Abstract
Objective:
Bevacizumab has been shown to be effective in the treatment of recurrent glioblastoma in combination with chemotherapy compared with historic controls but not in randomized trials.
Methods:
We conducted a retrospective analysis of patients treated for recurrent glioblastoma with bevacizumab vs a control group of patients, comparing progression-free survival (PFS) and overall survival (OS) between the two groups, and performed subgroup analysis based on age and performance status. Expression of vascular endothelial growth factor (VEGF) based on age was examined using DNA microarray analysis. We also evaluated the impact of bevacizumab on quality of life.
Results:
We identified 44 patients who received bevacizumab and 79 patients who had not been treated with bevacizumab. There was a significant improvement in PFS and OS in the bevacizumab-treated group. Patients of older age (≥55 years) and poor performance status (Karnofsky Performance Status ≤80) had significantly better PFS when treated with bevacizumab, and bevacizumab-treated older patients had significantly increased OS. VEGF expression was significantly higher in older glioblastoma patients (aged ≥55 years). Patients treated with bevacizumab also required less dexamethasone use and maintained their functional status longer than the control group.
Conclusions:
Bevacizumab in combination with chemotherapy may be a more effective treatment for recurrent glioblastoma and warrants further randomized prospective studies to determine its effect on survival. Bevacizumab also has more effect in those with older age and might reflect biologic differences in glioblastoma in different age groups as seen with the expression of vascular endothelial growth factor.
GLOSSARY
- GBM
= glioblastoma;
- HR
= hazard ratio;
- KPS
= Karnofsky Performance Status;
- OS
= overall survival;
- PFS
= progression-free survival;
- VEGF
= vascular endothelial growth factor.
Glioblastoma (GBM) is the most fatal and most common type of primary brain cancer. After standard therapy with surgical resection, radiation therapy, and concurrent chemotherapy, the median survival for patients with GBM is approximately 15 months.1 No standard therapy is available at recurrence. Further treatments in clinical trials only lead to a progression-free survival (PFS) of approximately 8 weeks and overall survival (OS) of 25–30 weeks.2-4
Antiangiogenesis is an attractive option in the treatment of GBMs because they are densely vascularized tumors and have increased expression of vascular endothelial growth factor (VEGF) compared with normal brain.5-7 Antiangiogenesis agents can also reduce peritumoral edema and reduce corticosteroid use.8,9
Recently, the antiangiogenic agent bevacizumab (Avastin®; Genentech, South San Francisco, CA) a humanized monoclonal antibody that binds to VEGF, has been used in the off-label setting and phase 2 clinical trials in the treatment of GBM. These studies have found impressive tumor response and prolonged survival compared with historic controls.10-14 However, these trials were not randomized trials of bevacizumab treatment against a control group of patients.
In this retrospective report, we assess the survival benefit of bevacizumab by comparing patients treated at our institution with bevacizumab vs patients who never received bevacizumab. Molecularly, we identify any differences in VEGF expression that might correlate with survival. We also evaluate the quality of life of our bevacizumab-treated patients vs the control group by looking at changes in functional status and corticosteroid use, because corticosteroids can cause long-term adverse effects15 and decrease quality of life.16
METHODS
Study design.
We performed a retrospective chart review of all patients treated for a recurrent GBM at the UCLA Neuro-Oncology Program and at our sister institution, Kaiser Permanente Los Angeles Neuro-Oncology. For the treated cohort, we identified 44 patients who received treatment with off-label bevacizumab for recurrent GBM between July 2005 and July 2006. These patients received bevacizumab at 5 mg/kg every 2 weeks. Most patients, 31 of 44, received concurrent irinotecan, 8 patients received carboplatin, 3 patients received lomustine, and 2 patients received etoposide. Twenty-seven of these patients also continued on with bevacizumab at progression, in combination with other chemotherapeutic agents.
