Abstract
Objective:
The Women's Health Initiative Memory Study (WHIMS) hormone therapy (HT) trials reported that conjugated equine estrogen (CEE) with or without medroxyprogesterone acetate (MPA) increases risk for all-cause dementia and global cognitive decline. WHIMS MRI measured subclinical cerebrovascular disease as a possible mechanism to explain cognitive decline reported in WHIMS.
Methods:
We contacted 2,345 women at 14 WHIMS sites; scans were completed on 1,424 (61%) and 1,403 were accepted for analysis. The primary outcome measure was total ischemic lesion volume on brain MRI. Mean duration of on-trial HT or placebo was 4 (CEE+MPA) or 5.6 years (CEE-Alone) and scans were conducted an average of 3 (CEE+MPA) or 1.4 years (CEE-Alone) post-trial termination. Cross-sectional analysis of MRI lesions was conducted; general linear models were fitted to assess treatment group differences using analysis of covariance. A (two-tailed) critical value of α = 0.05 was used.
Results:
In women evenly matched within trials at baseline, increased lesion volumes were significantly related to age, smoking, history of cardiovascular disease, hypertension, lower post-trial global cognition scores, and increased incident cases of on- or post-trial mild cognitive impairment or probable dementia. Mean ischemic lesion volumes were slightly larger for the CEE+MPA group vs placebo, except for the basal ganglia, but the differences were not significant. Women assigned to CEE-Alone had similar mean ischemic lesion volumes compared to placebo.
Conclusions:
Conjugated equine estrogen–based hormone therapy was not associated with a significant increase in ischemic brain lesion volume relative to placebo. This finding was consistent within each trial and in pooled analyses across trials.
GLOSSARY
- 3MSE
= modified Mini-Mental State Examination;
- BMI
= body mass index;
- CEE
= conjugated equine estrogen;
- CVD
= cerebrovascular disease;
- HT
= hormone therapy;
- MCI
= mild cognitive impairment;
- MPA
= medroxyprogesterone acetate;
- MRIQCC
= MRI Quality Control Center;
- ROI
= region of interest;
- WHIMS
= Women's Health Initiative Memory Study.
Dementia is increasing due to an aging population.1 Clinical stroke is often considered the first sign of cerebrovascular disease (CVD), but silent stroke and white matter lesions are more prevalent and commonly predate clinical CVD.2 Large cohort studies of older adults indicate that clinical and subclinical CVD increase the risk of cognitive decline3,4 and dementia.5
The Women's Health Initiative Memory Study (WHIMS) reported that hormone therapy (HT)—conjugated equine estrogen 0.625 mg/d (CEE) with or without medroxyprogesterone 2.5 mg/d (MPA)—increased the risk for dementia6,7 and global cognitive decline in women age 65 and older.8,9 The WHI reported that both CEE + MPA and CEE-Alone were associated with an increased risk of clinical stroke.10–12 These results question whether HT increased the prevalence of subclinical CVD as a possible mechanism for cognitive decline in WHIMS. The main objective of the WHIMS Magnetic Resonance Imaging Study (WHIMS-MRI) was to determine whether HT compared to placebo, randomly assigned at enrollment to the WHI hormone trials, was associated with volumetric subclinical CVD on post-trial brain MRI.
The primary outcome measure of WHIMS MRI was total ischemic lesion volume on brain MRI. Lesion volumes in the basal ganglia and in the white and gray matter outside the basal ganglia were secondary outcomes. We hypothesized that women randomized to CEE-based HT would have significantly increased ischemic lesion volumes on brain MRI. A companion article by Resnick at al.13 reports whether regional and total brain volumes differed by WHI treatment assignment.
METHODS
The ClinicalTrials.gov Identifier for this study is NCT00000611.
Design of WHI and WHIMS.
The WHI randomized HT trials were designed to evaluate postmenopausal HT and prevention of disease, with coronary heart disease the primary outcome. Hip fracture, other fractures, other cardiovascular disease, and endometrial, colorectal, and other cancers were secondary outcomes.14 A geographically diverse group of postmenopausal women aged 50–79 were enrolled in the parallel CEE+MPA (n = 16,608) or CEE-Alone (n = 10,739 women with prior hysterectomy) randomized placebo-controlled trials.15
Ancillary to the WHI, WHIMS followed women from these two trials to examine the effects of postmenopausal CEE-based HT on the risk of all-cause dementia and global cognitive functioning in healthy women who were 65–79 years old at WHIMS enrollment. Of age-eligible participants who were approached, 4,532 (92.6%) consented to participate in the WHIMS CEE+MPA trial and 2,947 (92.1%) consented to participate in the WHIMS CEE-Alone Trial. The WHIMS study designs, eligibility criteria, and recruitment procedures were described previously.16 WHIMS participants underwent cognitive screening with the modified Mini-Mental State Examination (3MSE)17 at enrollment and annually. Women who scored below an education-adjusted cutpoint on the 3MSE underwent a more comprehensive cognitive evaluation,18 assessment of mood, and a neuropsychiatric examination. If probable dementia was suspected, the participant underwent laboratory tests and non-contrast x-ray computerized tomography of the brain. Final classifications (normal, mild cognitive impairment [MCI], or probable dementia) were adjudicated at the WHIMS Clinical Coordinating Center at the Wake Forest University Health Sciences.
