Abstract
Background:
In some prospective studies, associations of serum vitamin B12 and homocysteine concentrations with cognitive decline have been reported but few have examined the role of methylmalonic acid, a more specific marker of vitamin B12 deficiency than homocysteine.
Objective:
The aim of the study was to determine whether serum concentrations of vitamin B12 or selected metabolites are related to cognitive decline.
Methods:
A total of 516 subjects were selected in a stratified random sampling design from among Chicago Health and Aging Project participants for clinical evaluation. We used linear mixed models to examine the association of blood markers of vitamin B12 status to change in cognitive scores over 6 years. Cognitive function was assessed every 3 years and measured as the sum of standardized scores on four tests.
Results:
Probable vitamin B12 deficiency was observed in 14.2% of the sample. Elevated serum concentrations of homocysteine were present in 19.2% of subjects, and of methylmalonic acid, in 36.4%. Higher serum methylmalonic acid concentrations were predictive of faster rates of cognitive decline (β = −0.00016, SE = 0.0001, p = 0.004) and higher serum vitamin B12 concentrations were associated with slower rates of cognitive decline (β = +0.00013, SE < 0.0001, p = 0.005) in multivariable adjusted mixed models. Serum concentrations of homocysteine had no relationship to cognitive decline.
Conclusions:
Serum methylmalonic acid and vitamin B12 concentrations may be the more important risk factors for cognitive decline when compared to serum homocysteine concentrations, particularly in older populations exposed to food fortification and possible supplements containing folic acid.
GLOSSARY
- CHAP
= Chicago Health and Aging Project;
- CI
= confidence interval;
- FFQ
= food frequency questionnaire;
- NHANES
= National Health and Nutrition Examination Survey;
- OR
= odds ratio.
Since the mandatory folic acid fortification of all grain products in the United States, the number of persons with elevated serum folate concentrations (>45.3 nmol/L) increased from approximately 7% prior to fortification to 38% postfortification.1 The significance of elevated folate concentrations is not clearly understood, but concern about adverse effects of high folic acid intake on neurologic function in people with undiagnosed vitamin B12 deficiency has delayed mandatory fortification in the United Kingdom.2 In a sample of older subjects from the 1999–2002 National Health and Nutrition Examination Survey (NHANES),3 those with low vitamin B12 status and elevated serum folate concentrations (>59 nmol/L) were more likely to manifest impaired cognitive performance than those with low vitamin B12 status but normal serum folate concentrations (≤59 nmol/L).
Previously, in the Chicago Health and Aging Project (CHAP), we reported greater cognitive decline in persons with folate intakes exceeding 400 μg per day compared to those with lower intakes.4 Biochemical markers for vitamin status were not measured. While the relationship of elevated homocysteine concentrations on cognitive changes has been examined,5–7 there is limited information on other vitamin markers such as serum methylmalonic acid. Thus, in an effort to further understand the complex relationships between vitamin B12 and folate and age-related cognitive decline, we examined whether biochemical indicators of vitamin B12 (serum vitamin B12, methylmalonic acid, homocysteine) and folate (serum homocysteine) insufficiency were associated with cognitive decline.
METHODS
Study population.
Study subjects were participants in CHAP, an ongoing cohort study of older residents on the south side of Chicago. Exactly 6,158 participated in in-home interviews that included four cognitive tests (79% participation overall; 81% among black subjects, 75% among white subjects). Follow-up interviews including cognitive assessments were conducted in 3-year cycles on all participants (figure). Stratified random samples from the study population were drawn at each cycle for clinical neurologic evaluations during which phlebotomies were performed.8 For the present study, biochemical analyses were performed on nonfasting bloods drawn from clinically evaluated participants at cycle 2 (1996–1999), and related to cognitive changes from cycle 2 to cycle 4. As shown in the figure, of the 842 participants clinically evaluated at cycle 2, 516 had cognitive assessments at cycle 3 or at cycle 4 or both. Serum vitamin B12 was measured immediately following the participant's clinical evaluation because this measurement was part of a routine diagnostic panel. All other vitamin B12 metabolites were analyzed from additional aliquots of blood that had been frozen for a period of 7–10 years. By chance, 174 of these were also selected for the cycle 3 clinical evaluation sample. Cycle 3 blood samples were analyzed for vitamin B12 metabolites to examine the change in metabolite concentrations in relation to changes in cognitive function. This study was approved by the Institutional Review Board of Rush University Medical Center; all participants gave written informed consent.
