Abstract
Background:
Parkinson disease (PD) is a common neurodegenerative disease affecting up to 1 million individuals in the United States. Depression affects up to 50% of these patients and is associated with a variety of poor outcomes for patients and their families. Despite this, there are few evidence-based data to guide clinical care.
Methods:
An NIH-funded, randomized, controlled trial of paroxetine CR, nortriptyline, and placebo in 52 patients with PD and depression. The primary outcomes were the change in the Hamilton Depression Rating Scale (HAM-D) and the percentage of depression responders at 8 weeks.
Results:
Nortriptyline was superior to placebo for the change in HAM-D (p < 0.002); paroxetine CR was not. There was a trend for superiority of nortriptyline over paroxetine CR at 8 weeks (p < 0.079). Response rates favored nortriptyline (p = 0.024): nortriptyline 53%, paroxetine CR 11%, placebo 24%. In planned contrasts of response rates, nortriptyline was superior to paroxetine CR (p = 0.034). Nortriptyline was also superior to placebo in many of the secondary outcomes, including sleep, anxiety, and social functioning, while paroxetine CR was not. Both active drug treatments were well tolerated.
Conclusions:
Though relatively modest in size, this is the largest placebo-controlled trial done to date in patients with Parkinson disease (PD) and depression. Nortriptyline was efficacious in the treatment of depression and paroxetine CR was not. When compared directly, nortriptyline produced significantly more responders than did paroxetine CR. The trial suggests that depression in patients with PD is responsive to treatment and raises questions about the relative efficacy of dual reuptake inhibitors and selective serotonin reuptake inhibitors.
GLOSSARY
- ARR
= absolute risk reduction;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- HAM-A
= Hamilton Anxiety Scale;
- HAM-D
= Hamilton Depression Rating Scale;
- MMSE
= Mini-Mental State Examination;
- NNT
= number needed to treat;
- PD
= Parkinson disease;
- PDQ
= Parkinson’s Disease Questionnaire;
- PSQI
= Pittsburgh Sleep Quality Index;
- SCID
= Structured Clinical Interview;
- SSRI
= selective serotonin reuptake inhibitor;
- TCA
= tricyclic antidepressant;
- UPDRS
= Unified Parkinson’s Disease Rating Scale.
Parkinson disease (PD) is the second most prevalent neurodegenerative illness in the United States, affecting approximately 1 million individuals. While the illness is defined by the motor triad of tremor, rigidity, and bradykinesia, non-motor features are both common and functionally important. Depression, dementia, drug-induced psychosis, impulsivity, and sleep disturbances may all complicate the course and management of PD and are associated with a variety of poor short and long-term outcomes.1
Depression is one of the most common nonmotor aspects of PD, with a reported prevalence of approximately 40% to 50%.2 It is of particular importance to these patients because, in addition to personal suffering, depression is associated with a faster progression of physical symptoms, a greater decline in cognitive skills and ability to care for oneself, poorer treatment compliance and quality of life, as well as greater caregiver distress.3–5 In fact, depression appears to be more predictive of distress than motor disability.6
Despite the prevalence and importance of depression in PD, there are few well-designed studies that inform treatment.7 There are a variety of open label trials and some older, methodologically flawed controlled trials, but a recent Cochrane Review concluded that there are insufficient data on the effectiveness and safety of any antidepressant therapies in PD to allow recommendations for their use.8
Nevertheless, antidepressants are apparently widely used for these patients, with selective serotonin reuptake inhibitors (SSRIs) being the most commonly used medications. A survey of physicians in the Parkinson Study Group found that 26% of the patients with PD were on antidepressants for depression and 51% of the physicians used SSRIs as their first line therapy.9 A more recent Veterans Affairs database study found that 63% of patients with PD and depression were taking SSRIs, while only 7% were taking tricyclic antidepressants (TCAs).10
In order to begin to provide clinical guidance for these patients, we conducted an NIH-funded, controlled trial of nortriptyline, paroxetine CR, and placebo in patients with PD and depression. The primary outcomes were the change in the Hamilton Depression Rating Scale (HAM-D), from baseline to endpoint, and the percent responders to each treatment.
METHODS
Study design.
Clintrials.gov identifier: NCT 00062738. Dr. Buyske, Department of Statistics at Rutgers University, carried out the statistical analysis.