The WHI hormone trials were terminated earlier than planned due to significantly more non-cognitive adverse events associated with HT compared to placebo.10,11 As a consequence, the ancillary WHIMS CEE+MPA and CEE-Alone trials were also prematurely terminated.6–9 Post-trial follow-up of participants continued annually for cognitive assessment and adjudication of MCI and probable dementia in the WHIMS Extension Study.
Design of WHIMS-MRI.
WHIMS-MRI was designed to compare neuroradiologic outcomes among women with an average on trial HT exposure of 4.0 years (CEE+MPA) or 5.6 years (CEE-Alone). Brain scanning was conducted, on average, 8.02 years (CEE+MPA) or 7.97 (CEE-Alone) years following randomization and 3.0 years (CEE+MPA) or 1.4 years (CEE-Alone) after termination of the WHIMS HT trials.
WHIMS-MRI was conducted in 14 of the 39 WHIMS clinical sites. Following Institutional Review Board approval, WHIMS-MRI recruitment began in January 2005 and was completed in April 2006. Women who provided consent underwent screening to determine if they were acceptable candidates for MRI. Exclusion criteria included the presence of items that would make the MRI procedure hazardous (pacemakers, prohibited medical implants, and foreign bodies); shortness of breath or inability to lie flat; and conditions that can be exacerbated by stress (anxiety panic disorders, claustrophobia) severe enough to preclude MRI.
WHIMS participants who enrolled in WHIMS-MRI tended to be younger and more highly educated than those who did not; they also had been relatively healthier at the time they enrolled in the WHI and tended to have experienced less on-trial decline in global cognition.19
MRI protocol.
The MRI scanning protocol was developed by investigators at the MRI Quality Control Center (MRIQCC) in the Department of Radiology, University of Pennsylvania. Scanning and reading were done by individuals masked to treatment assignment. The scans were conducted by a standardized protocol; scanning pulse sequences were performed in the following order:
Series one: three-plane gradient echo localizer for positioning.
Series two: sagittal T1-weighted spin echo midslice image to demonstrate anatomic location of the AC/PC for slice angle and slice position.
Series three: oblique axial spin density/T2-weighted spin echo images from the vertex to skull base parallel to the AC/PC plane.
Series four: oblique axial FLAIR T2-weighted spin echo images matching slice positions in series three.
Series five: oblique axial three-dimensional T1-weighted gradient echo images from the vertex to the skull base parallel to the AC/PC plane. The field of view was 22 cm and the acquisition matrix was 256 × 256 for series three, four, and five. Scans obtained in series three, four, and five were used for analyses of ischemic lesions.
The WHIMS-MRI technologist at each site immediately reviewed all scans for protocol compliance and technical problems. Imaging data were transmitted by an encrypted DICOM image transfer mechanism to the permanent study archive via the Web.
WHIMS-MRI primary outcome measure.
The primary outcome measure for WHIMS-MRI is total ischemic lesion volume, measured in cubic centimeters, detected from a standardized imaging and reading protocol. Secondarily, ischemic lesion volumes in the basal ganglia and the cerebral white and gray matter outside the basal ganglia were measured.20
Ischemic lesion volume defined and identified by this methodology generally corresponds to what has been called small vessel ischemic disease (ischemic white matter disease and lacunar infarctions). Basically unknown before the advent of MRI, this process is now accepted as a non-necrotic, ischemic effect on myelin that is secondary to the effects of aging, hypertension, and other small vessel pathologic processes of the brain.21,22 The earliest reports of this vasculopathy were by anecdotal observations which were quickly superseded by semiquantitative, human observer scoring systems such as those used in the Cardiovascular Health Study23 and the Rotterdam Study.24 While these systems are strongly correlated with each other in terms of rank order, their scores are not directly comparable, and these manual systems have limited reproducibility and restricted dynamic ranges.23,25 The methodology for detecting and quantifying ischemic tissue used in this report reflects the evolution in image processing from manual human observer to automatic, quantitative computerized digital image analytical techniques that are not only correlated with human observers and the semiquantitative scoring systems, but are very reproducible and offer a greater dynamic range.26,27
Our methodology classifies all brain tissue into either normal or ischemic gray or white matter and assigns the tissue type to each of 92 anatomic regions of interest (ROIs) of the cerebrum. These are organized in an anatomically hierarchal system that is collapsed into three ROIs for this analysis: the separate volumes of cerebral gray and white matter and basal ganglia (gray and white matter). Abnormal matter from these three ROIs form the basis for our secondary outcomes; the sum of the three is the primary outcome.
