Abstract
Adhesion to and subsequent extravasation through the endothelial lining of blood vessels is critical for tumor cells to establish metastases. Recent studies have indicated that polymorphonuclear neutrophils (PMNs) may enhance melanoma adhesion to the endothelium (EC) and subsequent extravasation under dynamic flow conditions. However, little is known about hydrodynamics involved in the tumor microenvironment within the microcirculation. In this study, effects of hydrodynamic flow on regulating melanoma cell adhesion to the EC have been investigated. Results indicate that under flow conditions, interactions between melanoma cells and the EC are distinctly different from PMN-EC interactions. Without expressions of surface integrins or sialylated molecules, most melanoma cells that express a high-level of intercellular adhesion molecule (ICAM-1) are not able to effectively adhere to the inflamed EC by themselves. Binding of melanoma cells and PMNs through ICAM-1 on melanoma cells and β2 integrins on PMNs has been shown to enhance melanoma cell arrest on the EC. Although PMN tethering on the EC is regulated by both the shear rate and shear stress, melanoma cell adhesion to the EC and subsequent extravasation via tethering PMN on the EC is predominantly regulated by shear rate, which partly is due to the shear-rate-dependent PMN-melanoma aggregation in shear flow. These findings provide a rationale and mechanistic basis for understanding of leukocyte-tumor cell interactions under flow conditions during tumor cell extravasation and metastasis.
Keywords: Shear flow, Neutrophil, β2 integrins, ICAM-1, Melanoma metastasis
INTRODUCTION
Malignant melanoma is the most deadly form of skin cancer due to its highly metastatic nature. To date, no effective treatment exists to prevent melanoma metastasis.34,39 Melanoma metastasis requires that tumor cells detach from a primary site and invade the surrounding stroma, survive immune defenses and turbulence of the blood circulation, extravasate through the endothelial lining of blood vessels, and finally form a new colony in the surrounding tissue. However, it is still not clear regarding how melanoma cells adhere to the endothelium (EC) and subsequently extravasate through it under the flow conditions in the microcirculation.
Significant progress has been made in the past decades toward understanding the mechanisms used by polymorphonuclear neutrophils (PMNs), which comprise 50-70% of circulating leukocytes, to adhere to the vascular EC and subsequently migrate to sites of inflammation. Studies indicate that endothelial selectins, including E-selectin, mediate PMN capture from the blood stream and rolling along the vascular wall,20,21 whereas the firm adhesion to the EC is mediated by β2 integrins (e.g., CD11a/CD18 or LFA-1, and CD11b/CD18 or Mac-1) on PMNs and intercellular cell adhesion molecule-1 (ICAM-1) on endothelial cells.40 Some studies also show that colon carcinoma cell adhesion to the EC involves sialylated molecules and integrins.4 However, not all melanoma cells express integrins or sialylated molecules at levels effective to adhere to the inflamed EC by themselves.37
During their passage through the circulatory system, tumor cells undergo extensive interactions with various host cells including PMNs.23 Although several recent studies have shown that PMNs may enhance melanoma transendothelial migration,37,43 little is known about mechanisms involved. Miele et al.27 reported that a dose- and time-dependent increase in surface expression of ICAM-1 upon stimulation of TNF-α was found in human malignant melanoma cells. They also found that inhibiting ICAM-1 reduced melanoma lung metastasis in vivo. Both endothelial and melanoma cells express ICAM-1, the potential ligand for β2 integrins on PMNs. Therefore, PMN-EC and PMN-melanoma cell adhesions could play important roles in bringing tumor cells into close proximity to the EC, thus facilitating their subsequent migration through the EC. Recent studies have quantified the strength and kinetics of LFA-1 and Mac-1 in PMN heterotypic aggregation to transfected cells expressing ICAM-1 in a shear flow.15,31 These studies showed that PMN adhesion to ICAM-1 is a cooperative and sequential process of LFA-1-dependent initial endothelial capture of PMNs followed by Mac-1-mediated stabilization. A similar adhesion mechanism has been observed for the interactions between PMNs and ICAM-1-expressing colon carcinomas.18,19 However, situations regarding how shear forces regulate PMN-melanoma interactions and subsequent melanoma cell extravasation through the EC are not entirely understood.
In the present study, we utilized a cone-plate viscometer to characterize the PMN-melanoma cell aggregation, a parallel-plate flow chamber to investigate PMN-mediated melanoma cell adhesion to the EC, and a flow-migration assay to examine melanoma cell extravasation under shear-flow conditions. Specifically, different roles of hydrodynamic shear stress, shear rate, and β2 integrins/ICAM-1 binding mechanisms were examined. Our results indicate that aggregation between PMNs and melanoma cells through β2 integrins/ICAM-1 binding is regulated by shear rate only. Although PMN tethering on the EC is affected by both shear rate and shear stress, melanoma cell adhesion to the EC and subsequent extravasation facilitated by tethering PMN on the EC is predominantly regulated by shear rate. These findings provide a rationale and mechanistic basis for understanding of leukocyte-tumor cell interactions under flow conditions, which also provide insights into potential therapeutic targets to melanoma extravasation and subsequent metastasis development.
MATERIALS AND METHODS
Reagents
Formyl-methionyl-leucyl-phenylalanine (fMLP) was purchased from Sigma (St. Louis, MO). Mouse anti-human LFA-1, mouse anti-human Mac-1, and mouse anti-human ICAM-1 monoclonal antibodies (mAbs) were purchased from Invitrogen (Carlsbad, CA).
Cell Preparations
Two different human melanoma cell lines WM9 and C8161.c9 were used in this study to correlate tumor metastatic potentials in terms of invasiveness, chemotactic migration, and adhesiveness as described earlier.9,37 C8161.c9 and WM9 melanoma cells were maintained and prepared as described previously.22 Prior to each experiment, melanoma cells were detached when confluent using 0.05% trypsin/versene (Invitrogen) and washed twice with fresh medium. Then the cells were re-suspended in fresh medium and allowed to recover for 1 h while being rocked at 8 rpm at 37 °C.
Fibroblast L-cells that had been transfected to express human E-selectin and ICAM-1 (EI cells) were maintained in culture as described elsewhere.35 EI cells constitutively express stable ICAM-1 comparable with IL-1β stimulated human umbilical vein endothelial cells14 and were used as an EC substrate for cell adhesion studies.