For controls, we searched the database for all patients who were treated for GBM in the first recurrence between June 2001 and July 2005 and found 79 patients who met all inclusion criteria (see below). The control patients never received bevacizumab, even in later recurrences. We chose only the first recurrence for control patients to avoid any selection bias because we did not choose a specific treatment for comparison. A large portion of subjects received a chemotherapy drug at recurrence (2 irinotecan, 25 lomustine, 8 carboplatin, 1 carmustine), and the others were patients enrolled in various phase 2 clinical trials (1 isotretinoin, 5 AEE788, 5 temsirolimus, 5 cilengitide, 1 gefitinib, 16 erlotinib, 1 SU5416 [semaxanib], 4 sirolimus [rapamycin], 2 tamoxifen, 2 thalidomide, 1 tipifarnib).
All patients had tissue diagnosis of GBM and were treated with radiation therapy and concurrent temozolomide chemotherapy at the time of diagnosis. Patients must have received the recurrent treatment at least 8 weeks from the completion of radiation therapy and did not have a recent resection for tumor progression. All patients had close and regular follow-ups in our clinics, with a median time of 268 days for the treated cohort and 182 days for controls. All subjects had signed consent forms to participate in a database containing all clinical information, approved by the institutional review board at both institutions.
The primary objective of this study was to compare PFS and OS between bevacizumab-treated vs control groups. As a secondary objective, we compared changes in functional status and dexamethasone use to assess quality of life. Functional status was measured at each clinic visit using the Karnofsky Performance Status (KPS), and a significant change was defined as a decrease of ≥20 points if baseline KPS ≥80, or a decrease of ≥10 points if baseline KPS <80.
Statistical analysis.
The Student t test was used to compare differences for age and KPS at diagnosis between bevacizumab-treated and control patients. The Kaplan–Meier method was used to calculate survival functions, and differences were assessed with the weighted log-rank statistic.17 Univariate and multivariate survival analyses were performed using the Cox proportional hazards regression model. Survival tree analyses (in S-PLUS) were run to find out the cut points of age and KPS for subgroup analysis. All analyses except survival tree analysis were performed with SAS software (version 9.1.3, Service Pack 3).
Evaluation of VEGF expression.
Clinical data, including histopathology and age from diagnosis, were retrieved from 174 primary GBMs reported in studies for which .CEL files (Affymetrix, Santa Clara, CA) were publicly available as well as patients treated in the UCLA Brain Tumor Program.18-21 Tissues were collected from the initial diagnostic surgery, before any treatments. Patient age at the time of diagnosis was available for all 174 patients and ranged from 22 to 86 years. The tumor tissues were collected before the availability of bevacizumab; these patients did not have treatment with bevacizumab and do not overlap with patients in this study.
Combination of microarray data.
All .CEL files used for this study were retrieved from the Celsius microarray database, which contains more than 140,000 .CEL files on various Affymetrix array platforms (genomics.ctrl.ucla.edu/wiki/index. php/Celsius). Methods for the processing of samples are previously described.22 Briefly, using default parameters, RMA from the Bioconductor R library was used to quantify and normalize the samples relative to other microarrays of the same Affymetrix platform with a comparison group of 50 samples randomly selected from the database.23,24
Expression analysis.
Gene expression analysis performed for the 174 GBM samples was executed with DNA-Chip Analyzer (dChip; www.dchip.org) software build 11-18-07. Analysis settings were set at default unless otherwise specified. Comparisons between tumors from patients younger and older than 55 years were performed across the four available VEGFA probe sets using fold change thresholds of greater than 1.2, t test p value thresholds of 0.05 or less, and 100 sample name permutations for false discovery rate determination. Clustering parameters were set at default where row standardization was subtracted by “Mean” and divided by “Standard Deviation.” Distances were set for “Pre-calculated,” and a “1-Correlation” metric where “Centroid” was used as the linkage method.
VEGF gene voting.
The activation status of VEGFA for the two probe sets found to be significantly activated (210512_s_at and 212171_x_at) was analyzed for each tumor. Briefly, the mean value of each VEGFA probe set was evaluated from all samples within each of the two microarray platforms U133A and U133 Plus 2.0 separately. Subsequently, the probe sets from each sample were assigned “ON” or “OFF” status if each sample’s individual probe set’s value was above or below the aforementioned mean within its platform. Such a gene voting strategy was used to assess the relative activation of each probe set to allow for cross-platform comparability and Fisher exact statistical tests.
