Abstract
Objective:
We previously used exploratory analyses across the entire cortex to determine that mild Alzheimer disease (AD) is reliably associated with a cortical signature of thinning in specific limbic and association regions. Here we investigated whether the cortical signature of AD-related thinning is present in individuals with questionable AD dementia (QAD) and whether a greater degree of regional cortical thinning predicts mild AD dementia.
Methods:
Participants included 49 older adults with mild impairment consistent with mild cognitive impairment (Clinical Dementia Rating [CDR] = 0.5) at the time of structural MRI scanning. Cortical thickness was measured in nine regions of interest (ROIs) identified previously from a comparison of patients with mild AD and controls.
Results:
Longitudinal clinical follow-up revealed that 20 participants converted to mild AD dementia (progressors) while 29 remained stable (nonprogressors) approximately 2.5 years after scanning. At baseline, QAD participants showed a milder degree of cortical thinning than typically seen in mild AD, and CDR Sum-of-Boxes correlated with thickness in temporal and parietal ROIs. Compared to nonprogressors, progressors showed temporal and parietal thinning. Using receiver operating characteristic curves, the thickness of an aggregate measure of these regions predicted progression to mild AD with 83% sensitivity and 65% specificity.
Conclusions:
Thinning in specific cortical areas known to be affected by Alzheimer disease (AD) is detectable in individuals with questionable AD dementia (QAD) and predicts conversion to mild AD dementia. This method could be useful for identifying individuals at relatively high risk for imminent progression from QAD to mild AD dementia, which may be of value in clinical trials.
GLOSSARY
- AD
= Alzheimer disease;
- ADT
= AD signature thickness;
- AUC
= area under the curve;
- CDR
= Clinical Dementia Rating;
- CDR-SB
= CDR Sum-of-Boxes;
- eTIV
= estimated total intracranial volume;
- EV
= entorhinal volume;
- HV
= hippocampal volume;
- MCI
= mild cognitive impairment;
- MCT
= mean cortical thickness;
- MMSE
= Mini-Mental State Examination;
- MTL
= medial temporal lobe;
- MTLT
= medial temporal lobe thickness;
- OC
= older controls;
- QAD
= questionable AD dementia;
- ROC
= receiver operating characteristic;
- ROI
= region of interest;
- WBV
= whole brain volume.
Anatomic abnormalities of brain regions known to be early sites of Alzheimer disease (AD) pathology, such as medial temporal lobe (MTL) regions including the entorhinal cortex and hippocampal formation, can be detected in prodromal AD prior to dementia.1–3 Although it is well known that early in its clinical course AD affects non-MTL neocortical association regions,4,5 there has been little investigation of neocortical anatomy in prodromal AD prior to dementia. The few investigations of cortical anatomic abnormalities in mild cognitive impairment (MCI)6 or prodromal AD7–9 have used techniques that involve exploratory mapping of the entire cortex. While these techniques are well suited to disorders in which the localization of pathology is unknown, it is possible to use in vivo imaging and postmortem pathologic data from patients with AD dementia to predict the localization of anatomic abnormalities in prodromal AD. Such an approach employing disease-signature cortical regions of interest (ROIs) is powerful in that it enables hypotheses to be tested about the sequence of involvement of cortical regions in AD progression through comparison of effect sizes from cross-sectional data from patients at different clinical stages in the disease.
Furthermore, there are questions about abnormalities of cortical anatomy in prodromal AD that, to our knowledge, have received no investigation. How do cortical anatomic abnormalities relate to symptoms in the mildest predementia phases of the disease? Which neocortical measures are best in terms of sensitivity and specificity for early diagnosis, and do they improve upon the predictive power of well-accepted morphometric measures, such as volumes of the hippocampal formation, entorhinal cortex, or whole brain?