Fresh human blood was collected from healthy donors by venipuncture. PMNs were isolated using a Histopaque® (Sigma) density gradient by manufacturer’s instruction and kept at 4 °C in Dulbecco’s PBS (D-PBS) containing 0.1% human serum albumin for up to 4 h before use. For fMLP simulation, PMNs were incubated with 1 μM fMLP for 2 min at 37 °C. For blocking experiments, fMLP-stimulated PMNs were pre-treated for 30 min at 4 °C with blocking anti-LFA-1 or anti-Mac-1 mAbs at 5 μg/106 cells, respectively.
Dextran-supplemented Medium
RPMI 1640 medium was supplemented with 0.1% bovine serum albumin (BSA) and 1-4% ultra-high molecular weight dextran (2 × 106 MW; Sigma). A range of dextran-supplemented media were made to achieve a range of viscosities from 0.7cP (no dextran) to 7.0cP (4% dextran). Either the medium viscosity (μ) of the cell suspension, and thus the shear stress , or the shear rate could be held constant while the other be varied in order to determine the hydrodynamic shear effects on melanoma cell adhesion to PMNs and extravasation under flow conditions. Controlled tests assured the dextran-supplemented media did not affect the expression of adhesion molecules on PMNs, melanoma cells, and EI cells (data not shown). The osmolarity of dextran-supplemented media was also tested to verify there was not a significant increase due to the addition of dextran. Osmolarity increased by <1% in a 4% solution of dextran in RPMI 1640 compared to un-supplemented media.
Flow-migration Assays
The in vitro flow-migration device was recently developed and is a modified 48-well chemotactic Boyden chamber.37 In brief, the top and bottom plates of the polycarbonate chamber are separated by a 0.02-in.-thick silicon gasket (PharmElast, SF Medical, Hudson, MA). A 7 × 2 cm opening cut from the center of the gasket forms the flow field. The wall shear stress (τw) is related to the volumetric flow rate (Q) by τw = 6 μQ/wh2, where μ is the medium viscosity, h is height, and w is width of the flow field.
An endothelial monolayer was formed by growing EI cells to confluence on sterilized PVP-free polycarbonate filters (8 μm pore size; NeuroProbe, Gaithersburg, MD) coated with fibronectin (30 μg/mL, 3 h) (Sigma). The bottom side of the filter was scraped prior to use to remove any potential cell growth. Soluble type IV collagen (CIV; 100 μg/mL in RPMI 1640/0.1%BSA) was used as the chemoattractant in the center 12 wells and control wells were filled with medium (RPMI 1640/0.1%BSA). The chamber was assembled and the cells were then introduced into the chamber. Typical experiments involved cases such as: PMN only; melanoma cells only; PMN + melanoma cells (5 × 105 cells of each cell type). Flow of circulating medium was immediately perfused into the chamber, initially at a flow rate 2 mL/min, which was then increased to a desired experimental rate (0-20 mL/min). The entire chamber was placed in a 37 °C, 5% CO2 incubator for 4 h.
To quantify migration, the filter was removed from the chamber and immediately stained with HEMA-3 (Fisher Scientific, Pittsburgh, PA). The cells on the bottom side of each filter, which had been facing the chemoattractant wells, were imaged using an inverted microscope and recorded with NIH Image (v. β4.0.2). No cells were found in the chemoattractant wells after 4 h of migration. Three pictures were taken of each filter in different locations. The number of cells migrated was quantified and averaged for each filter. A minimum of three filters were analyzed for each data point. Background migration was subtracted from each sample as appropriate.
Parallel-plate Flow Assays
Experiments on WM9 adhesion to the EI monolayer in the presence of PMNs were performed in a parallel-plate flow chamber (Glycotech, Rockville, MD) mounted on the stage of a phase-contrast optical microscope (Diaphot 300, Nikon, Japan). A syringe pump (Harvard Apparatus, South Natick, MA) was used to generate a steady flow field in the flow chamber. A petri dish (35 mm) with a confluent EI cell monolayer (acting as a ligand-binding substrate) was attached to the flow chamber by vacuum. All experiments were performed at 37 °C. The field of view was 800 μm (direction of the flow) by 600 μm. The focal plane was set on the EI monolayer. The flow chamber was perfused with appropriate medium over the EI monolayer for 2-3 min at a shear rate of 40 s-1 for equilibration before the introduction of a predetermined concentration (1 × 106 cells/mL) of PMNs and WM9. PMNs were stimulated with 1 μM fMLP for 2 min before perfusion into the parallel-plate flow chamber. After allowing PMNs and WM9 cells to contact the EI monolayer at a shear stress of 0.1-0.3 dyn/cm2 for 2 min, shear stress was adjusted to the experimental range of 0.6-2 dyn/cm2 and kept constant for 6-7 min. Experiments were performed in triplicate and analyzed off-line. Variables quantified included the total number of PMNs which were tethered (rolling or arrested) on the EI monolayer; collisions between WM9 cells (from the free stream near the EI) and tethered PMNs; aggregation of WM9 cells with tethered PMNs as a result of collision; and final adhesion of WM9-PMN aggregates on the EI monolayer. Some PMNs which were arrested by a WM9-PMN aggregate close to the EI surface were counted as tethered PMNs. For the rare cases in which more than one tumor cell adhered to a PMN, we count such a case as two aggregates if two WM9 cells adhered to a PMN. The adhesion of WM9 on EI monolayer was quantified and normalized by the term as “WM9 adhesion efficiency”:
The numerator is the number of WM9 cells arrested on the EI monolayer at the end of the entire flow assay as a result of collision between entering WM9 cells and tethered PMNs. The denominator is the total number of WM9-PMN collisions near the EI monolayer surface and is counted as a transient accumulative parameter throughout the entire flow assay.