Fifty-two patients were enrolled in this 8-week, randomized, double-blind trial of nortriptyline, paroxetine CR, or placebo. The primary hypothesis was that patients on active drug would have a significantly greater decrease in the baseline to endpoint HAM-D scores and that there would be significantly more responders in the active drug groups.
Consent procedures and data collection.
All procedures used were approved by the University of Medicine and Dentistry of New Jersey’s Institutional Review Board. All patients were evaluated between October 2003 and July 2007.
Patients.
Patients were included if they were between the ages of 35 and 80, had a confirmed diagnosis of PD by research criteria,11 and had a diagnosis on the Structured Clinical Interview12 (SCID) for the Diagnostic and Statistical Manual of Mental Disorders 4th ed. (DSM-IV)13 of major depression or dysthymia.
Patients were excluded if they had cognitive impairment (Mini-Mental State Examination [MMSE]14 less than 26), were “off” for greater than 50% of the day, or had any current DSM-IV Axis I diagnosis other than a depressive or anxiety disorder. Patients were not allowed to take any psychotropic medications other than the study medication during the trial. Patients who had been nonresponsive to more than one trial of an adequate dose and length of an approved antidepressant were excluded. Patients were required to maintain a stable dose of the PD medication that they were on at the start of the trial and all evaluations were done in the “on” state.
Dosing.
Patients were randomized, in variable length blocks, to equivalent-appearing nortriptyline, paroxetine CR, or placebo. Dosing was flexible with decisions on dose being made at each visit (or between visits if the patient was having troublesome side effects) based on efficacy and tolerability. Paroxetine CR was started at 12.5 mg and could be increased up to 37.5 mg. Nortriptyline was started at 25 mg and could be increased up to 75 mg. Placebo was started at one pill and could be increased up to three pills.
Assessments.
The primary outcomes were the HAM-D 17-item15 change from baseline and the percent responders. Responders were defined as patients who had a 50% or greater decrease in their baseline HAM-D at last observation. All patients and study personnel were blind to drug assignment. Patients were assessed on primary outcomes at 2, 4, and 8 weeks.
Secondary outcomes included quality of life, which was assessed using the Parkinson’s Disease Questionnaire (PDQ-8)16 and the Medical Outcome Study Short Form (SF 36),17 the Clinical Global Impression Scale,18 the Hamilton Anxiety Scale (HAM-A),19 The Pittsburgh Sleep Quality Index (PSQI),20 and the Unified Parkinson’s Disease Rating Scale (UPDRS).21
We also administered a battery of cognitive tests including the MMSE,14 forward and backward digit span of the Wechsler Adult Intelligent Test–Third Edition,22 the Boston Naming Test,23 the word list recall and recognition subtests of the Wechsler Memory Scale–Third Edition24 verbal category fluency, and the Stroop color-word test.25 All secondary outcomes were assessed at baseline and at week 8 or at the last visit for those who terminated early.
Data analyses.
An intent-to-treat approach was used in all analyses and included all 52 patients. The primary outcome of HAM-D change was evaluated at baseline, 2, 4, and 8 weeks using a mixed-model repeated measures analysis of variance as implemented in the MIXED procedure of SAS Version 9.1 with restricted maximum likelihood estimation.26 This analysis assumes that the data that are missing, most of which are attributed to patient dropout, are missing at random. Fixed effects were treatment (placebo, nortriptyline, paroxetine CR), time of assessment (baseline, 2, 4, and 8 weeks), and the treatment by time interaction. Random subject intercepts were included in the model. In modeling covariance structure for all analyses, we chose a continuous AR1 structure as a function of the square root of days from baseline that a given assessment occurred for a given patient. This model yielded the lowest AIC among all covariate structures examined. The interaction between treatment and time in the mixed model was of most interest, since a time effect alone would only indicate improvement over time across all three groups, whereas a treatment by time interaction indicates differential improvement over time, depending on treatment.
For the responder analysis, an LOCF approach was used in which a clinical response was operationally defined as at least a 50% reduction in the HAM-D score from baseline to 8 weeks. Clinical response was cross-tabulated with treatment and Fisher exact test was used to distinguish differences among these groups.