To address those questions, we studied a sample of individuals with questionable dementia (Clinical Dementia Rating [CDR]10 0.5) whose symptoms and signs were largely similar to MCI. Longitudinal clinical data after the MRI scan were used to classify subjects into progressors to mild AD dementia (CDR 1) vs nonprogressors. A set of AD signature cortical ROIs—found previously in a separate sample of patients with mild AD (CDR 1) to be consistently affected11—was used to extract regional thickness measures which were employed in subsequent analyses. ROI measures were compared in the group of MCI progressors vs nonprogressors, and were also compared to controls and patients with mild AD. In addition, receiver operating characteristic (ROC) curves were generated for the various novel anatomic measures obtained, which were compared to ROC curves for standard morphometric measures. Across all subjects, ROI measures were studied in relation to clinical measures for purposes of clinical validation. Finally, an analysis was performed to demonstrate a map of the spatial pattern of thinning associated with prodromal AD across the cerebral cortex.
METHODS
Participants, clinical assessment, and MRI data acquisition.
Forty-nine volunteer participants (age 64–90, 20 women) were studied, data from some of whom have been published previously.12–15 Participants were recruited from the ongoing longitudinal sample of the Washington University Alzheimer’s Disease Research Center using procedures approved by Washington University’s human subjects committee, and assessed using procedures previously described.16
For the purposes of the present study, the clinical diagnostic categories included questionable dementia of the Alzheimer type (QAD) (CDR Rating = 0.5) and mild AD (CDR Rating = 1). All participants were classified as QAD at the time of baseline MRI scan. Based on annual clinical follow-up, 29 individuals remained with a CDR Rating of 0.5 (nonprogressors) while 20 converted to mild AD (declined to a CDR Rating = 1; progressors).
Multiple (three or four) structural T1-weighted magnetization-prepared rapid gradient echo images were acquired on a 1.5 T Siemens Vision scanner (Siemens Medical Systems, Erlangen, Germany) using the following parameters: repetition time/echo time/inversion time/delay = 9.7/4/20/200 msec, flip angle = 10º, 256 × 256 (1 mm × 1 mm) in-plane resolution, 128 sagittal 1.25 mm slices without gaps. These images were motion-corrected and averaged together during processing. These data are openly available to the community, thanks in part to resources provided by the Washington University Alzheimer’s Disease Research Center (http://www.oasis-brains.org/).
MRI morphometric data analysis: Automated tissue segmentation and surface reconstruction and alignment of participants.
These methods have been previously described in detail, including the surface-based cortical thickness processing and spherical registration to align subjects’ cortical surfaces.12,17–22 The methods are summarized in appendix e-1 on the Neurology® Web site at www.neurology.org. The Freesurfer software used to analyze and visualize data in this study is freely available (http://surfer.nmr.mgh.harvard.edu).
MRI morphometric data analysis: Quantification of magnitude of atrophy within regions of interest.
Automated volume measures for whole brain, hippocampus, and entorhinal cortex were adjusted for estimated total intracranial volume (eTIV, calculated as described previously13) by dividing regional volume by eTIV. Nine regions of interest (ROIs) constituting the cortical signature of AD derived from an exploratory cortical thickness analysis comparing 115 older controls to 29 subjects with AD11 were used to generate regional thickness measures for each subject in this study. These adjusted volume and regional thickness measures were used for descriptive purposes and to calculate the mean difference in values of each ROI between the progressor and nonprogressor groups, the percent thinning in progressors relative to nonprogressors ([mean thickness of nonprogressor group − mean thickness of progressor group]/mean thickness of nonprogressor group), and the Cohen d effect sizes of progression-related differences for each ROI. In addition, we analyzed a measure of the average thickness of these 9 ROIs (average AD-signature ROI thickness), previously shown to be sensitive to early presymptomatic effects of AD.11
Statistical analyses.