Statistical analysis.
Patricia E. Hogan, MS, Department of Biostatistical Sciences, Division of Public Health Sciences, Wake Forest University School of Medicine, was the statistician.
Characteristics at the time of randomization to the WHI HT trials and cognitive measures during follow-up were compared between women assigned to HT vs placebo using χ2 tests. Because the distributions of the ischemic lesion volumes were highly skewed, a logarithmic transformation was employed and back-transformed (geometric) means are presented.
Analysis of covariance was used to assess the associations of outcomes with cognitive risk factors and other participant characteristics after adjustment for clinical site, age at randomization, time from randomization to MRI, intracranial volume, trial, and treatment assignment (active vs placebo). Analysis of covariance was also used to contrast means of our outcome measures between treatment groups, with adjustments for clinical site, age at randomization, time from randomization to MRI scan, intracranial volume, and trial. Additional models also involved adjustment for other baseline factors associated with ischemic brain lesion volumes and factors affecting participation in WHIMS-MRI (education, smoking, body mass index [BMI], prior cardiovascular disease, hypertension, and diabetes). We also examined subgroups of women defined by baseline 3MSE scores and compliance to study medications for evidence of differential treatment effects.
Women were analyzed according to WHI randomization assignment. Treatment arm differences are presented within trial and pooled across trials. Because WHIMS-MRI was designed to examine potential mechanisms underlying the adverse findings of the WHIMS trials, Type I error was not controlled for multiple comparisons: individual inferences were conducted according to two-sided significance levels of 0.05. Differential effects of treatment were examined for subgroups defined by baseline factors including age, BMI, smoking, prior cardiovascular disease, diabetes, and hypertension; however, none reached significance.
RESULTS
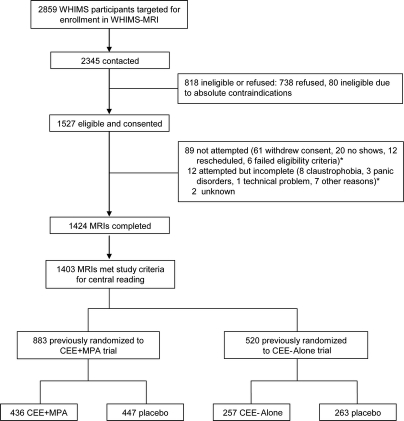
Of the 2,859 active WHIMS participants in the 14 WHIMS-MRI sites at the beginning of the study, 2,345 (82.0%) were contacted about enrollment in WHIMS-MRI, and 1,527 (65.1%) consented. MRI scans were completed on 1,424 participants (61%) and 1,403 met study criteria for central reading. Of 1,403 participants, 883 had previously been enrolled in the CEE+MPA trial (436 active and 447 placebo) and 520 in the CEE-Alone trial (257 active and 263 placebo) (figure).
Figure Enrollment and flow of participants through Women's Health Initiative Memory Study–MRI
*Multiple reasons were given, so totals are not additive.
There were no within or across trial treatment assignment differences in WHIMS-MRI participants at WHI baseline by age, years since menopause, education, ethnicity, smoking status, or alcohol consumption (table 1). At the time of MR scanning, the age range across both trials was 71 to 88 years and the mean age was 78.5 years.