Figure Timeline and protocol of selected samples from Chicago Health and Aging Project subjects at cycle 2, cycle 3, and cycle 4
At cycle 2, a random sample is obtained for clinical evaluations at which time bloods were drawn. Of the 666 cycle 2 participants with frozen sera and cognitive assessments, there were 516 subjects at cycle 2 for whom bloods were available as well as additional cognitive testing at cycles 3 or cycle 4 or both cycles. There were also 174 subjects with bloods that were available at both cycle 2 and cycle 3 or 174 pairs.
Biochemical analyses.
Bloods were drawn into red top Vacutainers, placed on ice, and centrifuged within 2 hours of phlebotomy. Sera was partitioned into aliquots and frozen at −80ºC. Aliquots were sent to the Metabolite Labs at the University of Colorado Health Sciences Center (Denver, CO) for analysis of homocysteine, methylmalonic acid, 2-methylcitric acid, and cystathionine. These metabolites were assayed as described previously by stable-isotope dilution and capillary gas chromatography-mass spectrometry.9–11 Intra-assay and interassay CVs for these metabolites averaged 2% and 5%, respectively (private communication, Dr. Sally Stabler). Serum creatinine measurements were performed by Rush University Medical Laboratories. Because homocysteine and methylmalonic acid are elevated when there is renal failure,9 adjustment for creatinine was included in all models to account for possible confounding. Vitamin B12 determinations were performed by automated competitive displacement immunoassay (Quest Laboratories, Wooddale, IL).
Cognitive function assessment.
The population interviews included administration of four cognitive tests during in-home interviews at each cycle. Tests included the East Boston tests of immediate and delayed recall,12 the Mini-Mental State Examination,13 and the Symbol Digit Modalities Test.14 Scores on each were expressed as z-scores and averaged for a global measure of cognitive function that was approximately normally distributed, and reduced the floor and ceiling effects and other measurement errors of the individual tests.
Other covariates.
Gender and race were obtained at the time of the census and verified at the baseline population interview. Race was determined by questions and categories of the 1990 US census. Information on non-dietary variables was collected at participants' baseline interview. Age was computed from self-reported birth date and date of baseline cognitive assessment. Education was computed from self-reported highest grade or years of formal education. A composite score reflecting the mean frequency of participation in seven cognitive activities was also constructed as described previously.15 Questions on cigarette smoking allowed for the computation of an indicator variable (never, former, or current smoker). Daily consumption of alcohol (grams per day) was based on three questions about usual consumption during the past year of beer, wine, and liquor. Dietary intakes of vitamin B12, folate, and other components were measured by a modified Willett food frequency questionnaire (FFQ).16 All dietary variables were energy-adjusted using the regression residual method.17 FFQs were obtained on all subjects at cycle 2—a median of 7 months before bloods were drawn.
Statistical analyses.
Possible sample bias between the incident sample drawn (n = 842) and the sample with available bloods and repeated cognitive testing over the 6-year period (n = 516) was examined by comparing characteristics of participants of both samples using χ2 and Wilcoxon rank sum test as appropriate. Similar difference tests were also conducted between those identified as probably deficient and those adequate with respect to vitamin B12. Pearson correlation tests were used to examine associations between biochemical markers of vitamin B12 status, age, and nutrient intake.
Our primary analyses were designed to estimate the effect of biochemical markers for vitamin B12 and folate on within-person rate of change in cognitive score using mixed-effects models.18 The adjusted model for change included terms for age (years), sex, race, education (years), participation in cognitive activities and serum creatinine concentrations, and the interaction between time and each covariate (basic model). Additional adjustment for dietary and lifestyle factors included energy-adjusted intakes of saturated fat, trans unsaturated fat, food vitamin E, total vitamin C, food niacin and fish intakes, smoking status, and alcohol use (multiple-adjusted model). The selection of dietary covariates is based on previous CHAP studies.19–21 The interaction terms with time represent the effects of the variables on the rate of change in cognitive score. Because a large portion of the sample was not included in the analyses, models were not weighted for the stratified random sampling design.