The eTIV-adjusted volume and regional thickness measures were normalized to a sample of age-matched controls by calculating a Z score based on the mean and SD of an age-matched group of older controls (OC; CDR = 0) as follows: Z = (x − μage-matched OCs)/σage-matched OCs. This Z score was used in all statistical analyses. All normalized (for age) thickness measurements were examined by analysis of variance, with clinical outcome as the independent variable (progressor vs nonprogressor). Thickness is not correlated with intracranial volume (data not shown), so is not adjusted for eTIV. Measures from both hemispheres were pooled to make one measure per region for each subject. Pearson correlations and a multiple linear regression analysis were performed to examine relationships between regional anatomic measures and the measures of symptom severity (the CDR Sum-of-Boxes [CDR-SB10]) and that of severity of impairment on cognitive testing (Mini-Mental State Examination [MMSE]23). ROC curves were used to assess the sensitivity and specificity of different measures. The ROC performance of particular morphometric measures was compared graphically, and for simplicity we highlight here the peak classification performance in which both sensitivity and specificity were greater than 50% (i.e., top left peak in non-shaded area in figure 2A). These statistical analyses were performed using SPSS 13.0 (SPSS, Chicago, IL).
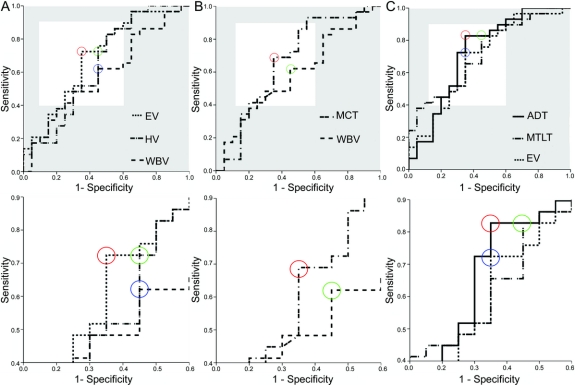
Figure 2 Receiver operating characteristic curves of the performance by various morphometric measures for classifying questionable Alzheimer dementia (QAD) progressors vs QAD nonprogressors
Optimal performance maximizing both sensitivity and specificity was determined by identifying the curve with the highest leftward point (white region of top graphs); bottom graphs show zoomed in views of areas in white from top graphs. (A) Comparing three standard volumetric variables, EV demonstrated highest sensitivity (72%) and specificity (65%). EV = entorhinal volume; HV = hippocampal volume; WBV = whole brain volume. (B) Comparing two global variables, mean cortical thickness demonstrated highest sensitivity (69%) and specificity (65%). MCT = mean cortical thickness. (C) Comparing two regional cortical thickness measures with EV, mean AD signature cortical thickness demonstrated highest sensitivity (83%) and specificity (65%). ADT = AD signature thickness; MTLT = medial temporal lobe thickness.
RESULTS
Clinical features.
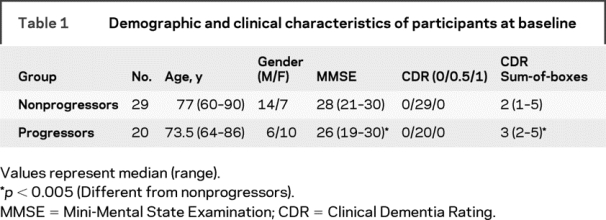
Participant characteristics are presented in table 1. For the entire sample, the average duration of clinical follow-up after the MRI scan was 2.7 (SD = 1.7) years with the progressor and nonprogressor groups being followed for 2.3 (SD = 1.1) and 3 (SD = 1.4) years. The progressor and nonprogressor groups did not differ on the basis of age [F(1) = 2.7, p = 0.11], but they did differ on CDR-SB [F(1) = 19.9, p < 0.001] and MMSE scores [F(1) = 10.1, p < 0.005].
Table 1 Demographic and clinical characteristics of participants at baseline
Morphometric correlates of symptom severity.