Table 1 WHI baseline demographic, socioeconomic status, and lifestyle characteristics of WHIMS MRI women by treatment assignment: Frequency (%)
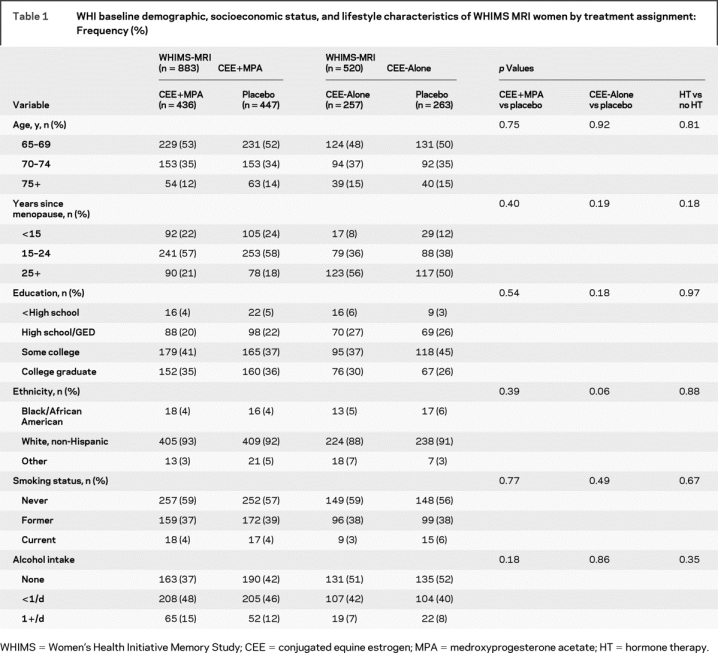
There were no differences between the active and placebo groups within or across the two trials by BMI, hypertension, prior cardiovascular disease (stroke, myocardial infarction, and/or history of angina, angioplasty, or coronary artery bypass graft surgery), diabetes, prior use of hormones, or baseline 3MS scores (table 2). In participants who showed MCI or probable dementia during or after WHIMS, there were increased but not significantly more cases of probable dementia in the treatment arm vs placebo of the CEE+MPA trial, and these findings parallel results previously reported for the overall WHIMS trial.6
Table 2 WHI baseline clinical characteristics of WHIMS-MRI women by treatment assignment: Frequency (%)
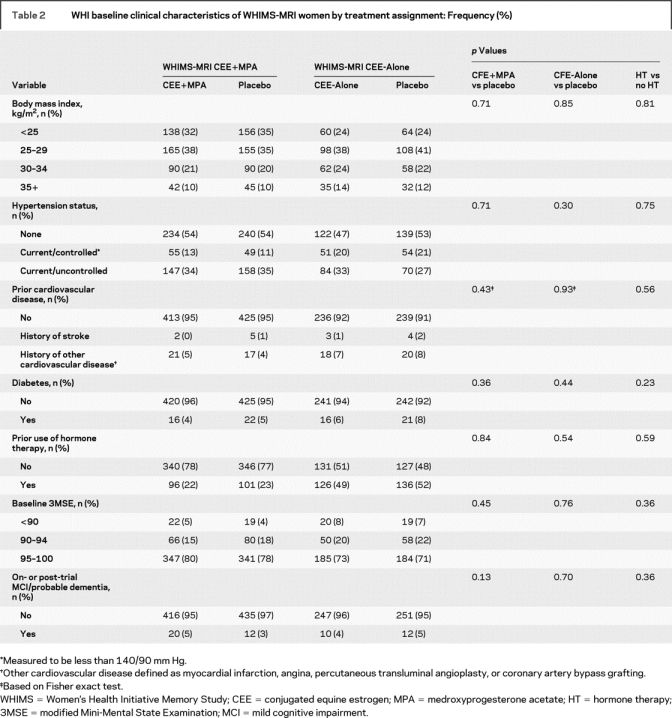
Age and hypertension were the strongest predictors of increasing geometric mean ischemic lesion volumes across all three measures (p < 0.0001) (table 3). Smoking was significantly associated while prior cardiovascular disease was of borderline significance across the three measures. Neither history of high cholesterol nor diabetes had a significant association with brain lesions, possibly due to small numbers of cases. Increased BMI was significantly associated with smaller ischemic lesion volumes except for those in the basal ganglia.