Secondary analyses were performed on 174 sample participants who had biochemical measurements at both cycle 2 and cycle 3. We used a linear regression model in which global cognitive score at cycle 3 was regressed on a dichotomous variable for homocysteine increase, baseline global cognitive scores, and the basic model variables.
RESULTS
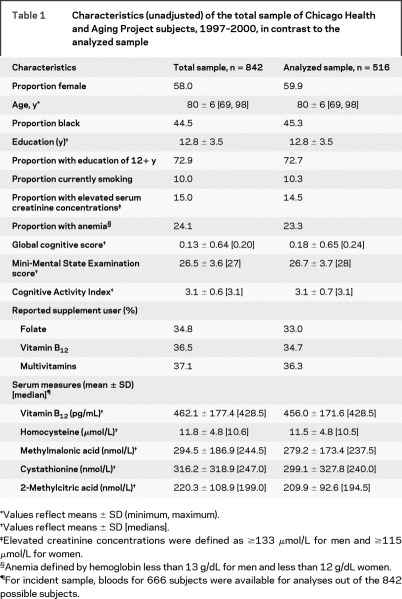
There were no significant differences in demographic, biochemical, and lifestyle characteristics between the full and analyzed samples (table 1). The analyzed sample comprised nearly 60% women, 45% black, with an average age of 80 years. Renal impairment was evident for 14% of incident sample participants as indicated by elevated serum creatinine concentrations. Exactly 19.2% of the analyzed sample had elevated homocysteine values (>13.9 μmol/L) and 36.4% had elevated methylmalonic acid concentrations (>271 nmol/L).
Table 1 Characteristics (unadjusted) of the total sample of Chicago Health and Aging Project subjects, 1997–2000, in contrast to the analyzed sample
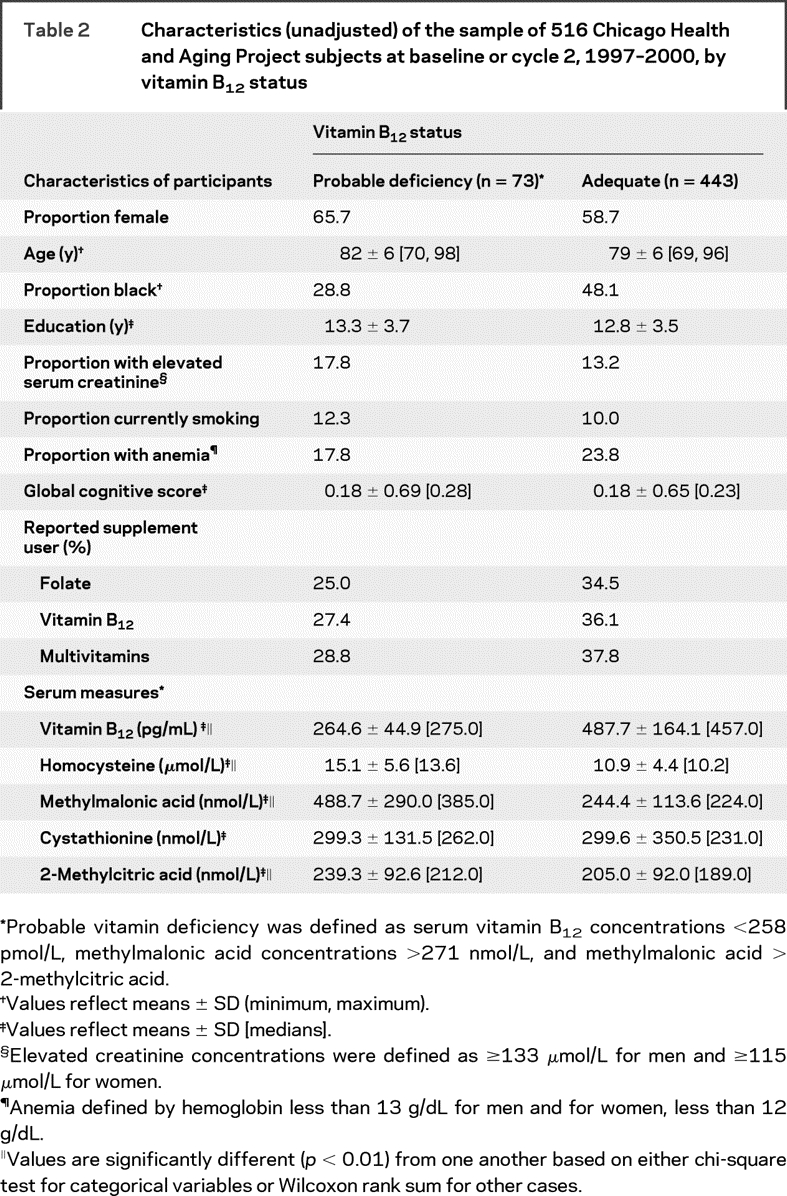
Only 1% of participants met the criteria for definite vitamin B12 deficiency (serum vitamin B12 <148 pmol/L, methylmalonic acid concentrations >271 nmol/L, and methylmalonic acid > 2-methylcitric acid).9–11 Probable vitamin B12 deficiency was observed for 14.2% of the sample (table 2, table e-1 on the Neurology® Web site at www.neurology.org). Those probably deficient were more likely white (p = 0.002), and consumed lower amounts of total vitamin C, total folate, total vitamin B12 as well as less fish per week. Global cognitive scores did not differ between the groups, but serum concentrations of homocysteine (p < 0.0001) and methylmalonic acid (p < 0.0001) were higher among the probably deficient group.
Table 2 Characteristics (unadjusted) of the sample of 516 Chicago Health and Aging Project subjects at baseline or cycle 2, 1997–2000, by vitamin B12 status
