology of melanomas taken from hybrid fish. A. and C. Representative examples of melanomas taken in cross section through the body of two fish. Note the melanoma infiltrating the muscular structure. 4x magnification. B. and D. High magnification of the same tumors. Note the muscle bundle compressed by the invasion of the melanoma. 40x magnification.
The increased incidence of MNU-induced tumor formation among BC1 fishes was hypothesized to be due to the activity of the two different CDKN2AB alleles (X. maculatus vs. X. helleri) present in BC1 offspring. Previous work (Nairn et al., 1996a, 1996b) has shown that spontaneous and UV-induced melanoma formation was correlated with the homozygous CDKN2AB genotype where tumor-bearing individuals were more likely to be homozygous for the X. helleri allele. In order to examine this association in the case of MNU-induced melanoma formation, gills from tumor bearing BC1 individuals were used to establish the genotype of each fish at the CDKN2AB locus. Unlike the data for spontaneous and UV-induced melanoma, no association between melanoma formation and CDKN2AB genotype was found when fish bearing MNU-induced tumors were analyzed (Table 2). Our results confirm those of Kazianis et al., (2001) who also reported lack of a genetic linkage in MNU-induced tumors.
Table 2.
Association of melanoma formation with CDKN2AB genotype
| Treatment | Genotyped fish with melanomas | Heterozygotes | Homozygotes | Recomb. % | lod* |
|---|---|---|---|---|---|
| Control† | 49 | 8 | 41 | 16.3 | 5.3 |
| UVB† | 64 | 14 | 50 | 21.9 | 4.7 |
| MNU | 36 | 12 | 24 | 33.3 | 0.9 |
Previously reported data included for comparison (Kazianis et al., 2001)
A lod value above 3.0 is considered significant evidence for linkage association
It was previously hypothesized that a direct mutation in the X. maculatus CDKN2AB allele might be masking any genetic association between tumor formation and genotype (Kazianis et al., 2001). Such a direct mutation within the X. maculatus CDKN2AB allele could lead to an excess of melanomas in heterozygous individuals. Direct sequencing of two regions encompassing the two exons of the X. maculatus CDKN2AB allele was performed in order to test for such direct mutations. Both MNU and UV-induced tumors as well as spontaneous tumors were sequenced but no mutations were found among any of the groups analyzed (Table 3) suggesting the mechanism of MNU-induced melanoma formation does not involve direct mutation of the candidate tumor suppressor gene CDKN2AB.
Table 3.
CDKN2AB sequence analysis
| No. tumors analyzed * | Total sequence reactions analyzed ** | Mutations found | |
|---|---|---|---|
| Control BC1 | 3 | 36 | 0 |
| UVB treated BC1 | 33 | 396 | 0 |
| MNU treated BC1 | 10 | 120 | 0 |
| MNU treated F1 | 8 | 96 | 0 |
Only tumors from individuals heterozygous at CDKN2AB locus were analyzed
Includes both primer sets
Discussion
The Xiphophorus fish model is an excellent example of an inducible model of cancer and study of this model has been important for understanding the impact of UV irradiation on melanoma formation in humans (Setlow et al., 1989, 1993). In this study, we confirmed (with a larger sample size) previously reported results (Kazianis et al., 2001) describing the induction of melanoma using the direct-acting carcinogen MNU on progeny from the UV-inducible X. maculatus Jp 163 B and X. helleri cross. In our study, the BC1 offspring of this cross exhibited a 3.5-fold increase in MNU-induced melanoma susceptibility compared to untreated individuals. These results suggest a genetic component to the induction of melanomas by MNU whereby backcross progeny are more likely to develop melanoma than parental or F1 generation offspring.