Table 3 Geometric mean (SE) ischemic brain lesion volumes (cubic centimeters) by demographics, socioeconomic status, lifestyle, and clinical characteristics
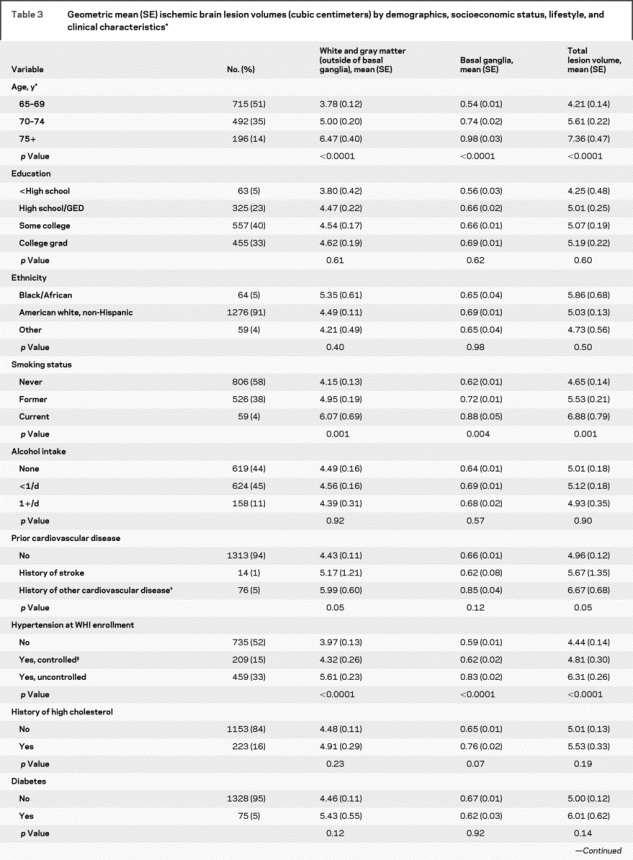
Table 3 Continued
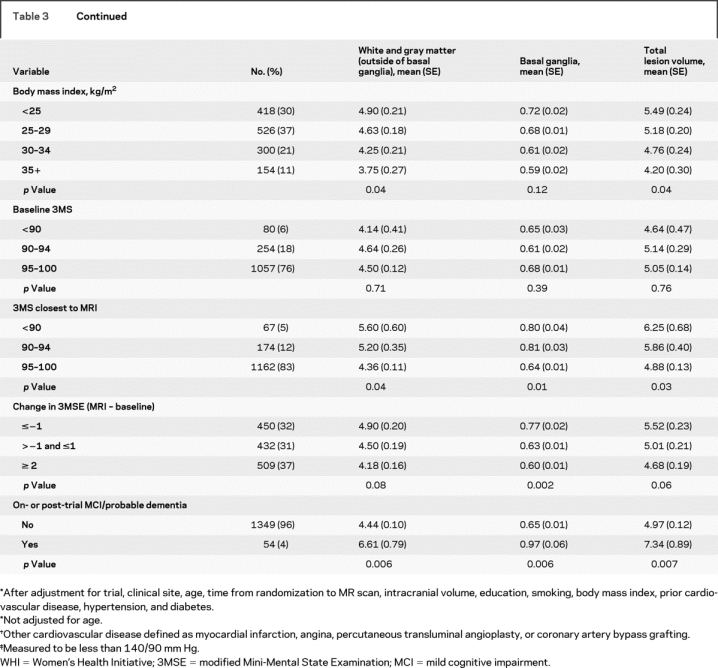
There was no significant association between global cognition (3MSE scores) at WHI baseline and cerebral ischemic lesion volumes on MRI. However, women who scored highest on the 3MSE obtained nearest to the MRI scan date had the smallest mean ischemic lesion volumes across all measures (p < 0.05). We also examined changes in 3MSE scores over time, from WHI baseline to the WHIMS cognitive testing session nearest the MRI scan. Women whose test scores improved by at least 2 points had the smallest lesions while women with worsening scores were more likely to have the largest lesions. This pattern was significant within the basal ganglia (p < 0.01). Women who developed on or post-trial MCI or probable dementia had larger ischemic lesion volumes, which were significant across all measures.
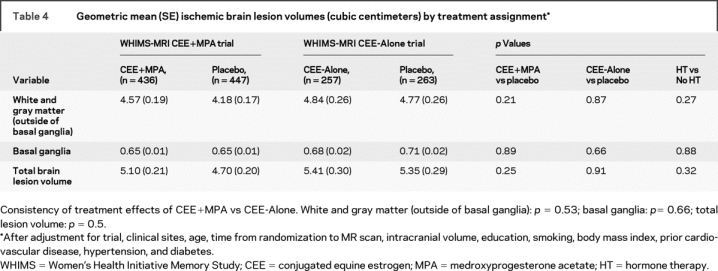
Table 4 shows there were no significant differences between geometric mean lesion volumes by treatment arm within or across trials, after initial adjustment for clinical site, age at randomization, time from randomization to MRI scan, and intracranial volume. After controlling for education, smoking, BMI, prior cardiovascular disease, hypertension, and diabetes (plus the previously mentioned variables), the results were very similar. Mean ischemic volumes were slightly but not significantly larger in the CEE+MPA group compared to placebo, except in the basal ganglia. We also examined subgroups of women defined separately by baseline 3MSE scores and compliance to study medication; no differential treatment effects were detected.