Serum vitamin B12 was correlated with serum homocysteine (r = −0.33, p < 0.0001) and methylmalonic acid (r = −0.23, p < 0.0001), but not with age (r = 0.001, p = 0.97). Serum methylmalonic acid and homocysteine concentrations were correlated with age (r = 0.27, p < 0.0001, and r = 0.09, p = 0.042, respectively). Serum homocysteine concentrations were associated with energy-adjusted intakes of total vitamin B12 (r = −0.12, p < 0.01) and of total folate (r = −0.13, p < 0.01). Serum vitamin B12 values were correlated with total intakes of vitamin B12 and of folate (both r = 0.17, p < 0.0001).
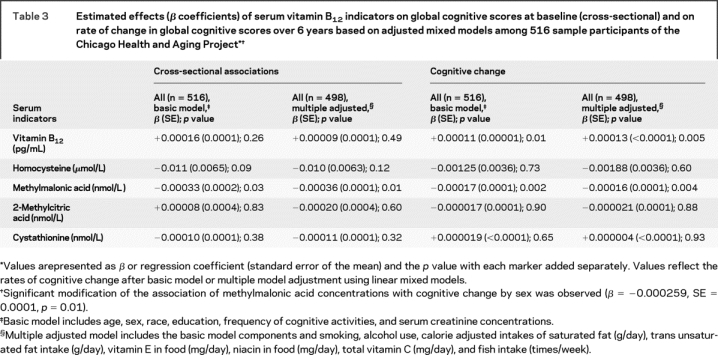
In the mixed effects models to examine the associations of each biochemical marker with cognition separately, only serum methylmalonic acid was associated with global cognitive scores cross-sectionally in basic (β = −0.00033, SE = 0.0002, p = 0.03) and multiple adjusted models (β = −0.00036, SE = 0.0001, p = 0.01) (table 3). Over the 6-year interval, higher concentrations of serum vitamin B12 were associated with slower rates of decline (β = +0.00011, SE < 0.0001, p = 0.01), and higher concentrations of methylmalonic acid were associated with faster rates of cognitive decline (β = −0.00017, SE = 0.0001, p = 0.002) in basic models. Estimates of effect did not change materially with further adjustment for smoking, alcohol use, or intakes of fats, fish, and vitamins E and C and niacin. Circulating concentrations of homocysteine, 2-methylcitric acid, or cystathionine were not predictive of cognitive change in basic or multiple adjusted models. When all three biomarkers were incorporated in the models simultaneously, serum methylmalonic acid remained predictive of cognitive decline for the basic model (β = −0.000149, SE = 0.0001, p = 0.01) and for the fully adjusted model (β = −0.000136, SE = 0.0001, p = 0.03). Serum vitamin B12 was not significantly associated with cognitive change in the basic model (β = +0.000078, SE < 0.0001, p = 0.12), but with adjustment for additional confounding factors, the effect estimate increased substantially and attained statistical significance (β = +0.000103, SE = 0.0001, p = 0.04).