Cone-plate Viscometer Assay
To measure the interactions between PMNs and WM9 cells, a heterotypic aggregation assay was performed using a cone-plate viscometer,15,31 which consists of a stationary plate placed beneath a rotating cone (1° angle) maintained at 37 °C (RotoVisco 1, Haake, Newington, NH). Mixed suspensions of PMNs and WM9 cells, respectively pre-labeled with LDS-751 (red) and TRITC (orange), were placed on the plate at a concentration ratio of 1:1 (106 cells/mL, 500 μL each) and allowed to equilibrate for 1 min. Thereafter, the heterotypic cell suspension was stimulated with 1 μM fMLP for 2 min before the application of shear. Exposure of cell suspensions to a linear velocity gradient resulted in collisions between the faster moving cells near the rotating cone and slower moving cells near the stationary plate.15 Stable aggregates formation occurred when the strength of adhesive bonds formed during collision contact outweighed the tensile forces experienced by aggregates in the shear field. Shear rate was varied from 62.5 to 800 s-1, typical for microcirculation,42 for pre-set shear duration ranging from 30 to 300 s. To understand relative shear rate and shear stress effects on PMN-melanoma aggregation, the medium viscosity was varied from 1.0 to 3.2 cP by adding different amounts of dextran polymer into the medium. After shear, aliquots were immediately fixed with 1% formaldehyde at room temperature and subsequently analyzed in GUAVA personal cytometer (GUAVA Technologies Inc., Burlingame, CA). The size distribution and cellular composition of aggregates generated in the cone-plate viscometer were determined by a two-color flow cytometric methodology15,31 where LDS-751-stained PMNs and TRITC-stained WM9 cells were identified on the basis of their characteristic forward-scatter, side-scatter, and fluorescence profiles. Heterotypic aggregation was quantified as the percentage of bound WM9 cells in total WM9 cells:
Statistical Analysis
All results are reported as the mean ± standard error of the mean (SEM) unless otherwise stated. One-way ANOVA analysis was used for multiple comparisons and t-tests were used for comparisons between two groups. p < 0.05 was considered significant.
RESULTS
PMN Facilitated Melanoma Cell Extravasation Under Flow Conditions
Effects of shear rate and shear stress on PMN-mediated melanoma adhesion and migration have been compared using a novel flow-migration assay (Fig. 1a). Cases in which shear stress was held constant and shear rate varied from 55.5 to 555 s-1 yielded dramatic variation in C8161 cell extravasation (183 ± 24 cells/mm2 at 555 s-1 to 290 ± 37 cells/mm2 at 55.5 s-1) that was inversely proportional to the shear rate (p = 0.041; Fig. 1b). In contrast, cases in which shear rate was held constant and shear stress ranged from 0.4 to 40 dyne/cm2 resulted in C8161 migration levels that were not statistically different (Fig. 1c). These results suggest that PMN-facilitated migration of melanoma cells is affected by local hydrodynamic shear rates that regulate the contact duration.
FIGURE 1.
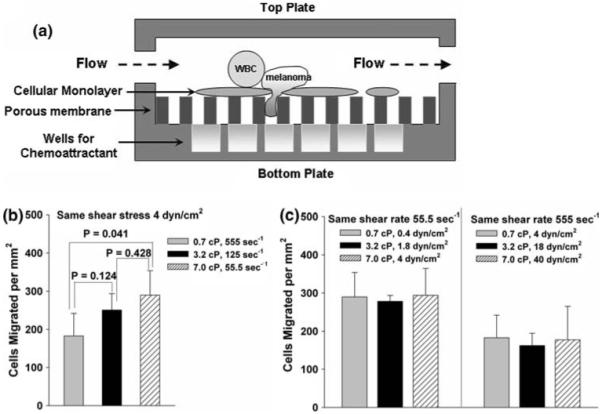
Effects of shear rate and shear stress on melanoma extravasation through EI monolayer. Shear rate and shear stress were isolated by varying viscosity with dextran-supplemented medium (0-4%). (a) Cross-section view of the flow-migration chamber shows the schematic of PMN-facilitated melanoma extravasation through EI monolayer in a shear flow. (b) Migration varies under constant shear stress but increasing shear rate. (c) Migration is unchanged over an order of magnitude of shear stress when shear rate is constant. All values are mean ± SEM for N ≥ 3.
Shear Rate Affects Melanoma Cell Adhesion to the EC Through Tethered PMNs
Adhesion of melanoma cell to the EC is the first step for its subsequent extravasation. In order to investigate the mechanism for shear-rate-dependent extravasation, the adhesion of melanoma cells to the EI monolayer in the presence of PMNs was investigated over a range of flow conditions using a parallel-plate flow chamber (Fig. 2). Results indicated that PMN tethering on the EI monolayer was significantly affected by both shear rate and shear stress (Fig. 2a). For a fixed shear rate 62.5 s-1, elevation of shear stress from 0.625 to 2 dyn/cm2 led to an increase in tethering frequency (Fig. 2a, left panel). At a constant shear stress of 2 dyn/cm2, the tethering frequency was greatest when shear rate was the lowest (62.5 s-1) and decreased with increasing shear rates (Fig. 2a, right panel). PMN tethering frequency was determined experimentally as the number of PMNs that adhered to the EI monolayer per unit time and area in the parallel-plate flow chamber assay, including both rolling and firmly-arrested cells. This frequency was normalized by cell flux to the surface to compensate for the different concentration of cells passing the same area of substrate at different shear rates. This normalization followed the procedure of Rinker et al.33 based on equations derived by Munn et al.29
FIGURE 2.
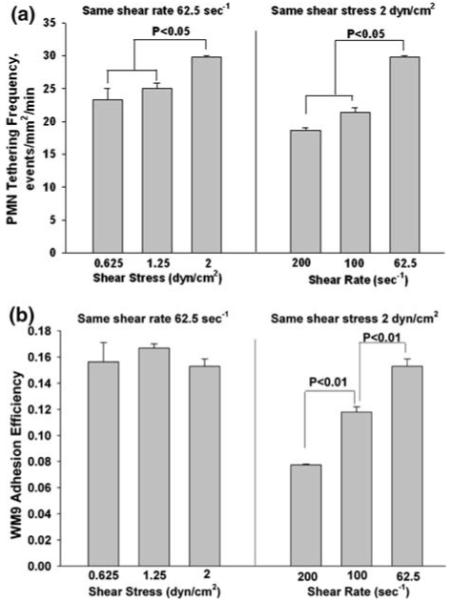
Effects of shear rate and shear stress on melanoma adhesion to the EI monolayer. (a) PMN tethering frequency under flow conditions. (b) Melanoma cell adhesion to the EI monolayer under flow conditions. WM9 adhesion efficiency and data correction procedure are defined in “Materials and Methods”. All values are mean ± SEM for N ≥ 3.