Mixed models analyses were used to analyze secondary variables. Nominal p values are given for secondary outcomes.
RESULTS
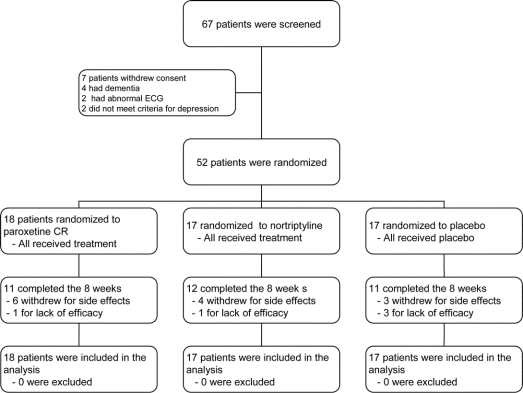
Sixty-seven patients signed informed consent and 15 were screened out for a variety of reasons (figure 1). Thus, 52 patients, 27 men and 25 women, entered the trial. Fifty of the patients had major depression, two had dysthymia in addition to major depression, and two had only dysthymia. The mean age was 62.8 and the mean duration of PD was 6.6 years. The average dose of medication was 28.4 mg for paroxetine CR, 48.5 mg for nortriptyline, and 2.7 pills for placebo. Other clinical information on the sample is found in table 1.
Figure 1 Patient flow diagram
Table 1 Clinical information on patients
Primary outcomes.
HAM-D.
The mixed effects analysis of the primary outcome measure, the HAM-D, resulted in a significant effect for treatment by time interaction [F(6,119) = 2.99, p = 0.009], using repeated measures collected at baseline and all available follow-up assessments for all 52 subjects. We then examined this treatment by time interaction for three distinct slices of the data set: 2 weeks vs baseline, 4 weeks vs baseline, and 8 weeks vs baseline (table 2). Table 2 shows the nominal significance level of treatment at each of these three slices (p values are 0.028, 0.022, and 0.005). Table 2 also shows, for each study group, least-squares means adjusting for other parameters in the model, standard deviations, and significance levels for three planned contrasts (nortriptyline vs placebo, paroxetine CR vs placebo, and nortriptyline vs paroxetine CR).
Table 2 Hamilton Depression Rating Scale (HAM-D) changes
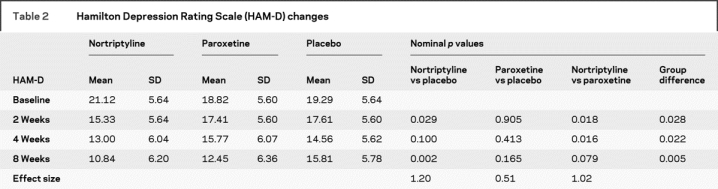
In planned contrasts, nortriptyline was superior to placebo at 2 weeks (p < 0.029) and at 8 weeks (p < 0.002). The 8-week comparison of nortriptyline and placebo remained significant (p < 0.05) after a Bonferroni correction for multiple comparisons. Paroxetine CR was not superior to placebo at any point. Nortriptyline was (nominally) superior to paroxetine CR at 2 weeks (p < 0.018) and 4 weeks (p < 0.016) but not 8 weeks (p < 0.079).
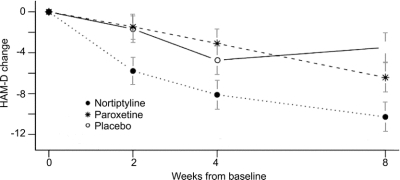
Table 2 also lists the Cohen’s effect sizes, which are 1.2 for nortriptyline vs placebo, 1.02 for nortriptyline vs paroxetine CR, and 0.51 for paroxetine CR vs placebo. The change in the HAM-D scores is shown graphically in figure 2.
Figure 2 Change in Hamilton Depression Rating Scale
Covariates.
We examined the effect of patient age, gender, and length of illness by adding these data to the mixed model. There were no significant effects (all p values >0.30) for any of these variables.
Responders.