Table 3 Estimated effects (β coefficients) of serum vitamin B12 indicators on global cognitive scores at baseline (cross-sectional) and on rate of change in global cognitive scores over 6 years based on adjusted mixed models among 516 sample participants of the Chicago Health and Aging Project
In further analyses of methylmalonic acid and cognitive decline, we observed effect modification by sex (β = −0.000259, SE = 0.0001, p = 0.01), but not by age (β = −0.000012, SE < 0.0001, p = 0.14) or race (β = +0.000061, SE = 0.0001, p = 0.61). When stratified by sex, the inverse estimate of the relation between methylmalonic acid concentrations and cognitive decline was of stronger magnitude among CHAP men (β = −0.00030, SE = 0.0001, p = 0.0002) as compared with CHAP women (β = −0.00006, SE = 0.0001, p = 0.39). There were no remarkable differences in the distributions of methylmalonic acid concentrations of men in contrast to those for women.
We explored the possibility that cognitive decline may result from increases in serum homocysteine concentrations as previously reported5,6 through an examination of the repeat biochemical markers in 174 sample participants. Nearly three quarters (70.5%) of subjects experienced an increase in serum homocysteine concentrations from cycle 2 to cycle 3; all increases exceeded 3 μmol/L. However, there was no association between the indicator variable for increased serum homocysteine concentration from cycle 2 to cycle 3 and cycle 3 cognitive score (β = −0.08, SE = 0.08, p = 0.36) in the basic model.
DISCUSSION
In this biracial study of older adults, higher serum concentrations of vitamin B12 were associated with a slower rate of cognitive decline and higher concentrations of methylmalonic acid were associated with a faster rate over a 6-year interval (table 3). Serum homocysteine concentrations were not significantly related to cognitive change.
Inadequate vitamin B12 nutriture in the elderly may occur with conditions (e.g., atrophic gastritis) or drugs that reduce absorption (e.g., metformin).22 Thus, while few CHAP participants reported inadequate intakes of vitamin B12, 14.2% showed evidence of metabolic or preclinical deficiency. There is no widely accepted cutoff for marginal or preclinical vitamin B12 deficiency.23,24 Use of several blood indicators in addition to vitamin B12 may improve differential diagnosis.7,9 Methylmalonic acid concentration reflects intracellular vitamin stores and exhibits higher specificity for low vitamin B12 status than any other metabolite including homocysteine.9 However, this indicator adds considerable cost when compared to the screening measure, serum vitamin B12.
Serum vitamin B12 concentrations have not been consistently associated with cognitive change in prospective analyses.25–29 Few groups examined cognition in relation to measures that reflect preclinical or metabolic vitamin B12 deficiency. In a cross-sectional UK survey of 1,000 individuals aged 75 years or older, cognitive impairment (defined as a Mini-Mental State Examination score <22) was more strongly associated with holotranscobalamin, a tissue transport protein for vitamin B12, homocysteine, and methylmalonic acid than with serum vitamin B12.30 In a recent prospective UK study of elders in whom three or more cognitive assessments were obtained, both methylmalonic acid and holotranscobalamin concentrations were predictive of cognitive decline.7 In these studies, serum vitamin B12 concentrations were lower (mean ± SD, 274 ± 139 pmol/L [370 ± 188 pg/mL, 2003 sample]) than those observed in either the present CHAP incident sample (n = 842) or subsample (n = 516) (table 1). Serum methylmalonic acid (390 ± 390 nmol/L) and homocysteine (16.5 ± 6.2 μmol/L) concentrations in the United Kingdom were also higher than those observed for our CHAP subjects. In a representative post-fortification US sample of older adults, homocysteine levels ranged from 8 to 10.7 μmol/L31; in two prospective studies of older adults,28,33 values were similar to those reported in the present study, which spans a pre- to perifortification period.