RESULTS
Patient characteristics.
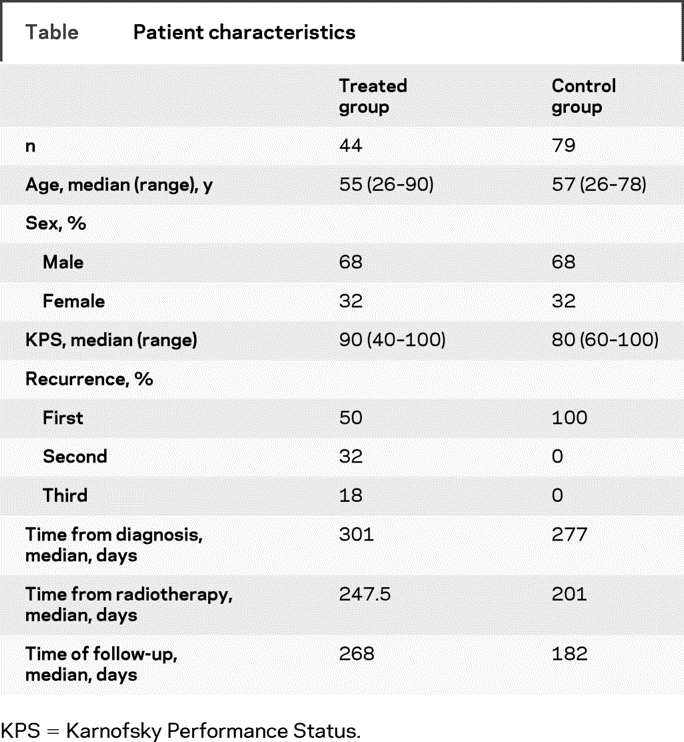
We identified 44 patients who were treated with bevacizumab for the treated group (table). The control group consisted of 79 patients. All subjects were treated at least 1 year before their records were analyzed. The median age was 55 (range 26–90) years for the treated group and 57 (range 26–78) years for the control group. The median KPS was slightly higher in the treated group, at 90 (range 40–100), in comparison with 80 (range 60–100) for the control group. There were no significant differences in subgroup analysis for age, KPS, or time from diagnosis. Half of the treated group received bevacizumab in the first recurrence, 32% in the second recurrence, and 18% in the third recurrence. Although we looked at outcomes in the control group only in the first recurrence, 68% of these patients did go on treatment for a second recurrence, and 38% received treatment for a third recurrence.
Table Patient characteristics
Clinical results.
There was a significant difference in median PFS for the treated group at 4.25 months, compared with 1.82 months in the control group (figure 1). The difference in median OS was also significant between the treated (9.01 months) and control (6.11 months) groups. The 6-month PFS was 41% for the treated group and 18% for the control group. Survival after failure was not significantly different between the two groups, at 4.32 months for the treated group and 4.4 months for the control group.

Figure 1 Kaplan–Meier survival curves for all patients
(A) Progression-free survival and (B) overall survival of bevacizumab-treated patients vs control group.
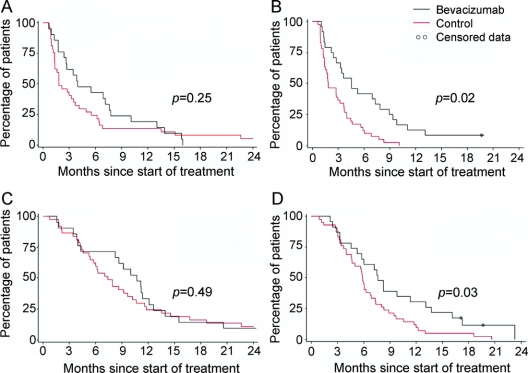
Interestingly, in subgroup analysis based on median age (age ≥55 or <55 years), there was only a significant difference in PFS and OS between the treated and control groups in patients aged ≥55 years (figure 2). When analyzed using 50 years as the age cutoff, significance was also found only in those aged ≥50 years (data not shown). Similarly, only those with poor performance status (KPS <80) had a significant improvement in PFS, but not OS, when treated with bevacizumab.