The response rates at endpoint, based on a 50% change in the HAM-D total score, also significantly favored nortriptyline: nortriptyline 53%, paroxetine CR 11%, placebo 24% (Fisher exact p = 0.024). In planned contrasts, adjusted for multiple comparisons, nortriptyline was superior to paroxetine CR (p = 0.034). While not an a priori outcome, we did examine remitter rates, using a HAM-D of 7 or less as a definition of remitter. The remitter rates were different (nortriptyline 41%, paroxetine CR 17%, placebo 12%) but they did not differ statistically (p = 0.154).
Number needed to treat.
The number needed to treat (NNT), based on responders, for nortriptyline compared to placebo is 3.5, with an absolute risk reduction (ARR) of 29%. NNT for nortriptyline compared to paroxetine CR is 2.4 with an ARR of 42%.
Secondary outcomes.
Quality of life.
The total SF36 score was not different between groups; however, patients on nortriptyline reported better social functioning at endpoint (drug by time interaction p = 0.0142; planned contrast nortriptyline vs placebo p = 0.016). No differences between groups were found on the PDQ 8.
Disease measures.
There were no significant differences between groups found on the UPDRS.
Sleep.
Patients taking nortriptyline reported significant improvements in sleep (PSQI) compared to placebo (drug by time interaction, p = 0.0021; planned contrast nortriptyline vs placebo, p = 0.008).
Anxiety.
A significant time by drug interaction was noted on the HAM-A (p < 0.0001). In the planned contrasts, nortriptyline was significantly better than paroxetine CR (p < 0.007) and placebo (p < 0.0001) in alleviating anxiety. Paroxetine CR showed a trend advantage over placebo (p = 0.0740).
Overall improvement.
There was a significant effect between treatment arms in the Clinical Global Improvement scale (Fisher exact, p = 0.0297).
Cognition.
There was no significant time by drug interaction on the neuropsychological measures.
Integrity of the blind.
To test the integrity of the blinding we asked both the patient and the rater to guess what treatment the patient was taking. There was no indication that the blinding was compromised.
Tolerability.
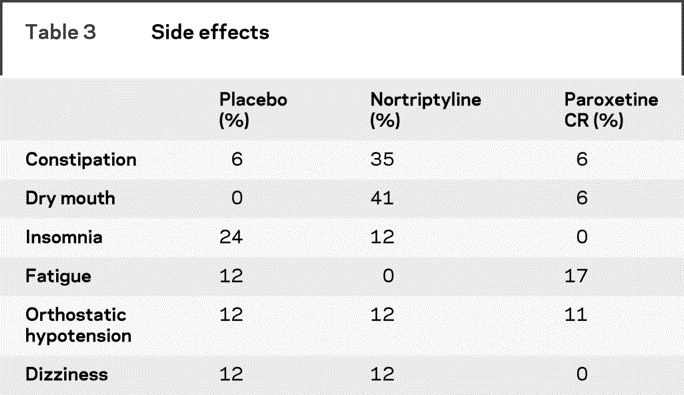
There were no differences in the dropout rates among the three arms of the trial: nortriptyline 29%, paroxetine CR 39%, and placebo 35%. Side effects of the treatments were generally mild or moderate. There was a significantly higher average number of side effects in the paroxetine CR group compared to placebo (Tukey contrasts, p = 0.0281) but no significant differences between nortriptyline and placebo. The most common side effects are listed in table 3.
Table 3 Side effects
Safety.
Safety measures in the trial included measurement of the QTc interval in patients on nortriptyline, nortriptyline levels, vital signs, and serious adverse events. The QTc of patients on nortriptyline decreased nonsignificantly from baseline (423 msec) to endpoint (422 msec). There was one patient with a QTc of greater than 450 msec at baseline and two at endpoint. No patient had a QTc above 500 msec. Nortriptyline levels ranged between 32 and 138 with an average of 74.88. There were no significant baseline to endpoint changes in vital signs in any of the groups. Six patients developed orthostatic hypotension at at least one visit in the trial: two on placebo, two on paroxetine CR, and two on nortriptyline. None of these patients had falls and none stopped the trial because of this side effect. There were three serious adverse events: one patient on paroxetine CR was hospitalized for fainting—no cause was discovered—and one patient on placebo had a severe worsening of rigidity due to a Parkinson medication change and another patient on placebo had delirium.