Within the entire sample, several cortical thickness measures correlated with the relative severity of cognitive impairment as measured by CDR-SB, including medial temporal (r = −0.36, p < 0.01), inferior temporal (r = −0.30, p < 0.04), and superior parietal cortex (r = −0.32, p < 0.03), with trends (p < 0.1) for temporal pole (r = −0.24) and angular gyrus (r = −0.28). In addition, CDR-SB correlated with average AD-signature thickness (r = −0.38, p < 0.01), and with mean thickness across the entire cortical mantle (r = −0.32, p < 0.03).
Similarly, a number of cortical thickness measures correlated with MMSE score: angular gyrus (r = 0.45, p < 0.001), superior frontal gyrus (r = 0.34, p < 0.02), superior parietal lobule (r = 0.38, p < 0.01), supramarginal (r = 0.32, p < 0.03), mean cortical thickness (r = 0.34, p < 0.02), and average AD-signature thickness (r = 0.35, p < 0.02).
As for volumetric measures, the only correlation with CDR-SB or MMSE was observed with entorhinal cortex volume, which showed a trend-level (r = −0.24, p < 0.1) effect.
Morphometric differences between progressors and nonprogressors.
Mean cortical thickness was 3.2% thinner in progressors than nonprogressors [nonprogressors = 2.11 (SD = 0.10) mm, progressors = 2.05 (SD = 0.14) mm; F(1) = 4.6, p = 0.037, Cohen d = 0.54].
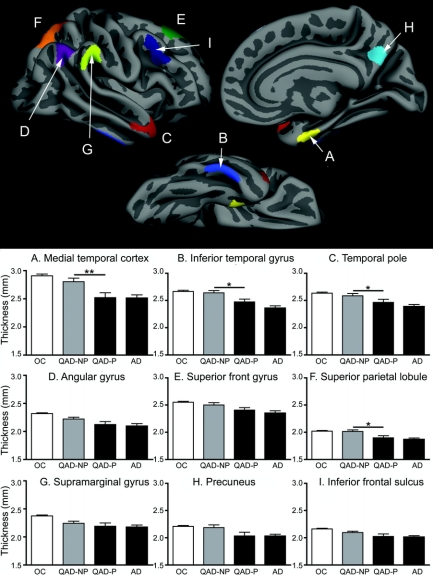
As mentioned above, figure 1 (top) illustrates the localization of the nine AD-signature cortical ROIs, derived from a separate sample of 115 OC vs 29 AD.11 Thinning in prodromal AD (the progressor group) was most prominent in the rostral medial temporal cortex, with thinning (compared to nonprogressors) of 0.28 mm (10.1%, Cohen d = 0.79). Three other regions differed (p < 0.05) between the two groups: inferior temporal gyrus (0.17 mm [6.3%] thinning, Cohen d = 0.73), superior parietal lobule (0.12 mm [5.8%] thinning, Cohen d = 0.70), and temporal pole (0.12 mm [4.7%] thinning, Cohen d = 0.51). Two regions showed trends (p < 0.1) toward thinning in prodromal AD: precuneus (0.15 mm [7.03%] thinning, Cohen d = 0.52), and angular gyrus (0.1 mm [4.43%] thinning, Cohen d = 0.48). Weaker effects in the same direction were observed in the other three ROIs. Group mean and standard error data are shown in figure 1. Additional details are shown in table 2.
Figure 1 Regions of interest (ROIs) derived from previous exploratory analysis that identified foci of thinning in mild Alzheimer disease,11 and thinning in cortical ROIs in patients with questionable AD who later progressed to mild AD dementia (QAD-P) compared to QAD nonprogressors (QAD-NP)
Top: ROIs derived from previous exploratory analysis that identified foci of thinning in mild AD.11 (A) Medial temporal cortex, (B) inferior temporal gyrus, (C) temporal pole, (D) angular gyrus, (E) superior frontal gyrus, (F) superior parietal lobule, (G) supramarginal gyrus, (H) precuneus, (I) inferior frontal sulcus. Bottom: Thinning in cortical ROIs in QAD-P compared to QAD-NP. Bar graphs show mean cortical thickness within each ROI in the two groups (middle two bars) and, for comparison purposes, in a sample of 115 older controls (OC, left bar) and 29 patients with mild AD (AD, right bar). Error bars indicate 1 SEM. *p < 0.05, **p < 0.005. See table 2 for additional detail.