An interaction between serum concentrations of vitamin B12 and folate on cognitive score was observed in a large cross-sectional survey of 1,302 NHANES 1999–2002 participants aged 60 years or more.3 Those with low vitamin B12 status and high serum folate concentrations (>59 nmol/L) were at greater risk of cognitive impairment (odds ratio [OR] = 2.6, 95% confidence interval [CI] 1.1–6.1) when compared to those with low vitamin B12 status and normal serum folate concentrations. In contrast, among those seniors with normal vitamin B12 status, high serum folate concentrations were associated with less cognitive impairment (OR = 0.4, 95% CI 0.2–0.9) when compared to those with normal serum folate concentrations. These findings partially support an earlier CHAP report in which a faster rate of cognitive decline was observed among participants with high folate intakes (>400 μg/day) from either supplements or food.4 In a multiple adjusted model that included folate intakes, we observed a positive interaction (p = 0.009) between total vitamin B12 intakes with older age on cognitive decline in CHAP.4 Rates of cognitive decline for the average 80-year-old who consumed 20 μg per day of vitamin B12 were 25% slower than rates of a similar 80-year-old who consumed the recommended daily allowance of 2.4 μg. For the average 70-year-old, rates of decline did not vary with such widely different vitamin B12 intakes.4 In the present study, although we measured several serum markers of vitamin B12 status, we did not determine serum folate concentrations. Thus, the interaction of serum folate concentrations with biochemical indicators of vitamin B12 deficiency on cognitive function/decline could not be investigated.
In the present study, serum homocysteine concentrations were not associated with cognitive performance or with cognitive decline. Nor did CHAP participants with increases in serum homocysteine concentrations over a 3-year period manifest significant cognitive decline. An association may not have been observed because subjects may have had adequate folate status but confirmation by measurements of serum or erythrocyte folate concentrations is needed. Serum homocysteine concentrations were not predictive of cognitive decline among the Leiden 85-Plus study participants26 nor the 70- to 79-year-old MacArthur Studies of Successful Aging participants.28 Elevated homocysteine concentrations were associated with declines among Framingham study participants but at a time prior to mandatory folic fortification,6 in select cognitive domains of French older persons33 and among aging male veterans.29 Among Mexican American seniors from 1997 to 1999, homocysteine concentrations were associated with greater risk of dementia and cognitive impairment, but this was modified by serum vitamin B12 concentrations; rates of decline were greater for those with lower vitamin B12 concentrations (<340 pg/mL) (HR = 1.61, p for interaction = 0.04) as compared to those with higher concentrations (>340 pg/mL but ≤498 pg/mL).32
Clinical trial evidence supportive of a role for homocysteine and cognitive decline is still equivocal. Most trials were performed where folate fortification has not been mandated, and in subjects with adequate serum vitamin B12 concentrations.34–36 In many, the intervention was a combination of B vitamins. In the longest trial (3 years), a large sample and a single vitamin intervention (800 μg folic acid daily), folate supplements significantly improved memory, information processing speed, and sensorimotor speed among Dutch adults.34
Although some cross-sectional evidence exists for a methylmalonic acid and cognition association,30,37–40 to our knowledge, there is limited study of its relation to cognitive decline. In the Oxford Healthy Aging Project, greater cognitive decline (over 10 years) was associated with lower holotranscobalamin and higher methylmalonic acid concentrations after adjustment for a number of vitamin markers.7 In our study, we observed a similar relationship—cognitive decline remained significantly associated with serum concentrations of methylmalonic and vitamin B12 after adjustment for vitamin markers. Thus, there is evidence for subtle vitamin B12 deficiency in two large prospective cohorts of elders in whom deleterious changes in cognition were related to sensitive markers for vitamin B12 inadequacy.