DISCUSSION
This is the largest placebo-controlled medication trial to date in depressed patients with PD, and the first to compare a dual reuptake inhibitor (nortriptyline) and an SSRI (paroxetine CR). It is also the largest controlled trial to show that depression in patients with PD responds to antidepressant treatment. Nonetheless, it is not large by clinical trial standards and must be seen as preliminary.
While preliminary, this trial yielded results that are perhaps surprising and may have significant clinical implications. The treatment effect of nortriptyline was significant for both the overall change in the HAM-D and in the percent responders, while paroxetine CR was not. Furthermore, nortriptyline, when compared directly to paroxetine CR, produced significantly more responders. Nortriptyline was also superior to placebo on many of the secondary outcomes while paroxetine CR was not. The effect size of nortriptyline was large (1.20, based on HAM-D) and the number needed to treat for nortriptyline was 3.5 based on response status. Both active treatments were well tolerated, although paroxetine CR did have significantly more side effects than placebo and nortriptyline did not.
Nortriptyline has been widely used in treatment of depression in the elderly27 and was found in one study to be superior to an SSRI in patients with post-stroke depression.28 The one previous study done with nortriptyline in depressed patients with PD, a small crossover study which used an outcome instrument that is not widely validated, did suggest that the drug is efficacious.29
The etiology of depression in patients with PD is unclear and any explanation of the apparent superiority of nortriptyline would be speculative. While there are significant psychosocial stressors intrinsic to the illness, there are also numerous studies that point to a neurobiological etiology. There is evidence implicating dopaminergic, serotonergic, noradrenergic, and cholinergic dysfunction.30 In addition, changes in trophic and inflammatory factors, such as nerve growth factor and cytokines, which seem to play a role in clinical depression, have also been associated with PD.31
A 2005 PET study30 provided evidence of a loss of noradrenergic neurons in the limbic system in patients with PD and depression, perhaps providing a possible way of understanding these results. Nortriptyline is a dual reuptake inhibitor, that is, it inhibits reuptake of both serotonin and norepinephrine, whereas paroxetine CR, an SSRI, inhibits the reuptake of only serotonin. Thus, it is possible that the mechanism of the apparent superiority of nortriptyline is its effect on norepinephrine.
Another, admittedly speculative, explanation for these results may lie in the role of norepinephrine transporters in the prefrontal cortex. The norepinephrine reuptake transporter is responsible for removing dopamine from the synapse in this area of the brain.32 Blockade of these transporters by agents such nortriptyline can acutely increase dopamine levels in the frontal cortex,33 facilitating dopaminergic function.
At present, it appears that the SSRIs are the first-line choice for depression in PD in clinical practice and that the TCAs are not commonly used.9,10 This study, however, suggests that the SSRI paroxetine CR is not superior to placebo in patients with PD and depression and may be inferior to nortriptyline. The effect size for paroxetine CR on the HAM-D was medium (0.51); a study with a larger sample size or of longer duration might have found a significant effect on this outcome, although paroxetine produced fewer responders than did placebo. These results are consistent with the two previous controlled trials with SSRIs34,35 (sertraline and paroxetine) in PD which, while admittedly methodologically flawed, did not support the efficacy of these compounds. However, there are also numerous open-label studies of paroxetine (non CR)36 and other SSRIs7 in this population that have shown good efficacy and low dropout rates.
While only an 8-week trial, the improvement in the secondary outcomes in this trial is noteworthy. Patients on nortriptyline had significant improvements in physician-rated overall improvement, social functioning, sleep, and anxiety. These improvements are consistent with the descriptive literature in depression that suggests that depression negatively affects these aspects of the patient’s condition. Furthermore, sleep and anxiety are strongly correlated with overall quality of life5 and improvements in these symptoms may be, by themselves, valuable outcomes.
The tolerability and safety assessment included in the trial indicated that both drugs were well tolerated. Both drug treatment arms had similar dropout rates and, although dropout rates of this size are not unusual in psychopharmacologic trials, their size may limit the generalizability of the trial. The side effects were consistent with side effects seen in non-PD populations. Nortriptyline can increase the P–R interval, QRS duration, and Q–Tc interval and has been associated with cardiac arrhythmias.37 There were, however, no significant effects of nortriptyline on cardiac conduction in this trial. There were also no significant changes in vital signs in any of the groups and neither active drug had negative effects on cognition.