Since WM9 cells do not express ligands for either E-selectin or ICAM-1 expressed on the EI monolayer, WM9 cells did not adhere to the EI by themselves (data not shown). In the presence of PMNs, WM9 cells were able to adhere to the EI monolayer through aggregation to tethered PMNs. However, WM9 adhesion efficiency was only affected by the shear rate, not by the shear stress (Fig. 2b), which was different from PMN tethering on the EI monolayer. When shear rate was held constant and shear stress ranged from 0.6 to 2 dyne/cm2, adhesion efficiency levels were not significantly altered (Fig. 2b, left panel). In contrast, when shear stress was held constant and shear rate varied from 62.5 to 200 s-1, the efficiency of melanoma cell adhesion to PMNs decreased dramatically, suggesting an inversely proportional relation between shear rate and melanoma adhesion efficiency (Fig. 2b, right panel). These results illustrate that PMN could facilitate melanoma cell adhesion to the EI monolayer, regulated by the local hydrodynamic shear rates, which suggest intercellular contact time (proportional to the inverse of wall shear rate) has a greater influence on the tumor cell adhesion efficiency than the wall shear stress.
Aggregation of PMNs and WM9 Cells is Shear-rate Dependent
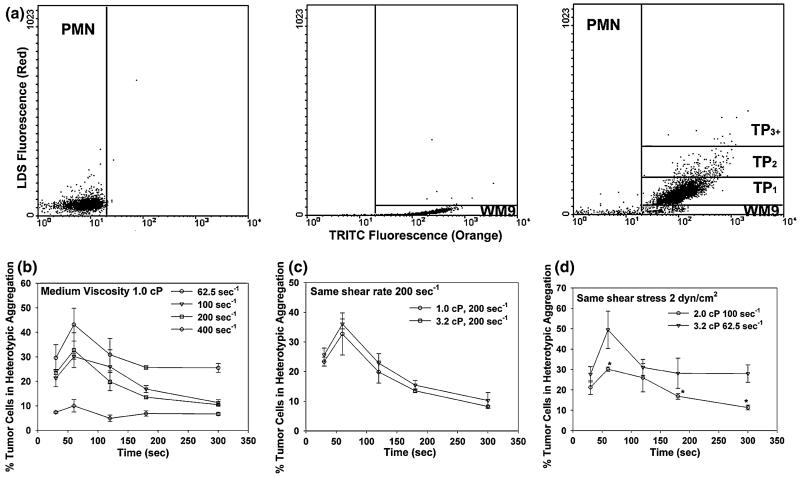
To study the interactions of melanoma cells and PMNs under shear conditions, the aggregation between WM9 cells and PMNs was characterized over a range of shear conditions using a cone-plate viscometer. Application of shear resulted in formation of heterotypic aggregation consisting of melanoma cell bound to PMNs, which could be detected by two-color flow cytometric technique (Fig. 3a). PMNs or WM9 cells alone fell into specific fluorescence channels in shear flow, which was different from the heterotypic aggregation between PMNs and WM9 cells. The population of WM9 cells was resolved into singlet and aggregates composed of a single WM9 cell bound to one, two, or more than two PMNs. The concentrations of these aggregates were represented by [WM9], [TP1],[TP2], and [TP3+], respectively. To quantify the time courses of PMN-WM9 aggregation kinetics, heterotypic aggregation percentage of PMNs and WM9 cells was measured at a given shear duration in different shear rates and medium viscosities. Results showed the aggregation between PMN and melanoma cells exhibited an ascending phase where it reached a maximum fraction at 60 s, followed by a descending phase at a given shear rate (Fig. 3b) at all shear rates tested varying from 62.5 to 400 s-1. More WM9 cells formed aggregations with PMNs (∼45%) at a low shear rate around 62.5 s-1, while the aggregation decreased to the baseline at high shear rates above 400 s-1 (Fig. 3b). Partial disaggregation of the heterotypic aggregates was observed from longer durations (100-300 s) of shear exposure at all shear rates examined. Maximum aggregation between WM9 and PMNs was maintained at shear rates to 200 s-1 and decreased to background levels as shear was increased to 400 s-1.
FIGURE 3.
The kinetics of PMN-WM9 aggregation under different shear conditions. (a) Detection of PMN-tumor cell aggregates by two-color flow cytometry. LDS-571-labeled PMNs (1 × 106/mL), TRITC-stained WM9 melanoma cells (1 × 106/mL), or both were sheared at 62.5 s-1 for 120 s in a cone-plate viscometer in the presence of 1 μM fMLP. Upon termination of shear, aliquots were immediately fixed with 2% formaldehyde and subsequently analyzed in a GUAVA flow cytometer. Left panel shows PMN only using flow cytometry; middle panel shows WM9 only; and right panel shows WM9-PMN heterotypic aggregates. The population of WM9 cells was resolved into singlet and aggregates composed of a single WM9 cell bound to one, two, or more than two PMNs. The concentrations of these aggregates were represented by [WM9], [TP1], [TP2], and [TP3+], respectively. The gating was based on the specific fluorescence channel where each population fell in. (b) The percentage of melanoma cell in the heterotypic aggregations at different shear rates with a medium viscosity 1.0 cP. (c) The percentage of melanoma cells in the heterotypic aggregations at a fixed shear rate 62.5 s-1 while shear stress varied from 2 to 6.4 dyn/cm2. (d) The percentage of melanoma cells in the heterotypic aggregations under a fixed shear stress 2 dyn/cm2 while shear rate varied from 62.5 to 100 s-1. Values are mean ± S.E.M. for N ≥ 3. *p < 0.05.
To test the regulation of hydrodynamic shear on PMN-WM9 aggregation, the dependence of aggregation percentage on shear stress and shear rate was compared respectively. The results indicated that the aggregation percentage remained the same when the shear stress varied from 2 to 6.4 dyn/cm2 at a fixed shear rate of 200 s-1 (Fig. 3c), but increased when shear rate was reduced from 100 to 62.5 s-1 at a fixed shear stress of 2 dyn/cm2 (Fig. 3d). These results suggest that the shear-induced aggregation of fMLP-stimulated PMNs and WM9 cells is shear-rate dependent.