Table 4 Geometric mean (SE) ischemic brain lesion volumes (cubic centimeters) by treatment assignment
DISCUSSION
In this analysis of older women enrolled in the WHIMS HT trials, who were evenly matched within trials on demographic and clinical characteristics, we found no marked differences within or across trials by treatment assignment (CEE+MPA or CEE-Alone vs placebo) on total ischemic lesion volume—the primary outcome of the WHIMS-MRI study. Further, we found no differences in ischemic lesion volumes in the basal ganglia or in the white and gray matter outside the basal ganglia.
We hypothesized that women assigned to HT would have significantly larger ischemic lesion volumes, providing a mechanism to explain the earlier reports from WHIMS that HT increased the risk of all-cause dementia6,7 and global cognitive decline in women age 65 years and older,8,9 and the association of HT and clinical stroke reported by the WHI.10–12 Instead, we found no evidence that HT exposure increased ischemic lesion volumes.
Increased lesion volumes on MRI demonstrated internal validity; they were associated with several vascular risk factors including increased age, smoking, history of cardiovascular disease, and hypertension at WHI baseline. Increased lesion volumes were also associated with development of on- or post-trial MCI and probable dementia in WHIMS and lower 3MSE scores at time of MRI scanning. History of stroke and diabetes were not associated with ischemic brain lesions in WHIMS-MRI due to few participants with these conditions. We found a protective association between increasing BMI and ischemic lesion volumes. Although recent MRI findings demonstrate the association of obesity with generalized and regional brain atrophy,28 and increased white matter volumes,29 the underlying mechanisms (diabetes with associated elevated insulin levels and metabolic syndrome) are not prevalent in the WHIMS-MRI cohort. Although the literature records a link between midlife obesity and risk for future dementia,30 as age increases, overweight and moderate obesity may have a neutral31 or protective effect on cognition in women,32,33 but not in men.34 The association of BMI (low and high) with brain MRI findings and the underlying mechanisms require further study.
Compared to the general age-matched US population, WHIMS-MRI participants have lower prevalence rates of risk factors for cardiovascular disease and stroke, including diabetes, hypercholesterolemia, or uncontrolled hypertension35,36 and history of cigarette smoking.37 Thus, HT may not have the same adverse effects in individuals with few or no vascular risk factors compared to those at high risk.38
Post hoc calculations indicate that WHIMS-MRI provided 80% statistical power (based on a two-sided test with α = 0.05) to detect a mean difference of 19% in the primary outcome, total ischemic lesion volume, between the active and placebo arms. The mean we observed was a nonsignificant difference of only 5.7%. To put this in perspective, we examined the cross-sectional age increases in total lesion volume observed in the placebo group. The geometric means were 3.93 cm3 and 6.03 cm3 for the 70–74 and 80+ age groups, respectively, after adjusting for trial differences. This corresponds to a 53% change over 10 years or approximately 5.3% per year. Therefore, the mean treatment difference detected (5.7%) was equivalent to about a 1-year age increase, whereas we were powered to detect an age increase of approximately 3.5 years. Additional analyses of sequences of pre-scan cognitive data provided reassurance that our null findings could not be attributed to differential enrollment between women who had been assigned to HT vs placebo. We repeated our principal analyses using propensity scores adjustment to account for potential differential enrollment,39 using all factors identified as having independent associations with enrollment21 which resulted in essentially identical results.
Our sample may have under-represented the participants most at risk for hormone-related ischemic brain lesions and cognitive decline. In addition, our sample size may be too small to measure the small differences in blood pressure by treatment assignment in the WHIMS trial.
The cross-sectional analysis in WHIMS-MRI limits the ability to measure the frequency of new ischemic brain lesions associated with prior assignment to HT compared to placebo. The difference in the frequency of new lesions may be masked by the much higher underlying prevalence of lesions at trial entry. This suggests the need for repeat scanning with longitudinal analyses of ischemic lesions.
Furthermore, the detrimental cognitive effects of HT reported in WHIMS may be primarily related not to vascular disease but to neurodegeneration, which would not have been detected by MRI data analysis limited to ischemic lesion volume measurements. HT may promote glial or neuronal damage, for instance, by triggering the accumulation of amyloid or other toxic compounds causing primarily cell death and brain atrophy. Perhaps older women have fewer estrogen receptors in the brain, and thus the vasodilatory effect of estrogen seen in younger women may be lost. This could cause a decrease in cerebral blood flow, which, according to the neurovascular hypothesis of Alzheimer disease, could cause long-term accumulation of amyloid and glial/neuronal loss.40 A companion article by Resnick et al.13 reports the association of HT and brain volumes by MRI.