Strengths of the present study include the prospective design, a biracial community sample, and use of a number of biochemical indicators of B vitamin status and of multiple tests to measure cognitive function. Moreover, the present analyses were adjusted for many dietary and lifestyle confounders. As stated previously, a limitation of the present study is the lack of serum folate determinations. Because of the observational study design, we must caution against a causal interpretation of findings. Further study of these complex interrelationships between vitamin B12, folate, and cognitive changes is warranted, but multiple biochemical indices of folate and vitamin B12 should be determined. Greater attention must be paid to plausibility of subtle vitamin B12 deficiency among our senior citizens, especially when higher folate intakes are possible.
ACKNOWLEDGMENT
The authors thank Drs. Robert Allen and Sally Stabler for their expertise and vitamin B12 metabolite determinations. They also thank Cheryl Bibbs for study coordination and Todd Beck for programming and the CHAP interviewers and participants.
Supplementary Material
Supplemental data at www.neurology.org
Address correspondence to Dr. Christine C. Tangney, Department of Clinical Nutrition 425 TOB, 1700 West Van Buren St., Chicago, IL 60612 ctangney@rush.edu
Supported by grants (AG11101 and AG13170) from the National Institute on Aging.
Disclosure: The authors report no disclosures.
Received July 11, 2008. Accepted in final form October 15, 2008.
REFERENCES
- 1.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999-2000. Am J Clin Nutr 2005;82:442–450. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health. London: Scientific Advisory Committee on Nutrition; 2006.
- 3.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr 2007;85:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris MC, Evans DA, Bienias JL, et al. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol 2005;62:641–645. [DOI] [PubMed] [Google Scholar]
- 5.Ravaglia G, Forti P, Maioli F, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr 2005;82:636–643. [DOI] [PubMed] [Google Scholar]
- 6.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med 2002;346:476–483. [DOI] [PubMed] [Google Scholar]
- 7.Clarke R, Birks J, Nexo E, et al. Low vitamin B-12 status and risk of cognitive decline in older adults. Am J Clin Nutr 2007;86:1384–1391. [DOI] [PubMed] [Google Scholar]
- 8.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP). J Alzheimer Dis 2003;5:349–355. [DOI] [PubMed] [Google Scholar]
- 9.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J 1993;7:1344–1353. [DOI] [PubMed] [Google Scholar]
- 10.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Elevation of 2-methylcitric acid I and II levels in serum, urine, and cerebrospinal fluid of patients with cobalamin deficiency. Metabolism 1993;42:978–988. [DOI] [PubMed] [Google Scholar]
- 11.Stabler SP, Lindenbaum J, Savage DG, Allen RH. Elevation of serum cystathionine levels in patients with cobalamin and folate deficiency. Blood 1993;81:3404–3413. [PubMed] [Google Scholar]
- 12.Albert MS, Smith LA, Scherr PA. Use of brief cognitive tests to identify individuals in the community with clinically-diagnosed Alzheimer's disease. Intl J Neurosci 1991;57:167–178. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 14.Smith A. Symbol Digit Modalities Test Manual—Revised. Los Angeles, CA: Western Psychological;1984. [Google Scholar]
- 15.Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci 1999;54: P155–P160P160. [DOI] [PubMed] [Google Scholar]
- 16.Morris MC, Colditz GA, Evans DA. Response to a mail nutritional survey in an older bi-racial community population. Ann Epidemiol 1998;8:342–346. [DOI] [PubMed] [Google Scholar]
- 17.Willett WC. Total energy intake and nutrient composition: dietary recommendations for epidemiologists. Int J Cancer 1990;46:770–771. [DOI] [PubMed] [Google Scholar]
- 18.Laird NM, Ware JH. Random effects models for longitudinal data. Biometrics 1982;38:963–974. [PubMed] [Google Scholar]
- 19.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol 2005;62: 1849–18531853. [DOI] [PubMed] [Google Scholar]