Binding of β2 Integrin and ICAM-1 Regulates PMN-Melanoma Aggregation
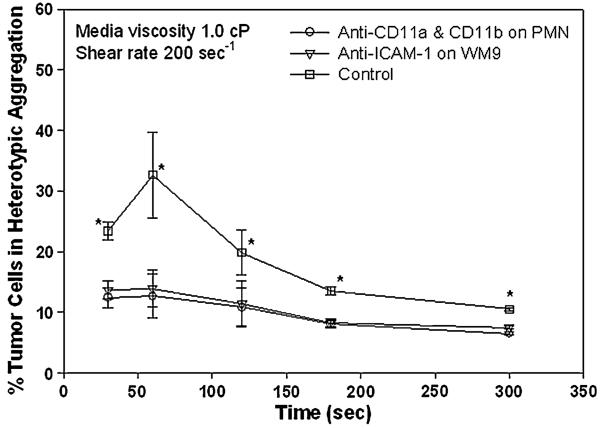
Recent studies have quantified the strength and kinetics of LFA-1 and Mac-1 in PMN heterotypic aggregation to transfected cells expressing ICAM-1 and ICAM-1-expressing colon carcinomas in a shear flow.15,18,19,31 To further understand the underlying mechanisms of interacting molecules that mediate the PMN and ICAM-1-expressing melanoma cells aggregation, functional-blocking mAbs directly against LFA-1 and Mac-1 on PMNs or ICAM-1 on WM9 were used to elucidate specificity of the aggregation of PMNs and WM9 cells. As shown in Fig. 4, the aggregation percentage of PMNs and WM9 cells was present in the absence of blocking mAbs but was abolished to the baseline fraction when anti-LFA-1 and -Mac-1 mAbs or anti-ICAM-1 mAbs were present in all shear durations tested, suggesting that the specific β2 integrin-ICAM-1 interactions support the PMN-WM9 aggregation.
FIGURE 4.
Effects of β2 integrin-ICAM-1 binding on PMN-WM9 aggregation. Blocking of β2 integrin on PMNs significantly reduced PMN-WM9 aggregation compared with control. *p < 0.05 compared with the other two blocking cases. Values are mean ± SEM for N ≥ 3.
PMN Tethering on the EC is Critical for Melanoma Cells Adhesion to the EC
Recent studies have indicated PMNs can enhance melanoma cell transendothelial migration.37,43 However, the mechanism involved is not entirely understood. Cone-plate viscometry assay indicated an ability of melanoma cells to form heterotypic aggregations with PMNs under shear conditions. Present results showed blocking either LFA-1 or Mac-1 on PMNs, or ICAM-1 on the EI monolayer decreased melanoma adhesion efficiency significantly compared with the control (Fig. 5). Both treatments blocked the binding between PMNs and EI cells and thus reduced PMN tethering on the EI monolayer (data not shown), suggesting that PMN tethering to the EI monolayer is critical for melanoma cell to form close contact with the EI monolayer. Blocking ICAM-1 on WM9 significantly reduced melanoma adhesion efficiency compared with both the control and anti-ICAM-1 on EI cases (Fig. 5), which indicates β2 integrins/ICAM-1 binding between PMNs and melanoma cells is essential for successful melanoma cell arrest on the EI monolayer.
FIGURE 5.
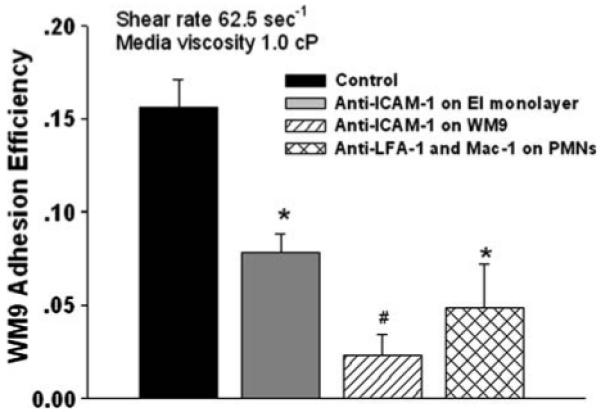
Effects of PMN tethering on melanoma cell adhesion to the EI monolayer. PMNs were treated with mAb to functionally block LFA-1 and Mac-1 (30 min, 4 °C); EI monolayer was treated with mAb against ICAM-1 (30 min, 4 °C); WM9 cells were treated with mAb against ICAM-1 (30 min, 4 °C). After each treatment, the excess of mAb was washed out by centrifuging cells down and re-suspending them in fresh medium. Cells were then injected into the parallel plate flow chamber for the adhesion assay. Blocking ICAM-1 on WM9 significantly reduced melanoma adhesion efficiency compared with both the control and anti-ICAM-1 on EI cases. *p < 0.05 compared with control samples. #p < 0.05 with respect to concurrent ICAM-1 blocking on the EI cells under the same shear condition. Values are mean ± SEM for N ≥ 3.
Population Ratio of PMNs to Melanoma Cells Affects Melanoma Aggregation, Adhesion, and Extravasation
The ratio of melanoma cells to PMNs was examined to determine whether changing the relative concentrations would change the probability of a tumor cell colliding with and binding to a PMN, which in turn will affect melanoma adhesion and extravasation. Cone-plate assay results indicated that an increase in population ratio of PMN to WM9 from 1:1 to 4:1 would significantly increase the aggregation between melanoma cells and PMNs after applying shear for 300 s (data not shown). However, after normalizing the aggregation data to the collision frequency calculated by using the methods previously published,15 our results indicated that although the absolute number of PMN-WM9 aggregations increased, an increase in PMN concentration did not really change the normalized PMN-WM9 heterotypic aggregations (Fig. 6a) in a cone-plate flow assay where the wall effect on cell-cell aggregation were absent. Interestingly, the parallel-plate flow assay showed WM9 adhesion efficiency increased significantly after an increase in the ratio of PMNs to WM9 cells (Fig. 6b), resulting in more PMN-facilitated melanoma adhesion to the EI monolayer. Higher number of PMNs increased the number of tethered PMNs on the EI monolayer and there were formations of PMN clusters on the EI monolayer surface. Therefore, in the same area and same duration, the existence of higher concentration of PMNs resulted in more melanoma cells collisions in short time period and thus increased melanoma adhesion efficiency. Moreover, flow migration data also indicated that PMN-mediated C8161 extravasation increased significantly with more PMN present (Fig. 6c). These results strongly suggest an important role of substrate adhesion in PMN-melanoma cell aggregation under flow conditions.
FIGURE 6.
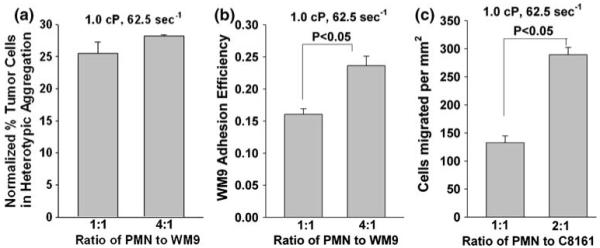
Population ratio effects on: (a) PMN-WM9 aggregation; (b) WM9 adhesion to the EI monolayer; and (c) C8161 extravasation under various shear conditions. Increase in ratio of PMN to WM9 significantly promotes PMN-WM9 aggregation, WM9 adhesion to the EI monolayer; and increase in ratio of PMN to C8161 increases subsequent extravasation through the EI monolayer. Values are mean ± SEM for N ≥ 3.