APPENDIX
WHIMS-MRI Clinical Centers. Albert Einstein College of Medicine, Bronx, NY: Sylvia Wassertheil-Smoller, Mimi Goodwin, Richard DeNise, Michael Lipton, James Hannigan; Medical College of Wisconsin, Milwaukee: Jane Morley Kotchen, Diana Kerwin, John Ulmer, Steve Censky; Stanford Center for Research in Disease Prevention, Stanford University, CA: Marcia L. Stefanick, Sue Swope, Anne Marie Sawyer-Glover; The Ohio State University, Columbus: Rebecca Jackson, Rose Hallarn, Bonnie Kennedy; University of California at Davis, Sacramento: John Robbins, Sophia Zaragoza, Cameron Carter, John Ryan; University of California at Los Angeles: Lauren Nathan, Barbara Voigt, Pablo Villablanca, Glen Nyborg; University of Florida, Gainesville/Jacksonville: Marian Limacher, Sheila Anderson, Mary Ellen Toombs, Jeffrey Bennett, Kevin Jones, Sandy Brum, Shane Chatfield; University of Iowa, Davenport: Jennifer Robinson, Candy Wilson, Kevin Koch, Suzette Hart; University of Massachusetts, Worcester: Judith Ockene, Linda Churchill, Douglas Fellows, Anthony Serio; University of Minnesota, Minneapolis: Karen Margolis, Cindy Bjerk, Chip Truwitt, Margaret Peitso; University of Nevada, Reno: Robert Brunner, Ross Golding, Leslie Pansky; University of North Carolina, Chapel Hill: Carol Murphy, Maggie Morgan, Mauricio Castillo, Thomas Beckman; University of Pittsburgh, PA: Lewis Kuller, Pat McHugh, Carolyn Meltzer, Denise Davis.
WHIMS-MRI Clinical Coordinating Center. Wake Forest University Health Sciences, Winston-Salem, NC: Sally Shumaker, Mark Espeland, Laura Coker, Jeff Williamson, Debbie Felton, LeeAnn Andrews, Steve Rapp, Claudine Legault, Maggie Dailey, Julia Robertson, Patricia Hogan, Sarah Jaramillo, Pam Nance, Cheryl Summerville, Josh Tan.
WHIMS-MRI Quality Control Center. University of Pennsylvania, Philadelphia: Nick Bryan, Christos Davatzikos, Lisa Desiderio.
WHIMS-MRI Working Group. Wake Forest University Health Sciences, Winston-Salem, NC: LeeAnn Andrews; University of Pennsylvania, Philadelphia: Nick Bryan; Wake Forest University Health Sciences, Winston-Salem, NC: Laura Coker; Wake Forest University Health Sciences, Winston-Salem, NC: Mark Espeland; Wake Forest University Health Sciences, Winston-Salem, NC: Debbie Felton; University of Pittsburgh, PA: Lew Kuller; University of Minnesota, Minneapolis: Karen Margolis; University of Minnesota, Minneapolis: Anne Murray; National Institute on Aging, Baltimore, MD: Susan Resnick; Wake Forest University Health Sciences, Winston-Salem, NC: Sally Shumaker; Wake Forest University Health Sciences, Winston-Salem, NC: Jeff Williamson.
US NIH. National Institute on Aging, Bethesda, MD: Neil Buckholtz, Susan Molchan, Susan Resnick; National Heart, Lung, and Blood Institute, Bethesda, MD, Jacques Rossouw, Linda Pottern.
Received June 13, 2008. Accepted in final form August 28, 2008.
Address correspondence and reprint requests to Dr. Laura H. Coker, Division of Public Health Sciences, Wake Forest University Health Sciences, Medical Center Blvd., Winston-Salem, NC 27157 lcoker@wfubmc.edu
See page 135
*See the appendix for details about the WHIMS centers.
Authors' affiliations are listed at the end of the article.
The Women's Health Initiative is funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health, US Department of Health and Human Services. The Women's Health Initiative Memory Study was funded in part by Wyeth Pharmaceuticals, Inc., St. Davids, PA.
Disclosure: The authors report no disclosures.