Table 2 Quantitative metrics of thinning by region
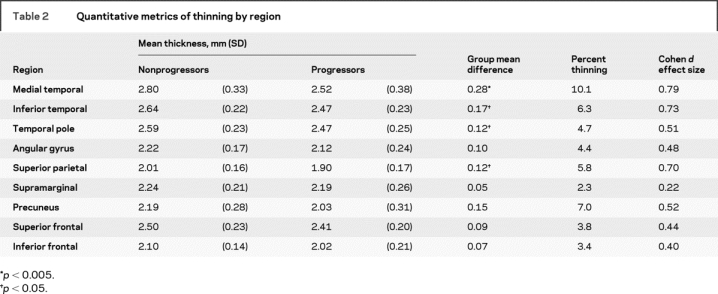
The progressor and nonprogressor groups did not differ for whole brain volume (p > 0.3), but both hippocampal [F(1) = 4.5, p < 0.04, Cohen d = 0.4] and entorhinal [F(1) = 6.4, p < 0.02, Cohen d = 0.6] volumes were smaller in the progressors than nonprogressors, as expected.
ROC analysis.
The goal of this analysis was to provide data to assist in the comparison of the cortical thickness measures of primary interest in this study with morphometric measures employed more widely in previous studies of the prediction of progression to dementia. Rather than discriminant function or logistic regression analysis, ROC was chosen to explore the full spectrum of performance across a range of sensitivity and specificity. Full curves are presented in figures, and here we compare measures by focusing on the peak performance point (highlighted in the figures) that optimized both sensitivity and specificity.
Of the standard volumetric measures, both hippocampal and entorhinal volume outperformed whole brain volume across a wide range of the ROC curve, with the best peak performance by entorhinal cortex (sensitivity and specificity of 72.4% and 65%; hippocampal sensitivity and specificity were 83% and 50%; figure 2A). Area under the curve (AUC) measures for whole brain volume (WBV), hippocampal volume, and entorhinal volume are 0.59, 0.65, and 0.70.
Since AD is primarily a gray matter disease with important degenerative effects on the cerebral cortex, we hypothesized that, among global measures, mean cortical thickness would be more sensitive than whole brain volume, which reflects white matter volume as well as gray matter volume. Therefore, an ROC comparison was made of the predictive classification performance of mean cortical thickness vs whole brain volume. Mean cortical thickness demonstrated predictive classification performance that was better than whole brain volume, with a peak sensitivity of 69% and specificity of 65% (figure 2B) as compared to the peak sensitivity and specificity for whole brain volume of 62% and 55%. AUC measures for WBV and mean cortical thickness are 0.59 and 0.67.
Next, we performed similar analyses of the regional cortical thickness measures. Among all regional thickness measures (figure e-1 and appendix e-1), superior parietal, precuneus, and inferior frontal cortices showed relatively high sensitivity and medial temporal, temporal pole, and superior frontal cortices showed relatively high specificity. Of the nine cortical ROI measures, the medial temporal lobe thickness had the best peak performance, with a sensitivity and specificity of 83% and 55%.
Finally, we compared the predictive classification performance of the average AD-signature thickness vs MTL thickness alone vs entorhinal volume (figure 2C). The AD signature measure performed best, with a sensitivity of 83% and specificity of 65%. AUC measures for hippocampal volume, MTL thickness, and mean AD signature thickness are 0.65, 0.72, and 0.73.