Figure 2 Kaplan–Meier survival curves by age
(A) Progression-free survival for patients aged <55 years, (B) progression-free survival for patients aged ≥55 years, (C) overall survival for patients aged <55 years, and (D) overall survival for patients aged ≥55 years.
Multivariate analysis of factors, including age, KPS, and treatment type, revealed that only treatment with bevacizumab significantly impacted PFS (hazard ratio [HR] = 0.624). For OS, age was the only significant factor (HR = 1.51). Table e-1 on the Neurology® Web site at www.neurology.org contains a summary of all survival data.
VEGF expression analysis.
Comparing the expression levels of VEGFA between GBMs derived from patients younger than 55 years (n = 84) and older than 55 years (n = 90), we observed that patients older than 55 years showed a 1.4-fold higher expression of VEGFA (probe sets 210512_s_at and 212171_x_at; p ≤ 0.01). Moreover, the expression of VEGFA was assessed as “ON” or “OFF” based on whether each sample’s two probe sets indicated values above or below the mean from all (n = 175) tumors. Twenty-six percent (22/84) of the tumors derived from patients younger than 55 years showed VEGFA activation, whereas 44% (40/90) of the tumors from patients older than 55 years showed likewise activation (figure 3). The Fisher exact test indicated that the higher levels of expression observed in the older patients vs the younger patients was significant.
Figure 3 Vascular endothelial growth factor (VEGF) expression by age via DNA microarray analysis
Quality-of-life analysis.
Of patients who were receiving dexamethasone at the start of treatment, 54% of patients in the bevacizumab-treated group were able to reduce their dexamethasone dosages during the course of treatment, with eight patients completely discontinuing dexamethasone (figure 4A). Only 33% of patients in the control group reduced their dexamethasone, and more than half increased their dexamethasone dose. Control patients had to increase dexamethasone in a shorter amount of time (130.5 median days) than the treated group (149 median days) (p = 0.04; figure 4B). Patients treated with bevacizumab also maintained their functional status longer than control patients (252 vs 120 median days; p = 0.006; figure 4C.
Figure 4 Quality-of-life analysis
(A) Percent change in dexamethasone from baseline in bevacizumab (Bev)–treated group (top) and control group (bottom) from the start of treatment to 1 month after failure. (B) Time to dexamethasone increase in control vs bevacizumab-treated group. (C) Time to decrease in Karnofsky Performance Status between control vs bevacizumab-treated group.
Subgroup analysis of patients by age also revealed that patients older than 55 years had more significant differences in days to dexamethasone change and KPS change between the treated and control groups. The treated group patients aged ≥55 years consistently had longer time before an increase in dexamethasone dose (182 median days vs 130 days for control group; p = 0.03) or a decrease in functional status (217 median days vs 85 days; p = 0.02) than the control group patients aged ≥55 years (figure e-1). We did not see any significant difference between the groups with age <55 years (data not shown).
DISCUSSION
From this retrospective study, we found that patients with recurrent GBM treated with bevacizumab may have better PFS and OS than a control group of patients not receiving bevacizumab. The increase in PFS and OS with bevacizumab treatment is greater in patients older than 55 years. Patients who received bevacizumab also had less dexamethasone use and longer stability in functional status.
The ability to reduce dexamethasone overtime should lead to a better quality of life for patients with GBM. This higher reduction in dexamethasone in patients treated with bevacizumab might also be a factor that led to the longer maintenance of functional status, as seen by our data. In our analysis, we looked at dexamethasone reduction during the treatment period as well as after treatment failure because many of our patients continued on with bevacizumab in later recurrences. However, we did not find a significant difference in survival after treatment failure. Similarly, one report did show that continuing on bevacizumab after initial failure does not prolong survival.25 However, this study did not look at dexamethasone changes or functional status after failure. The benefit of maintaining quality of life might outweigh the lack of survival benefit in this population.