REFERENCES
- 1.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health 1998;88:1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryan RN, Cai J, Burke G, et al. Prevalence and anatomic characteristics of infarct-like lesions on MR images of middle-aged adults: The Atherosclerosis Risk in Communities Study. Am J Neuroradiol 1999;20:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 3.Mosley TH Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: The Atherosclerosis Risk in Communities Study. Neurology 2005;64:2056–2062. [DOI] [PubMed] [Google Scholar]
- 4.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005;128:2034–2041. [DOI] [PubMed] [Google Scholar]
- 5.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology 2003;22:13–22. [DOI] [PubMed] [Google Scholar]
- 6.Shumaker SA, Legault C, Rapp SR, et al. The effects of estrogen plus progestin on the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2651–2662. [DOI] [PubMed] [Google Scholar]
- 7.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 2004;291:2947–2958. [DOI] [PubMed] [Google Scholar]
- 8.Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: The Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2663–2672. [DOI] [PubMed] [Google Scholar]
- 9.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA 2004;291:2959–2968. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 11.Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. [DOI] [PubMed] [Google Scholar]
- 12.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women. JAMA 2003;289:2673–2684. [DOI] [PubMed] [Google Scholar]
- 13.Resnick SM, Espeland MA, Jaramillo SA, et al., for the Women's Health Initiative Memory Study. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology 2009;72:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol 2003;13:S5–17. [DOI] [PubMed] [Google Scholar]
- 15.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group Controlled Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 16.Shumaker SA, Reboussin BA, Espeland MA. The Women's Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Controlled Clin Trials 1998;19:604–621. [DOI] [PubMed] [Google Scholar]
- 17.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–318. [PubMed] [Google Scholar]
- 18.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989;39:1159–1165. [DOI] [PubMed] [Google Scholar]
- 19.Jaramillo SA, Felton D, Andrews L, et al. Enrollment in a brain magnetic resonance study: Results from the Women's Health Initiative Memory Study Magnetic Resonance Imaging Study (WHIMS-MRI). Acad Radiol 2007;14:603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lao Z, Shen D, Liu D, Jawad AF, Melhem ER, Launer LJ, Bryan RN, Davatzikos C. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol 2008;15:300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: A review. Stroke 1997;28:652–659. [DOI] [PubMed] [Google Scholar]
- 22.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. Am J Neuroradiol 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 23.Bryan RN, Manolio TA, Schertz LD, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain: The Cardiovascular Health Study. Am J Neuroradiol 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 24.de Groot JC, de Leeuw FE, Oudkerk M, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 2002;52:335–341. [DOI] [PubMed] [Google Scholar]
- 25.Mantyla R, Erkinjuntti T, Salonen O, et al. Variable agreement between visual rating scales for white matter hyperintensities on MRI. Comparison of 13 rating scales in a poststroke cohort. Stroke 1997;28:1614–1623. [DOI] [PubMed] [Google Scholar]
- 26.Anbeek P, Vincken KL, van Osch MJ, Bisschops RH, van der Grond J. Automatic segmentation of different-sized white matter lesions by voxel probability estimation. Med Image Anal 2004;8:205–215. [DOI] [PubMed] [Google Scholar]
- 27.Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology 2006;67:2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jagust W. What can imaging reveal about obesity and the brain? Curr Alzheimer Res 2007;4:135–139. [DOI] [PubMed] [Google Scholar]
- 29.Haltia LT, Viljanen A, Parkkola R, et al. Brain white matter expansion in human obesity and the recovering effect of dieting. J Clin Endocrinol Metab 2007;92:3278–3284. [DOI] [PubMed] [Google Scholar]
- 30.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005;62:1556–1560. [DOI] [PubMed] [Google Scholar]
- 31.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. The implications of body fat mass and fat distribution for cognitive function in elderly women. Obes Res 2004;12:1519–1526. [DOI] [PubMed] [Google Scholar]
- 32.Brownbill RA, Ilich JZ. Cognitive function in relation with bone mass and nutrition: cross-sectional association in postmenopausal women. BMC Womens Health 2004;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo HK, Jones RN, Milberg WP, et al. Cognitive function in normal-weight, overweight, and obese older adults: An analysis of the Advanced Cognitive Training for Independent and Vital Elderly cohort. J Am Geriatr Soc 2006; 54:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol Aging. 2005;26 Suppl 1:11–16. [DOI] [PubMed] [Google Scholar]
- 35.Arnold AM, Psaty BM, Kuller LH, et al. Incidence of cardiovascular disease in older Americans: the Cardiovascular Health Study. J Am Geriatr Soc 2005;53:211–218. [DOI] [PubMed] [Google Scholar]
- 36.Goff DC Jr, Bertoni AG, Kramer H, et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation 2006;113:647–656. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults–United States, 2004. Morb Mortal Weekly Rep 2005;54:1121-1124. [PubMed] [Google Scholar]
- 38.Petitti DB. Hormone replacement therapy for prevention: more evidence, more pessimism. JAMA 2002;288:99–101. [DOI] [PubMed] [Google Scholar]
- 39.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 40.Kalaria RN. The role of cerebral ischemia in Alzheimer's disease. Neurobiol Aging 2000;21:321–330. [DOI] [PubMed] [Google Scholar]