- 20.Morris MC, Evans DA, Bienias JL, et al. Dietary niacin and the risk of incident Alzheimer's disease and of cognitive decline. J Neurol Neurosurg Psychiatry 2004;75:1093– 1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology 2004;62:1573–1579. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson-Ehle H. Age-related changes in cobalamin (vitamin B12) handling: implications for therapy. Drugs Aging 1998;12:277–292. [DOI] [PubMed] [Google Scholar]
- 23.Stabler S, Lindenbaum J, Allen R. Vitamin B-12 deficiency in the elderly: current dilemmas. Am J Clin Nutr 1997;66:741–749. [DOI] [PubMed] [Google Scholar]
- 24.Saperstein DS, Wolfe GI, Gronseth GS, et al. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch Neurol 2003;60:1296–1301. [DOI] [PubMed] [Google Scholar]
- 25.Martin DC, Francis J, Protetch J, Huff FJ. Time dependency of cognitive recovery with cobalamin replacement: report of a pilot study. J Am Geriatr Soc 1992;40:168–172. [DOI] [PubMed] [Google Scholar]
- 26.Mooijaart SP, Gussekloo J, Frolich M, et al. Homocysteine, vitamin B-12, and folic acid and the risk of cognitive decline in old age: the Leiden 85-Plus study. Am J Clin Nutr 2005;82:866–871. [DOI] [PubMed] [Google Scholar]
- 27.Kang JH, Irizarry MC, Grodstein F. Prospective study of plasma folate, vitamin B12, and cognitive function and decline. Epidemiology 2006;17:650–657. [DOI] [PubMed] [Google Scholar]
- 28.Kado DM, Karlamangla AS, Huang MH, et al. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur Studies of Successful Aging. Am J Med 2005;118:161–167. [DOI] [PubMed] [Google Scholar]
- 29.Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A, 3rd. High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr 2005;82:627–635. [DOI] [PubMed] [Google Scholar]
- 30.Hin H, Clarke R, Sherliker P, et al. Clinical relevance of low serum vitamin B12 concentrations in older people: the Banbury B12 study. Age Ageing 2006;35:416–422. [DOI] [PubMed] [Google Scholar]
- 31.Pfeiffer CM, Osterloh JD, Kennedy-Stephenson J, et al. Trends in circulation concentrations of total homocysteine among US adolescents and adults: findings from the 1991–1994 and 1999–2004 National Health and Nutrition Examination Surveys. Clin Chem 2008;54:1–12. [DOI] [PubMed] [Google Scholar]
- 32.Haan MN, Miller JW, Aiello AE, et al. Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. Am J Clin Nutr 2007;85:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dufouil C, Alperovitch A, Ducros V, Tzourio C. Homocysteine, white matter hyperintensities, and cognition in healthy elderly people. Ann Neurol 2003;53:214–221. [DOI] [PubMed] [Google Scholar]
- 34.Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet 2007;369:208–216. [DOI] [PubMed] [Google Scholar]
- 35.Stott DJ, MacIntosh G, Lowe GD, et al. Randomized controlled trial of homocysteine lowering vitamin treatment in elderly patients with vascular disease. Am J Clin Nutr 2005;82:1320–1326. [DOI] [PubMed] [Google Scholar]
- 36.McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med 2006;354:2764–2772. [DOI] [PubMed] [Google Scholar]
- 37.Lewerin C, Matousek M, Steen G, Johanssen B, Steen B, Nilsson-Ehle H. Significant correlations of plasma homocysteine and serum methylmalonic acid with movement. Am J Clin Nutr 2005;81:155–162. [DOI] [PubMed] [Google Scholar]
- 38.McCracken C, Hudson P, Ellis R, McCaddon A. Medical Research Council Cognitive Function and Ageing Study: methylmalonic acid and cognitive function in the Medical Research Council Cognitive Function and Ageing Study. Am J Clin Nutr 2006;84:1406–1411. [DOI] [PubMed] [Google Scholar]
- 39.Lewis MS, Miller LS, Johnson MA, Dolce EB, Allen RH, Stabler SP. Elevated methylmalonic acid is related to cognitive impairment in older adults enrolled in an elderly nutrition program. J Nutr Elderly 2005;24:47–65. [DOI] [PubMed] [Google Scholar]
- 40.Garcia AA, Haron Y, Evans LR, Smith MG, Freedman M, Roman GC. Metabolic markers of cobalamin deficiency and cognitive function in normal older adults. J Am Geriatr Soc 2004;52:66–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.