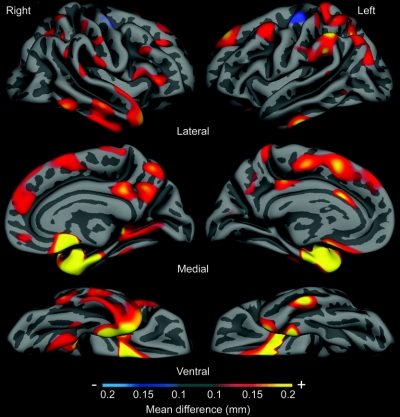
An exploratory analysis of cortical thickness across the entire mantle (figure 3) revealed a pattern of thinning in prodromal AD similar to, but expressed to a lesser degree than, that seen in mild AD.11
Figure 3 Magnitude of cortical thinning in prodromal Alzheimer disease (AD) in millimeters, derived from sample of 29 questionable Alzheimer dementia (QAD) nonprogressor vs 20 progressor subjects
Map shows parameter estimate of amount of thinning across cerebral cortex from general linear model analysis of 49 subjects, showing areas where cortex is at least 0.1 mm thinner in prodromal AD dementia group (progressors) than in nonprogressors. Maps are presented on the semi-inflated cortical surface of an average brain with dark gray regions representing sulci and light gray regions representing gyri. Non-neocortical regions and regions that are not part of the cortical mantle (such as the corpus callosum and thalamus) have been excluded from the analysis. Color scale shows magnitude of thinning from 0.1 mm (red) through 0.2 mm (yellow).
DISCUSSION
In this study, we used existing knowledge about regional cortical thinning in a sample of patients with mild AD dementia to investigate, using an a priori ROI-based approach, regional cortical thinning in a sample of patients with questionable AD dementia, many characteristics of which are similar to those of patients with MCI. The magnitude of thinning of certain regions correlates with the relative severity of very mild symptoms in daily life (as measured by CDR-SB) and signs on performance testing (MMSE). Furthermore, the thickness of particular regions of the cerebral cortex known to undergo thinning in mild AD provides a useful measure for the prediction of progression from questionable AD dementia (CDR 0.5) to mild AD dementia (CDR 1).
While extensive investigation has been performed in MCI of certain morphometric measures, such as the volumes of the whole brain, ventricular system, hippocampal formation, and entorhinal cortex,1–3,24 there is scant evidence regarding anatomic abnormalities in other cortical brain regions. Most of the literature on cortical atrophy in MCI has focused on exploratory mapping of atrophy in MCI compared to controls or patients with AD,6,25,26 or in MCI converters to mild AD dementia compared to nonconverters.8,9,27
In the former studies of atrophy in MCI in which the diagnostic outcome is unknown, atrophy patterns are largely similar to, although of lesser magnitude, than those of mild AD dementia. Of the previous investigations that have followed patients clinically after scanning, two demonstrated distributed atrophy patterns in the MCI progressors that are typical of those of patients with AD when comparing MCI progressors to controls,7 but surprisingly one study showed more widespread atrophy when comparing MCI progressors to nonprogressors,7 while the other showed much less widespread differences involving only supramarginal, inferior frontal, and hippocampal regions.9 A third investigation identified focal ventromedial temporal atrophy.8 There are no previous comparable data regarding the magnitude of atrophy (% difference) between progressors vs nonprogressors. The present data are consistent with previous data indicating that, when AD symptoms are still incipient or very mild, atrophy is already present in a set of heteromodal and limbic cortical regions. These data build on previous results by showing that although a lesser magnitude of atrophy is present in these very mildly affected patients, the spatial pattern is predictable based on what is known about the cortical regions affected in mild AD dementia (when defined using an independent sample).