The association of bevacizumab with better survival in the older age group is an unexpected outcome, because age tends to be a poor prognostic factor in patients with malignant glioma.2 In a general population of patients with GBM, we found that age correlates with higher expression of VEGF-A, suggesting that GBMs in older age groups have more angiogenesis. This finding is consistent with a previous report that patients with proliferative or angiogenic/mesenchymal molecular profiles tend to be older than patients with the neuronal subclass.20 Our data on quality of life also support this finding because older patients (aged ≥55 years) seem to have longer benefits from bevacizumab, stay at a lower dose of steroids longer, and remain functional longer than control patients. Thus, an antiangiogenic agent might have more activity for the older age group than in patients of younger age or neuronal molecular profile.
This study is limited by its retrospective nature and small number of patients. We cannot compare each treated patient with a perfectly matched control. However, the cohorts are similar in their clinical characteristics. Because we only looked at survival at the first recurrence in the control group, survival data should be weighted in favor of this group instead of the heavily pretreated bevacizumab group. Also, a retrospective study does not allow us to collect and perform direct molecular studies on patient tissues, and the molecular difference in VEGF-A in this report had to be inferred from a more general population. There are not enough available tissues from the patients analyzed in this study.
Our VEGF-A data were also gleaned from newly diagnosed tissues, but the patients in this study were not treated until recurrence. One study indicated that tissues might shift from proliferative to mesenchymal pattern at recurrence,20 so tumor tissues taken at recurrence might have even higher VEGF-A expression. It is also unknown whether our data might have been less robust if these patients were treated in the up-front setting, where presumedly there would be fewer tumors with mesenchymal features. Future prospective trials of bevacizumab or other antiangiogenic agents should include molecular studies of different age groups and more extensive gene expression studies of other factors within the angiogenic pathways as well as study in both recurrent and up-front settings. More importantly, elderly patients, such as patients older than 70 years, should be studied with bevacizumab. These patients are often excluded from clinical trials.1,26,27 However, our data might indicate that these patients could receive significant benefit from bevacizumab. Our study does not have enough patients in this age group to analyze the effect of bevacizumab in this subgroup alone, and future prospective studies should be conducted for this age group.
AUTHOR CONTRIBUTIONS
Statisticians: W. Liu and R. Elashoff.
MEDICATIONS
Bevacizumab (Avastin®; Genentech, South San Francisco, CA); irinotecan (Camptosar®; Pfizer, New York, NY); carboplatin (Paraplatin®; Parenta, West Columbia, SC); lomustine (CCNU, CeeNU®; Bristol-Myers Squibb, Princeton, NJ); etoposide (VP-16, Vepesid®; Bristol-Myers Squibb, Princeton, NJ); carmustine (BCNU, BiCNU®; Ben Venue, Bedford, OH); isotretinoin (Accutane®; Roche, Nutley, NJ); AEE-788 (Novartis Pharma, Basel, Switzerland); temsirolimus (CCI-779, Torisel®; Wyeth, Philadelphia, PA); cilengitide (EMD 121974; Merck KGaA, Darmstadt, Germany); gefitinib (Iressa®; AstraZeneca, Wilmington, DE); erlotinib (Tarceva®; Genentech, South San Francisco, CA); SU5416 (semaxanib; no longer being manufactured); sirolimus (rapamycin, Rapamune®; Wyeth, Philadelphia, PA); tamoxifen (Andrx Pharmaceuticals, Ft. Lauderdale, FL); thalidomide (Thalomid®; Celgene, Summit, NJ); tipifarnib (R11577, Zarnestra™; J&JPRD, Raritan, NJ).
Supplementary Material
Address correspondence and reprint requests to Dr. Phioanh (Leia) Nghiemphu, UCLA Neuro-Oncology, 710 Westwood Plaza, RNRC 1-230, Los Angeles, CA 90095
Leian@ucla.edu
Supplemental data at www.neurology.org
This work received grant support from Brain Tumor Funders’ Collaborative and was also supported by the Harry Allgauer Foundation through The Doris R. Ullmann Fund for Brain Tumor Research Technologies, the Henry E. Singleton Brain Tumor Foundation, a generous donation from the Ziering Family Foundation in memory of Sigi Ziering, Art of the Brain, and the Roven Family Fund in memory of Dawn Steel.
Disclosure: T.F.C. receives research grant funding from Genentech and has received an honorarium from Genentech.
Medications: Medications used in this study are listed at the end of the article.