While nortriptyline, given its potential for cardiac conduction delay, needs to be used cautiously, the emerging evidence suggests that its benefits are substantial. The two non-TCA dual reuptake inhibitors that do not have significant cardiac effects, venlafaxine and duloxetine, have not been extensively evaluated in depression in PD. There are ongoing trials evaluating some of these compounds in PD, so we await, with interest, the results of these trials. Further studies, with larger numbers of patients, broader entrance criteria, and antidepressants that effect neurotransmitters other than serotonin, are needed.
Supplementary Material
Address correspondence and reprint requests to Dr. Matthew Menza, Robert Wood Johnson Medical School, D207A, 671 Hoes Lane, Piscataway, NJ 08854 menza@umdnj.edu
Editorial, page 868
e-Pub ahead of print on December 17, 2008, at www.neurology.org.
Supported by a grant from the National Institute of Neurological Disorders and Stroke (NINDS) RO1NS043144. GlaxoSmithKline provided free paroxetine CR and matching placebo.
Disclosure: Matthew Menza, MD—research support: National Institutes of Health (NINDS), Astra-Zeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Forest Laboratories, GlaxoSmithKline, Lilly, Pfizer, Sanofi-Aventis, Sepracor, Takeda Wyeth. Consultant: National Institutes of Health (NIMH, NINDS), GlaxoSmithKline, Kyowa, Lilly Research Laboratories, Pfizer, Sepracor, Takeda. Speaker: Sanofi-Aventis. Stocks: none. Other financial: none; Roseanne DeFronzo Dobkin, PhD—research support: National Institutes of Health (NINDS); Humberto Marin, MD—research support: National Institutes of Health (NINDS), GlaxoSmithKline, Lilly, Sanofi-Aventis, Sepracor, Takeda. Consultant: Lilly Research Laboratories; Margery Mark, MD: research support: Kyowa, Cephalon. Speaker: Allergan, Boehringer Ingelheim, GlaxoSmithKline, Valeant; Michael Gara, PhD—none; Steven Buyske, PhD—research support: National Institutes of Health (NIAAA); Karina Bienfait, PhD—none; Allison Dicke—none.
Received March 31, 2008. Accepted in final form August 22, 2008.
REFERENCES
- 1.Menza M, Marsh L, eds. Psychiatric Issues in Parkinson’s Disease: A Practical Guide. London: Taylor & Francis; 2006. [Google Scholar]
- 2.Reijnders MA, Ehrt U, Weber WEJ, Aarsland D, Leentjens AFG. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord Epub 2007 Nov 6. [DOI] [PubMed]
- 3.Ravina B, Camicioli R, Como PG, Marsh L, Jankovic J, Weintraub D, Elm J. The impact of depressive symptoms in early Parkinson disease. Neurology 2007;69:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whetten-Goldstein K, Sloan F, Kulas E, Cutson T, Schenkman M. The burden of Parkinson’s disease on society, family, and the individual. J Am Geriatr Soc 1997;45:844–849. [DOI] [PubMed] [Google Scholar]
- 5.Schrag A. Quality of life and depression in Parkinson’s disease. J Neurol Sci 2006;248:151–157. [DOI] [PubMed] [Google Scholar]
- 6.Global Parkinson’s Disease Steering Committee. Factors impacting on quality of life in Parkinson’s disease: results from an international survey. Mov Disord 2002;17:60–67. [DOI] [PubMed] [Google Scholar]
- 7.Weintraub D, Morales KH, Moberg PJ, et al Antidepressant studies in Parkinson’s disease: a review and meta-analysis. Mov Disord 2005;20:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghaxi-Noori S, Chung TH, Deane KHO, Rickards H, Clarke CE. Therapies for depression in Parkinson’s disease. Cochrane Database Syst Rev 2003;3:CD003465. [DOI] [PubMed] [Google Scholar]
- 9.Richard IH, Kurlan R. A survey of antidepressant drug use in Parkinson’s disease. Neurology 1997;49:1168-1170. [DOI] [PubMed] [Google Scholar]
- 10.Chen P, Kales HC, Weintraub D, Blow FC, Jiang L, Mellow AM. Antidepressant treatment of veterans with Parkinson’s disease and depression: Analysis of a national sample. J Geriatr Psychiatry Neurol 2007;20:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward CD, Gibb WR. Research diagnostic criteria for Parkinson’s disease. Adv Neurol 1990;53:245–249. [PubMed] [Google Scholar]