Besides comparisons between diagnostic patient groups, we also investigated the relationships between regional cortical thickness and relative severity of mild symptoms in daily life (as measured by CDR-SB) and signs of cognitive impairment (as measured by MMSE) within this group of participants. To our knowledge, only one study has investigated the relationship of MMSE to cortical atrophy within a sample restricted to MCI or prodromal AD, and no regions of correlation were found.9 We observed robust relationships between the average thickness of the nine AD-signature regions and MMSE performance as well as CDR-SB. Such similar relationships, while intuitive, are not inevitable given the frequent dissociation between symptoms in daily life and signs on cognitive testing, particularly in the mildest prodromal stages of the illness.28 The correlations were anatomically distinct, however, in that CDR-SB correlates most prominently with ventromedial temporal and superior parietal thickness whereas MMSE correlates with superior frontal and lateral parietal thickness. Overall, the strength of the correlations was relatively subtle, as has been the case in previous reports focusing on medial temporal lobe regions. Further investigation will be necessary to determine whether consistent localized brain–behavior relationships can be illuminated through the investigation of regional cortical thinning in neurodegenerative diseases.
Most importantly, measures of the thickness of specific cortical regions known to be affected by AD are useful in predicting progression from questionable dementia to mild AD dementia within a few years. These findings build on a large body of work focused on morphometric measures of the medial temporal lobe in predicting progression from incipient dementia/MCI to AD dementia, expanding now to include regions of the temporoparietal and frontal cortices. The use of ROC analysis illuminates the utility of these measures across a broad spectrum of sensitivity-specificity tradeoffs, allowing the clinician to choose cutpoints depending on the question at hand. Also, by comparing the novel cortical thickness methods to more widely used volumetric measures (the results of which are similar to those of previous studies2,29), these analyses further demonstrate the potential utility of thickness measures.
Of the global measures, the average thickness of the cerebral cortex outperforms whole brain volume substantially with respect to predicting progression. This is not surprising given that whole brain volume likely reflects large classes of tissue that are relatively unaffected by AD. Of the regional volume measures, entorhinal volume performed better than hippocampal volume, as has been seen previously.30 MTL thickness performed better yet in comparison to entorhinal volume, which may relate in part to past observations that thickness appears to be a purer reflection of the effects of AD on cortical morphology while surface area, and therefore volume, are altered substantially by aging.31 Finally, ROC analysis demonstrated that the average thickness of all nine AD-signature ROIs was the most powerful measure for predicting progression to mild dementia. Compared to MTL ROI measures, such a disease composite measure probably increases sensitivity by including individuals with thinning in additional brain regions involved in the disease and increases specificity by excluding individuals with relative preservation of thickness in those regions (whose relatively isolated MTL thinning may be due to other pathologies).
Several limitations of this study deserve mention. The diagnosis of AD was not confirmed by autopsy or any other imaging markers. Longitudinal MRI measures were not performed. Finally, to translate these measures into biomarkers for use in individual patient diagnostic classification, as has been done with other approaches to cortical morphometric analysis in AD,32,33 further work is necessary. Yet the robust effects observed provide optimism that this approach to cortical anatomic measurement may be able to achieve such goals.
AUTHOR CONTRIBUTIONS
Dr. Dickerson and Mr. Bakkour performed all statistical analyses.
ACKNOWLEDGMENT
The authors thank Dr. Randy Buckner for his insight as well as the faculty and staff of the Washington University Alzheimer’s Disease Research Center. They also thank the participants in this study and their families for their valuable contributions, without which this research would not have been possible.
Supplementary Material
Address correspondence and reprint requests to Dr. Brad Dickerson, MGH Gerontology Research Unit, 149 13th St., Suite 2691, Charlestown, MA 02129, bradd@nmr.mgh.harvard.edu
Supplemental data at www.neurology.org
Editorial, page 1038
e-Pub ahead of print on December 24, 2008, at www.neurology.org.
Supported by grants from the NIA K23-AG22509, R01-AG29411, R21-AG29840, P50-AG05681, and P01-AG03991, NCRR P41-RR14075, U24-RR021382, the Alzheimer’s Association, Howard Hughes Medical Institute, and the Mental Illness and Neuroscience Discovery (MIND) Institute.
Disclosure: The authors report no disclosures.
Received June 18, 2008. Accepted in final form October 3, 2008.