DISCUSSION
The initial step in melanoma extravasation is tumor cell adhesion to the EC under flow conditions. Many studies have suggested that melanoma cells inefficiently bind the EC under hemodynamic shear within the circulation and may recruit other cellular assistance for adhesion.3,7,25 Human leukocytes, including PMNs, actively participate in the inflammatory response through adhesion to the EC.40 PMNs typically use selectin molecules to form rolling attachments along the vessel wall and β2 integrins to anchor shear-resistant bonds, and therefore support onward transmigration through the EC.40 In contrast, melanoma cells do not express β2 integrins, sLex or other sialylated molecules at levels to effectively adhere to the EC within the circulation.45 In addition to tumor cell entrapment within the vasculature, in vivo studies have shown that the mechanisms utilized by metastatic tumor cells to adhere to a vessel wall prior to extravasation are very different from PMNs.5,24,44 Several ligands for inducible endothelial adhesion molecules have been identified on various types of tumor cells.44 Both endothelial and melanoma cells express ICAM-1, the potential ligand for the β2 integrins on PMNs; therefore, PMN-EC and PMN-melanoma cells adhesions may play very important roles in bringing tumor cells close to the EC surface and facilitating their subsequent extravasation through the EC in the presence of shear. However, little has been shown how hemodynamic flow plays a role in this process. In this study, the aggregation kinetics of melanoma cells and PMNs in a shear flow has been quantified, and melanoma cell adhesion to the EC through tethered PMNs under flow conditions has also been investigated. Results have indicated that shear rate, not shear stress, affects the formation of PMN-WM9 heterotypic aggregation, which may contribute to the shear-rate-dependent PMN-facilitated melanoma adhesion to the EC and subsequent extravasation.
In the current study, PMNs have been directly observed forming aggregates with WM9 cells through β2 integrins-ICAM-1 binding. Once E-selectin initiates PMN tethering on the EC, β2 integrins mediate stable arrest of PMNs to the EC. Since melanoma cells express a high-level of stable ICAM-1, collisions of melanoma cells with PMNs tethered on the EC could result in the formation of aggregates followed by melanoma cell arrest on the EC. PMNs form a two-way bridge by binding to ICAM-1 on both EC surface and melanoma cells through LFA-1 and Mac-1. Blocking LFA-1 and Mac-1 on PMNs or ICAM-1 on WM9 has shown to significantly reduce WM9-PMN aggregation. This finding is in contrast to a published study of PMN binding to ICAM-1-high/sLex-low expressing HCT-8 carcinoma cells, which showed that the ICAM-1 blocking alone could not substantially reduce PMN-HCT-8 cell aggregation in response to a longer shear exposure time.19 However, such differences could be due to the presence of another potential ligand for LFA-1 or Mac-1 with a higher affinity than ICAM-1 on the surface of HCT-8 cells.19 In addition, blocking ICAM-1 on the EI monolayer significantly reduced WM9 adhesion to the EI monolayer. Blocking ICAM-1 on WM9 cells is more influential in decreasing melanoma cell adhesion to the EC than blocking ICAM-1 on the EI cells, suggesting that PMNs can still attach to EI monolayer via other than ICAM-1.15 However, capture and firm adhesion of WM9 to PMN requires β2 integrins-ICAM-1 interaction and is therefore sufficient for maximal melanoma adhesion to the EC.
The trend of PMN-facilitated melanoma cell extravasation directly follows that of melanoma cell adhesion efficiency, which are both affected by the intercellular contact time (inversely proportional to shear rates). Shear rate modulates the cell-cell collision frequency and contact time affected by the relative velocities of cells with respect to the flow and possibly the local Reynolds near cells in the fluid field. Shear stresses determine the forces applied to cells individually and intermolecular bonds between cells. Because lower shear rate increases the contact time between colliding melanoma cells and PMNs and it appears to be a requisite of on-rate kinetic constant for an integrin bond to form,15 there would be higher probability of formation for WM9-PMN aggregations under low shear-rate conditions. Current results indicate that the collision and contact time between β2 integrins and ICAM-1 may play more important roles in cell aggregation than forces applied to the bonds. Previous studies have reported similar shear-rate dependent bond formation. For example, Goldsmith et al.13 showed shear-rate dependent PMN homotypic aggregations in a Couette flow; Chen and Springer6 reported individual PMNs expressing P-selectin glycoprotein ligand 1 (PSGL-1) rolled along and tethered to the P-selectin-immobilized substrate was shear rate dependent. Our results here show PMN-WM9 aggregation facilitates melanoma cell adherence to the EC, which is predominantly regulated by the shear rate. In contrast, PMN tethering on the EC is both shear-rate and shear-stress dependent. The difference suggests a “two-step adhesion” paradigm that involves initial PMN tethering on the EC and subsequent melanoma cells being captured by tethered PMNs. The formation of PMN-melanoma cell aggregates is a dominant step, which could be possibly due to the fundamental differences in the hydrodynamics of these two steps. PMN tethering on the EC is the interaction between a moving sphere and a wall. Under the same shear rate (inversely proportional to the cell-cell contact time), increasing the shear stress caused more rolling and firmly arrest PMNs. A similar finding was reported by Rinker et al.33 A possible mechanism is that an increased shear force could cause PMNs to deform to a greater extent, thereby creating a greater contact area leading to more bond formation for adhesion. The threshold effect reported for selectin mediated rolling of leukocytes on the EC is also a possible explanation.10 In contrast, capture of melanoma cells by tethered PMNs depends on the interaction between two moving spherical cells or adhesion between a moving melanoma cell and a firmly adherent and possibly flattened PMN. Thus, it is speculated that a shorter contact time is available in the case of capture in the flow field between two spherical cells.