Previously performed linkage analysis of UV-induced melanomas suggested an association between tumor formation and the DIFF candidate tumor suppressor CDKN2AB genotype where BC1 individuals homozygous for the X. helleri allele were more likely to develop melanomas than their heterozygous counterparts (Nairn et al., 1996a; 1996b). However, when this association was examined in the context of MNU-induced melanomas previously (Kazianis et al., 2001) and again in this study, no significant linkage was found between incidence of MNU-induced melanomas and CDKN2AB genotype. One possible reason for this lack of association, as postulated by Kazianis et al., (2001), was that direct mutations within the somatic X. maculatus CDKN2AB gene may be masking the linkage association by making heterozygous (one copy each of X. maculatus and X. helleri CDKN2AB alleles) individuals as likely to develop melanoma as those with two X. helleri alleles. Such a mutation could inactivate the X. maculatus allele resulting in functional expression of CDKN2AB from only the X. helleri allele. However, after sequence analysis of two regions containing the entire coding sequence of the X. maculatus CDKN2AB allele in heterozygous BC1 fish with UV and MNU-induced tumors, no such mutation was found, suggesting that a somatic mutation within the X. maculatus CDKN2AB allele was not the cause of the increased tumor incidence in BC1 hybrids.
It is hypothetically possible that a promoter mutation, or a mutation in the untranslated region (UTR) of the X. maculatus CDKN2AB allele, rather than in the coding sequence, could have been induced by MNU and resulted in inactivation; our analyses would not have detected such events. There are examples of human mutations in CDKN2A in which an aberrant initiation codon occurs upstream from the normal translational start site, and suppresses its expression presumably due to competition with the normal AUG (Liu et al., 1999, Harland et al., 2000). However, this is an example of a founder non-coding sequence mutation predisposing to melanoma, and it is extremely unlikely that in our experiments such mutations would have occurred exclusively, that is, in the absence of coding sequence mutations as well.
Previous work demonstrated a positive relationship between the XMRK oncogene and CDKN2AB expression in melanomas (Butler et al., 2007). A previously proposed hypothetical model explaining this relationship between CDKN2AB overexpression and XMRK-induced hyperproliferation in UV-induced melanomas depends on differential expression between the two CDKN2AB alleles (Nairn et al., 2001) as is observed in heterozygous individuals with UV-induced tumors (Kazianis et al., 1999). In this model, overexpression of the X. maculatus CDKN2AB allele relative to the X. helleri allele in heterozygous individuals provides sufficient CDKN2AB to compensate for the proliferation signals generated by elevated XMRK expression and fewer tumors result. In homozygous individuals with two alleles of X. helleri, the lower expression of CDKN2AB is insufficient to compensate for XMRK proliferation signals and tumors develop (Nairn et al., 2001). An analysis of the promoter structure for several Xiphophorus species has been performed and has revealed interesting differences, most notably the length of a (GT)n repeat sequence and the presence of a deletion in the X. helleri promoter relative to that of X. maculatus (Kazianis et al., 1999). If this differential expression were altered by the action of MNU on the promoter of the X. maculatus allele resulting in decreased expression of CDKN2AB, an increased incidence of melanomas may result.
An alternative explanation for the increased incidence of MNU-induced melanomas in the BC1 fish is that MNU is acting on a different genetic target responsible for regulation of pigment cell proliferation or transformation in interspecies crosses. It has been hypothesized that the DIFF locus (for which CDKN2AB is a candidate) may not solely regulate melanoma formation in the cross used in this study in contrast to co-inheritance of spontaneous melanoma and pigment patterns in the classic Gordon-Kosswig cross (Nairn et al., 1996b). Studies of mammalian systems using MNU to induce tumors in rats have shown that the oncogenes p53 and Ras may be specific targets for MNU-induced activating mutations (Sukumar et al., 1991; Matsumoto et al., 1997). Activating mutations in both p53 and Ras are thought to be important in the development of human malignant melanoma along with mutations in the CDKN2A gene (the human homologue for Xiphophorus CDKN2AB) (Benjamin et al., 2007). It is possible that MNU mutations in p53 and/or Ras or other genes may be contributing to the increase in MNU-induced melanomas in BC1 hybrids regardless of the zygosity at the CDKN2AB locus; however, in this case an upregulation of Ras would be unlikely to increase melanoma formation as XMRK (a upstream activator of Ras) is already overexpressed in tumors (Schartl, 1990). Additional studies may yield important information about how the genes in this complex model work together to result in the initiation of melanoma.