- 12.First MB, Spritzer RL, Gibbon M. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition, Biometrics Research Department. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 13.American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton M. Hamilton Depression Scale. In: ECDEU Assessment Manual for Psychopharmacology, revised edition. Rockville, MD: National Institute of Mental Health; 1976: 179–192. [Google Scholar]
- 16.Jenkinson CR. The PDQ-8: development and validation of a short-form Parkinson’s disease questionnaire. Psychol Health 1997;12:805–814. [Google Scholar]
- 17.Jenkinson C, Coulter A, Wright L. Short Form 36 (SF 36) Health Survey Questionnaire; normative data for adults of working age. BMJ 1993;306:1437–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guy W. ECDEU assessment manual for psychopharmacology. Washington, DC: U.S. Department of Health, Education, and Welfare; 1976. [Google Scholar]
- 19.Hamilton M. The assessment of anxiety status by rating. Br J Med Psychol 1959;32:50–55. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res 1998;45(1 Spec No):5–13. [DOI] [PubMed] [Google Scholar]
- 21.Fahn S, Elton RL, Members of the UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, eds. Recent Developments in Parkinson’s Disease, Vol. 2. Florham Park, NJ: Macmillan Health Care Information; 1987: 153–164. [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Test (Third Edition). New York: Psychological Corporation; 1997. [Google Scholar]
- 23.Kaplan EF, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lee & Febiger; 1983. [Google Scholar]
- 24.Wechsler D. Wechsler Memory Scale (Third Edition). New York: Psychological Corporation; 1997. [Google Scholar]
- 25.Golden CJ. Stroop Color and Word Test. Wood Dale, IL: Stoelting Co.; 1978:1–32. [Google Scholar]
- 26.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer; 2000. [Google Scholar]
- 27.Bump GM, Mulsant BH, Pollock BG, et al Paroxetine versus nortriptyline in the continuation and maintenance treatment of depression in the elderly. Depress Anxiety 2001;13:38–44. [DOI] [PubMed] [Google Scholar]
- 28.Robinson RG, Schultz SK, Castillo C, et al Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry 2000;157:351–359. [DOI] [PubMed] [Google Scholar]
- 29.Anderson J, Aabro E, Gulmann AE, Hjelmsted A, Pedersen HE. Anti-depressive treatment in Parkinson’s disease: a controlled trial of the effect of nortriptyline in patients with Parkinson’s disease treated with L-dopa. Acta Neurol Scand 1980;62:210–219. [DOI] [PubMed] [Google Scholar]
- 30.Remy P, Doder M, Lees A, Turjanki N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 2005;128:1314–1322. [DOI] [PubMed] [Google Scholar]
- 31.Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: role for cytokines. Current Pharma Des 2005;11:999–1016. [DOI] [PubMed] [Google Scholar]
- 32.Morón JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 2002;22:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valentini V, Frau R, Di Chiara G. Noradrenaline transporter blockers raise extracellular dopamine in medial prefrontal but not parietal and occipital cortex: differences with mianserin and clozapine. J Neurochem 2004;88:917–927. [DOI] [PubMed] [Google Scholar]
- 34.Wermuth L, Sorensen PS, Timm S, et al Depression in idiopathic Parkinson’s disease treated with paroxetine: a placebo-controlled trial. Nord J Psychiatry 1998;52:163–169. [Google Scholar]
- 35.Leentjens AFG, Vreeling FW, Luijckx GJ, Berhey FRJ. SSRIs in the treatment of depression in Parkinson’s disease. Int J Geriatric Psychiatry 2003;18:552–554. [DOI] [PubMed] [Google Scholar]
- 36.Ceravolo R, Nuti A, Piccinni A, et al Paroxetine in Parkinson’s disease: effects on motor and depressive symptoms. Neurology 2000;55:1216–1218. [DOI] [PubMed] [Google Scholar]
- 37.Glassman AH, Bigger JT Jr. Cardiovascular effects of therapeutic doses of tricyclic antidepressants: a review. Arch Gen Psychiatry 1981;38:815–820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.