Melanoma cell binding to PMNs has been found to be time dependent, which is evident by the fact that heterotypic aggregation peaked after about 60 s upon shear but start to decrease afterwards under all shear conditions. The binding of melanoma cells to PMNs may happen at the site of collisional contact because the predicted minimum contact duration at which aggregation between melanoma cells and PMNs was detected is on the order of 10 ms at 200 s-1 (e.g., tcontact ≈ 2.6/shear rate).12,41 This interval is insufficient for significant numbers of adhesion molecules to diffuse into the region of cell-cell contact.2 The onset of fluid shear may increase cell-cell interactions and aggregation through cell collisions; however, tensile forces may gradually outweigh cell-cell adhesive bonds and break up the aggregations when cells are under longer exposure to shear. This biphasic kinetic behavior agrees with previous studies that showed fMLP stimulation resulted in an increase in Mac-1 expression on PMNs but reached a plateau within 2 min.1,17 However, increased Mac-1 avidity was reversed over minutes unless increasing stimulus would be applied.17,26 Some studies also showed that CD18 expression could be down-regulated upon fluid shear.11
The mechanism for PMN-facilitated melanoma adhesion and subsequent extravasation is still under investigation. Studies have demonstrated that melanoma cells, such as C8161,37 A375SM,36 and WM932 can produce interleukin 8 (IL-8). IL-8 has a wide range of proinflammatory effects on the migration of PMNs from the circulation to inflammatory sites by activation of CXC chemokine receptors 1 and 2 (CXCR1 and CXCR2).16,30 Recent reports suggest that the expressions of chemokine and chemokine receptors by melanoma may contribute to its ability to escape tumor surveillance and may partially explain preferential patterns of melanoma metastasis.28 Previous studies have also reported that IL-8 is crucial for PMN-facilitated melanoma adhesion and subsequent extravasation, which are inhibited by functional blockade of CXCR1/2 receptors on PMNs.22,38 Our results here have shown important roles of β2 integrins-ICAM-1 binding in PMN and melanoma cell interactions. IL-8 has been shown to up-regulate the ligand binding activity of the β2 integrins on PMN.8 Furthermore, Mac-1 expression and IL-8 production from PMNs are founded to increase in the melanoma-PMN microenvironment.28,38 These results suggest that potential communication between the immune system and cancer cells may alter both the host’s physiology and tumor cell behavior by modulating the growth, invasion, migration, and metastasis of tumor cells.
In summary, this study has examined molecular interactions between the EC, PMNs and melanoma cells under well-defined hydrodynamic shear conditions. Results indicate that PMN-facilitated melanoma adhesion to the EC and subsequent extravasation are shear-rate dependent, which could be attributed to PMN-melanoma cell aggregation through binding of β2 integrins and ICAM-1. These findings provide a mechanistic basis for understanding leukocyte-tumor cell interactions mediated by specific receptor-ligand interactions, as well as subsequent tumor cell adhesion to the EC through tethered leukocytes. The present results shed light on potential targets to inhibit interactions of PMNs and melanoma cells, particularly by possible tumor-induced immunoediting mechanisms which in turn will prevent PMN-mediated melanoma adhesion and subsequent metastasis development. Obviously, a further understanding of multiple intercellular events regulating melanoma, PMN, and microvascular EC functions and validation of effectiveness of preventing melanoma sequestration will be critical to determine the efficiency of melanoma extravasation within the microcirculation and in fostering new approaches to cancer treatment through anti-inflammatory therapeutics.
ACKNOWLEDGEMENT
The authors thank Dr. M. Herlyn (Wistar Institute, Philadelphia, PA) for providing WM9 cells. This work was supported by grants from NSF (CBET-0729091), NIH (CA-97306 and CA125707), NIH-NSF BBSI EEC-0234026 (C. Dong), and UC Cancer Research Coordinating Committee AI47294 (S. I. Simon). Penn State GCRC was supported by NIH grants M01-RR-010732 and C06-RR-016499.
Footnotes
A special issue of the Annals of Biomedical Engineering, in honor of Dr. Harry Goldsmith.
REFERENCES
- 1.Bateman J, Parida S, Nash G. Neutrophil integrin assay for clinical studies. Cell Biochem. Funct. 1993;11:87–91. doi: 10.1002/cbf.290110203. [DOI] [PubMed] [Google Scholar]
- 2.Bell GI. Models for the specific adhesion of cells to cells. A theoretical framework for adhesion mediated by reversible bonds between cell surface molecules. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 3.Burdick M, McCarty O, Jadhav S, Konstantopoulos K. Cell-cell interactions in inflammation and cancer metastasis. IEEE Eng. Med. Biol. Mag. 2001;20:86–91. doi: 10.1109/51.932731. [DOI] [PubMed] [Google Scholar]
- 4.Burdick M, McCaffery J, Kim Y, Bochner B, Konstantopoulos K. Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. Am. J. Physiol. Cell Physiol. 2003;284:977–987. doi: 10.1152/ajpcell.00423.2002. [DOI] [PubMed] [Google Scholar]
- 5.Chambers A, MacDonald I, Schmidt E, Morris V, Groom A. Clinical targets for anti-metastasis therapy. Adv. Cancer Res. 2000;79:91–121. doi: 10.1016/s0065-230x(00)79003-8. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Springer T. Selectin receptor-ligand bonds: formation limited by shear rate and dissociation governed by the Bell model. Proc. Natl. Acad. Sci. USA. 2001;98:950–955. doi: 10.1073/pnas.98.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coussens L, Werb Z. Inflammation and cancer. Nature. 2001;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond M, Springer T. The dynamic regulation of integrin adhesiveness. Curr. Biol. 1994;4:506–507. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 9.Dong C, Slattery M, Liang S, Peng H. Melanoma cell extravasation under flow conditions is modulated by leukocytes and endogenously produced interleukin 8. Mol. Cell. Biomech. 2005;2:145–160. [PMC free article] [PubMed] [Google Scholar]