Received August 12, 2008. Accepted in final form December 16, 2008.
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 2.Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the New Approaches to Brain Tumor Therapy CNS Consortium phase I and II clinical trials. J Clin Oncol 2007;25:2601–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamborn KR, Yung WKA, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neurooncology 2008;10:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong E, Hess K, Gleason M, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 1999;17:2572. [DOI] [PubMed] [Google Scholar]
- 5.Carlson MRJ, Pope WB, Horvath S, et al. Relationship between survival and edema in malignant gliomas: role of vascular endothelial growth factor and neuronal pentraxin 2. Clin Cancer Res 2007;13:2592–2598. [DOI] [PubMed] [Google Scholar]
- 6.Lamszus K, Ulbricht U, Matschke J, Brockmann MA, Fillbrandt R, Westphal M. Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin Cancer Res 2003;9:1399–1405. [PubMed] [Google Scholar]
- 7.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 1992;359:845–848. [DOI] [PubMed] [Google Scholar]
- 8.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients cancer. Cell 2007;11:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 2008;70:779–787. [DOI] [PubMed] [Google Scholar]
- 10.Cloughesy T, Prados M, Mikkelsen T, et al A phase II randomised non-comparative clinical trial of the effect of bevacizumab (BV) alone or in combination with irinotecan (CPT) on 6-month progression free survival (PFS6) in recurrent treatment-refractory glioblastoma (GBM). In: 2008 ASCO Annual Meeting. Chicago. J Clin Oncol 2008; May 20 suppl. Abstract 2010b.
- 11.Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology 2006;66:1258–1260. [DOI] [PubMed] [Google Scholar]
- 12.Stark-Vance V. Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma. In: Proceedings of the World Federation of Neuro-Oncology Meeting. Edinburgh, UK: Duke University Press; 2005:91.
- 13.Vredenburgh JJ, Desjardins A, Herndon JE II, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 2007;13:1253–1259. [DOI] [PubMed] [Google Scholar]
- 14.Vredenburgh JJ, Desjardins A, Herndon JE II, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007;25:4722–4729. [DOI] [PubMed] [Google Scholar]
- 15.Batchelor TT, Byrne TN. Supportive care of brain tumor patients. Hematol Oncol Clin North Am 2006;20:1337–1361. [DOI] [PubMed] [Google Scholar]
- 16.Vecht C, Hovestadt A, Verbiest H, van Vliet J, van Putten W. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: a randomized study of doses of 4, 8, and 16 mg per day. Neurology 1994;44:675–680. [DOI] [PubMed] [Google Scholar]
- 17.Fleming TR, Harrington DP. Counting Processes and Survival Analysis. New York: John Wiley; 1991. [Google Scholar]
- 18.Freije WA, Castro-Vargas FE, Fang Z, et al. Gene expression profiling of gliomas strongly predicts survival. Cancer Res 2004;64:6503–6510. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Scheck AC, Cloughesy T, et al. Gene expression analysis of glioblastomas identifies the major molecular basis for the prognostic benefit of younger age. BMC Med Genomics 2008;1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006;9:157–173. [DOI] [PubMed] [Google Scholar]
- 21.Rich JN, Hans C, Jones B, et al. Gene expression profiling and genetic markers in glioblastoma survival. Cancer Res 2005;65:4051–4058. [DOI] [PubMed] [Google Scholar]
- 22.Day A, Carlson M, Dong J, O’Connor B, Nelson S. Celsius: a community resource for Affymetrix microarray data. 2007;8:R112. [DOI] [PMC free article] [PubMed]
- 23.Bolstad B, Irizarry R, Astrand M, Speed T. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003;19:185–193. [DOI] [PubMed] [Google Scholar]
- 24.Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 2004;5:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quant E, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on a bevacizumab-containing regimen. In: 2008 ASCO Annual Meeting. Chicago. J Clin Oncol 2008; May 20 suppl. Abstract.
- 26.Brandes AA, Rigon A, Monfardini S. Radiotherapy of the brain in elderly patients: contra. Eur J Cancer 2000;36:447–451. [DOI] [PubMed] [Google Scholar]
- 27.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061–2067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.