REFERENCES
- 1.Jack CR Jr., Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 1999;52:1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol 2000;47:430–439. [PubMed] [Google Scholar]
- 3.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging 2001;22:747–754. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Rub U, Schultz C, Del Tredici K. Vulnerability of cortical neurons to Alzheimer’s and Parkinson’s diseases. J Alzheimer Dis 2006;9:35–44. [DOI] [PubMed] [Google Scholar]
- 5.Scheff SW, Price DA. Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimer Dis 2006;9:101–115. [DOI] [PubMed] [Google Scholar]
- 6.Singh V, Chertkow H, Lerch JP, Evans AC, Dorr AE, Kabani NJ. Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer’s disease. Brain 2006;129:2885–2893. [DOI] [PubMed] [Google Scholar]
- 7.Whitwell JL, Shiung MM, Przybelski SA, et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology 2008;70:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chetelat G, Landeau B, Eustache F, et al. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage 2005;27:934–946. [DOI] [PubMed] [Google Scholar]
- 9.Bozzali M, Filippi M, Magnani G, et al. The contribution of voxel-based morphometry in staging patients with mild cognitive impairment. Neurology 2006;67:453–460. [DOI] [PubMed] [Google Scholar]
- 10.Morris JC, Ernesto C, Schafer K, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s Disease Cooperative Study experience. Neurology 1997;48:1508–1510. [DOI] [PubMed] [Google Scholar]
- 11.Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex Epub 2008 Jul 16. [DOI] [PMC free article] [PubMed]
- 12.Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cereb Cortex 2004;14:721–730. [DOI] [PubMed] [Google Scholar]
- 13.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage 2004;23:724–738. [DOI] [PubMed] [Google Scholar]
- 14.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 2005;25:7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology 2005;64:1032–1039. [DOI] [PubMed] [Google Scholar]
- 16.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology 2006;67:467–473. [DOI] [PubMed] [Google Scholar]
- 17.Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry 2003;60:878–888. [DOI] [PubMed] [Google Scholar]
- 18.Rosas HD, Liu AK, Hersch S, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology 2002;58:695–701. [DOI] [PubMed] [Google Scholar]
- 19.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9:195–207. [DOI] [PubMed] [Google Scholar]
- 20.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9:179–194. [DOI] [PubMed] [Google Scholar]
- 21.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000;97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 24.de Leon MJ, Golomb J, George AE, et al. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR Am J Neuroradiol 1993;14:897–906. [PMC free article] [PubMed] [Google Scholar]
- 25.Pennanen C, Testa C, Laakso MP, et al. A voxel based morphometry study on mild cognitive impairment. J Neurol Neurosurg Psychiatry 2005;76:11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apostolova LG, Steiner CA, Akopyan GG, et al. Three-dimensional gray matter atrophy mapping in mild cognitive impairment and mild Alzheimer disease. Arch Neurol 2007;64:1489–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitwell JL, Shiung MM, Przybelski SA, et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology 2008;70:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickerson BC, Sperling RA, Hyman BT, Albert MS, Blacker D. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry 2007;64:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devanand DP, Pradhaban G, Liu X, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology 2007;68:828–836. [DOI] [PubMed] [Google Scholar]
- 30.Killiany RJ, Hyman BT, Gomez-Isla T, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology 2002;58:1188–1196. [DOI] [PubMed] [Google Scholar]
- 31.Dickerson BC, Feczko E, Augustinack JC, et al. Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging Epub 2007 Sep 13. [DOI] [PMC free article] [PubMed]
- 32.Vemuri P, Gunter JL, Senjem ML, et al. Alzheimer’s disease diagnosis in individual subjects using structural MR images: validation studies. Neuroimage 2008;39:1186–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lerch JP, Pruessner J, Zijdenbos AP, et al. Automated cortical thickness measurements from MRI can accurately separate Alzheimer’s patients from normal elderly controls. Neurobiol Aging 2008;29:23–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.