- 10.Finger EB, Purl KD, Alon R, Lawrence MB, von Andrian UH, Springer T. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996;379:266–269. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda S, Schmid-Schonbein G. Regulation of CD18 expression on neutrophils in response to fluid shear stress. Proc. Natl. Acad. Sci. USA. 2003;100:13152–13157. doi: 10.1073/pnas.2336130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldsmith HL, Bell DN, Braovac S, Steinberg A, McIntosh F. Physical and chemical effects of red cells in the shear-induced aggregation of human platelets. Biophys. J. 1995;69:1584–1595. doi: 10.1016/S0006-3495(95)80031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldsmith HL, Quinn T, Drury G, Spanos C, McIntosh F, Simon SI. Dynamics of neutrophil aggregation in couette flow revealed by videomicroscopy: effect of shear rate on two-body collision efficiency and doublet lifetime. Biophys. J. 2001;81:2020–2034. doi: 10.1016/S0006-3495(01)75852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopalan P, Smith C, Lu H, Berg E, McIntire L, Simon SI. PMN CD18-dependent arrest on ICAM-1 in shear flow can be activated through L-selectin. J. Immunol. 1997;158:367–375. [PubMed] [Google Scholar]
- 15.Hentzen ER, Neelamegham S, Kansas GS, Benanti JA, McIntire LV, Smith CW, Simon SI. Sequential binding of CD11a/CD18 and CD11b/CD18 defines neutrophil capture and stable adhesion to intercellular adhesion molecule-1. Blood. 2000;95:911–920. [PubMed] [Google Scholar]
- 16.Holmes W, Lee J, Kuang W, Rice G, Wood W. Structure and functional expression of human interleukin-8 receptor. Science. 1991;253:1278–1280. [PubMed] [Google Scholar]
- 17.Hughes B, Hollers J, Crockett-Torabi E, Smith CW. Recruitment of CD11b/CD18 to the neutrophil surface and adherence-dependent cell locomotion. J. Clin. Invest. 1992;90:1687–1696. doi: 10.1172/JCI116041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadhav S, Bochner B, Konstantopoulos K. Hydrodynamic shear regulates the kinetics and receptor specificity of polymorphonuclear leukocyte-colon carcinoma cell adhesive interactions. J. Immunol. 2001;167:5986–5993. doi: 10.4049/jimmunol.167.10.5986. [DOI] [PubMed] [Google Scholar]
- 19.Jadhav S, Konstantopoulos K. Fluid shear-and time-dependent modulation of molecular interactions between PMNs and colon carcinomas. Am. J. Physiol. Cell Physiol. 2002;283:C1133–C1143. doi: 10.1152/ajpcell.00104.2002. [DOI] [PubMed] [Google Scholar]
- 20.Kunkel E, Chomas J, Ley K. Role of primary and secondary capture for leukocyte accumulation in vivo. Circ. Res. 1998;82:30–38. doi: 10.1161/01.res.82.1.30. [DOI] [PubMed] [Google Scholar]
- 21.Ley K, Tedder T. Leukocyte interactions with vascular endothelium. New insights into selectin-mediated attachment and rolling. J. Immunol. 1995;155:525–528. [PubMed] [Google Scholar]
- 22.Liang S, Slattery MJ, Dong C. Shear stress and shear rate differentially affect the multi-step process of leukocyte-facilitated melanoma adhesion. Exp. Cell Res. 2005;310:282–292. doi: 10.1016/j.yexcr.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lichtenstein A. Stimulation of the respiratory burst of murine peritoneal inflammatory neutrophils by conjugation with tumor cells. Cancer Res. 1987;47:2211–2217. [PubMed] [Google Scholar]
- 24.Liotta L. Cancer cell invasion and metastasis. Sci. Am. 1992;266:54–63. doi: 10.1038/scientificamerican0292-54. [DOI] [PubMed] [Google Scholar]
- 25.Liotta L, Kohn E. The microenvironment of the tumor-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 26.Lo S, Detmers P, Levin S, Wright S. Transient adhesion of neutrophils to endothelium. J. Exp. Med. 1989;169:1779–1793. doi: 10.1084/jem.169.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miele M, Bennett C, Miller B, Welch D. Enhanced metastatic ability of TNF-α treated malignant melanoma cells is reduced by intercellular adhesion molecule-1 (ICAM-1) antisense oligonucleotides. Exp. Cell Res. 1994;214:231–241. doi: 10.1006/excr.1994.1253. [DOI] [PubMed] [Google Scholar]
- 28.Muller A, Homey B, Soto H, Ge N, Carton D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 29.Munn L, Melder R, Jain R. Analysis of cell flux in the parallel plate flow chamber: implications for cell capture studies. Biophys. J. 1994;67:889–895. doi: 10.1016/S0006-3495(94)80550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy P, Tiffany H. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–1283. [PubMed] [Google Scholar]
- 31.Neelamegham S, Taylor A, Burns A, Smith C, Simon SI. Hydrodynamic shear shows distinct roles for LFA-1 and MAC-1 in neutrophil adhesion to intercellular adhesion molecule-1. Blood. 1998;92:1626–1638. [PubMed] [Google Scholar]
- 32.Peng H, Liang S, Henderson A, Dong C. Regulation of interleukin-8 expression in melanoma-stimulated neutrophil inflammatory response. Exp. Cell Res. 2007;313:551–559. doi: 10.1016/j.yexcr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinker K, Prabhakar V, Truskey G. Effect of contact time and force on monocyte adhesion to vascular endothelium. Biophys. J. 2001;80:1722–1732. doi: 10.1016/S0006-3495(01)76143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serrone L, Hersey P. The chemoresistance of human malignant melanoma: an update. Melanoma Res. 1999;9:51–58. doi: 10.1097/00008390-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Simon SI, Hu Y, Vestweber D, Smith C. Neutrophil tethering on E-selectin activates b2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J. Immunol. 2000;164:4348–4358. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- 36.Singh R, Varney M. Regulation of interleukin-8 expression in human malignant melanoma cells. Cancer Res. 1998;58:1532–1537. [PubMed] [Google Scholar]
- 37.Slattery MJ, Dong C. Neutrophils influence melanoma adhesion and migration under flow conditions. Int. J. Cancer. 2003;106:713–722. doi: 10.1002/ijc.11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am. J. Physiol. Cell Physiol. 2005;288:C831–C839. doi: 10.1152/ajpcell.00439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soengas M, Lowe S. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 40.Springer T. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 41.Taylor AD, Neelamegham S, Hellums JD, Smith CW, Simon SI. Molecular dynamics of the transition from L-selectin to β2 integrin-dependent neutrophil adhesion under defined hydrodynamic shear. Biophys. J. 1996;71:3488–3500. doi: 10.1016/S0006-3495(96)79544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turitto V. Blood viscosity, mass transport, and thrombogenesis. Prog. Hemost. Thromb. 1982;6:139–177. [PubMed] [Google Scholar]
- 43.Wu Q, Wang J, Condron C, Bouchier-Hayer D, Redmond H. Human neutrophils facilitate tumor cell transendothelial migration. Am. J. Physiol. Cell Physiol. 2001;280:C814–C822. doi: 10.1152/ajpcell.2001.280.4.C814. [DOI] [PubMed] [Google Scholar]
- 44.Yamada K. Introduction: adhesion molecules in cancer. Part I. Semin. Cancer Biol. 1993;4:215–218. [PubMed] [Google Scholar]
- 45.Zetter B. Adhesion molecules in tumor metastasis. Semin. Cancer Biol. 1993;4:219–229. [PubMed] [Google Scholar]