Acknowledgments
This work was supported by CA055245 and NIEHS Center grant ES07784. The authors would like to acknowledge the Science Park Molecular Biology Facility Core for performing the DNA sequencing.
Footnotes
This paper is derived from a presentation given at the 4th Aquatic Animal Models of Human Disease Conference: hosted by Duke University’s Nicholas School of the Environment and Earth Sciences, and Duke’s Comprehensive Cancer Center, Durham, NC, USA, January 31 - February 3, 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam D, Dimitrijevic N, Schartl M. Tumor suppression in Xiphophorus by an accidentally acquired promoter. Science. 1993;259:816–819. doi: 10.1126/science.8430335. [DOI] [PubMed] [Google Scholar]
- Anders F, Diehl H, Schwab M, Anders A. Contributions to an understanding of the cellular origin of melanoma in the Gordon-Kosswig xiphophorine fish tumor system. Pigment Cell Res. 1979;4:142–149. [Google Scholar]
- Anders F. Contributions of the Gordon-Kosswig melanoma system to the present concept of neoplasia. Pigment Cell Res. 1991;3:7–29. doi: 10.1111/j.1600-0749.1991.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Benjamin CL, Melnikova VO, Ananthaswamy HN. Models and mechanisms in malignant melanoma. Mol Carcinog. 2007;46:671–678. doi: 10.1002/mc.20353. [DOI] [PubMed] [Google Scholar]
- Butler AP, Trono D, Coletta LD, Beard R, Fraijo R, Kazianis S, Nairn RS. Regulation of CDKN2A/B and Retinoblastoma genes in Xiphophorus melanoma. Comp Biochem Physiol C. 2007;145:145–155. doi: 10.1016/j.cbpc.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Gomez A, Volff JN, Hornung U, Schartl M, Wellbrock C. Identification of a second egfr gene in Xiphophorus uncovers an expansion of the epidermal growth factor receptor family in fish. Mol Biol Evol. 2004;21:266–275. doi: 10.1093/molbev/msh017. [DOI] [PubMed] [Google Scholar]
- Gordon M. The Hereditary Basis for Melanosis in Hybrids of Mexican Killifishes. Proc Natl Acad Sci USA. 1931;17:276–280. doi: 10.1073/pnas.17.5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humm DG, Young RS. The embryological origin of pigment cells in platyfish-swordtail hybrids. Zoologica. 1956;41:1–10. [Google Scholar]
- Kazianis S, Gutbrod H, Nairn RS, McEntire BB, Della Coletta L, Walter RB, Borowsky RL, Woodhead AD, Setlow RB, Schartl M, Morizot DC. Localization of a CDKN2 gene in linkage group V of Xiphophorus fishes defines it as a candidate for the DIFF tumor suppressor. Genes Chromosomes Cancer. 1998;22:210–220. [PubMed] [Google Scholar]
- Kazianis S, Gimenez-Conti I, Trono D, Pedroza A, Chovanec LB, Morizot DC, Nairn RS, Walter RB. Genetic analysis of neoplasia induced by N-nitroso-N-methylurea in Xiphophorus hybrid fish. Mar Biotechnol (NY) 2001;3:S37–43. doi: 10.1007/s10126001-0025-2. [DOI] [PubMed] [Google Scholar]
- Kazianis S, Morizot DC, Coletta LD, Johnston DA, Woolcock B, Vielkind JR, Nairn RS. Comparative structure and characterization of a CDKN2 gene in a Xiphophorus fish melanoma model. Oncogene. 1999;18:5088–5099. doi: 10.1038/sj.onc.1202884. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Iwase T, Hirono I, Nishida Y, Iwahori Y, Hori T, Asamoto M, Takasuka N, Kim DJ, Ushijima T, Nagao M, Tsuda H. Demonstration of ras and p53 gene mutations in carcinomas in the forestomach and intestine and soft tissue sarcomas induced by N-methyl-N-nitrosourea in the rat. Jpn J Cancer Res. 1997;88:129–136. doi: 10.1111/j.1349-7006.1997.tb00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meierjohann S, Schartl M. From Mendelian to molecular genetics: the Xiphophorus melanoma model. Trends Genet. 2006;22:654–661. doi: 10.1016/j.tig.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Nairn RS, Kazianis S, Della Coletta L, Trono D, Butler AP, Walter RB, Morizot DC. Genetic analysis of susceptibility to spontaneous and UV-induced carcinogenesis in Xiphophorus hybrid fish. Mar Biotechnol (NY) 2001;3:S24–36. doi: 10.1007/s1012601-0004-7. [DOI] [PubMed] [Google Scholar]
- Nairn RS, Kazianis S, McEntire BB, Della Coletta L, Walter RB, Morizot DC. A CDKN2-like polymorphism in Xiphophorus LG V is associated with UV-B-induced melanoma formation in platyfish-swordtail hybrids. Proc Natl Acad Sci USA. 1996a;93:13042–13047. doi: 10.1073/pnas.93.23.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn RS, Morizot DC, Kazianis S, Woodhead AD, Setlow RB. Nonmammalian models for sunlight carcinogenesis: genetic analysis of melanoma formation in Xiphophorus hybrid fish. Photochem Photobiol. 1996b;64:440–448. doi: 10.1111/j.1751-1097.1996.tb03089.x. [DOI] [PubMed] [Google Scholar]
- Schartl A, Pagany M, Engler M, Schartl M. Analysis of genetic factors and molecular mechanisms in the development of hereditary and carcinogen-induced tumors of Xiphophorus. Rec Results Cancer Res. 1997;143:225–235. doi: 10.1007/978-3-642-60393-8_15. [DOI] [PubMed] [Google Scholar]
- Schartl A, Malitschek B, Kazianis S, Borowsky R, Schartl M. Spontaneous melanoma formation in nonhybrid Xiphophorus. Cancer Res. 1995;55:159–165. [PubMed] [Google Scholar]
- Schartl M. Platyfish and swordtails: a genetic system for the analysis of molecular mechanisms in tumor formation. Trends Genet. 1995;11:185–189. doi: 10.1016/S0168-9525(00)89041-1. [DOI] [PubMed] [Google Scholar]
- Schartl M. Homology of melanoma-inducing loci in the genus Xiphophorus. Genetics. 1990;126:1083–1091. doi: 10.1093/genetics/126.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M, Haas J, Abdo S, Ahuja MR, Kollinger G, Anders A, Anders F. Genetic basis of susceptibility for development of neoplasms following treatment with N-methyl-N-nitrosourea (MNU) or x-rays in the platyfish/swordtail system. Experientia. 1978;34:780–782. doi: 10.1007/BF01947324. [DOI] [PubMed] [Google Scholar]
- Setlow RB, Woodhead AD, Grist E. Animal model for ultraviolet radiation-induced melanoma: platyfish-swordtail hybrid. Proc Natl Acad Sci USA. 1989;86:8922–8926. doi: 10.1073/pnas.86.22.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci USA. 1993;90:6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar S, Armstrong B, Bruyntjes JP, Leav I, Bosland MC. Frequent activation of the Ki-ras oncogene at codon 12 in N-methyl-N-nitrosourea-induced rat prostate adenocarcinomas and neurogenic sarcomas. Mol Carcinog. 1991;4:362–368. doi: 10.1002/mc.2940040507. [DOI] [PubMed] [Google Scholar]
- Vielkind JR, Kallman KD, Morizot DC. Genetics of melanomas in Xiphophorus fishes. J Aquat Anim Health. 1989;1:69–77. [Google Scholar]