Abstract
Objective:
To examine whether antiplatelet medication use at onset of intracerebral hemorrhage (ICH) is associated with hemorrhage growth and outcome after spontaneous ICH using a large, prospectively collected database from a recent clinical trial.
Methods:
The Cerebral Hemorrhage and NXY-059 Treatment trial was a randomized, placebo-controlled trial of NXY-059 after spontaneous ICH. We analyzed patients in the placebo arm, and correlated antiplatelet medication use at the time of ICH with initial ICH volumes, ICH growth in the first 72 hours, and modified Rankin Score at 90 days. Patients on oral anticoagulation were excluded.
Results:
There were 282 patients included in this analysis, including 70 (24.8%) who were taking antiplatelet medications at ICH onset. Use of antiplatelet medications at ICH onset had no association with the volume of ICH at presentation, growth of ICH at 72 hours, initial edema volume, or edema growth. In multivariable analysis, there was no association of use of antiplatelet medications with any hemorrhage expansion (relative risk [RR] 0.85 [upper limit of confidence interval (UCI) 1.03], p = 0.16), hemorrhage expansion greater than 33% (RR 0.77 [UCI 1.18], p = 0.32), or clinical outcome at 90 days (odds ratio 0.67, 95% confidence interval 0.39–1.14, p = 0.14).
Conclusions:
Use of antiplatelet medications at intracerebral hemorrhage (ICH) onset is not associated with increased hemorrhage volumes, hemorrhage expansion, or clinical outcome at 90 days. These findings suggest that attempts to reverse antiplatelet medications after ICH may not be warranted.
GLOSSARY
- CHANT
= Cerebral Hemorrhage and NXY-059 Treatment;
- CI
= confidence interval;
- ICH
= intracerebral hemorrhage;
- IQR
= interquartile range;
- mRS
= modified Rankin Scale;
- RR
= relative risk;
- UCI
= upper limit of confidence interval.
Intracerebral hemorrhage (ICH) is a devastating stroke subtype. About 50% of patients die within the first month and only 20% of patients are living independently at 6 months.1,2 Larger volume of hemorrhage predicts poor outcome.1 Over one-third of patients have significant expansion of their hemorrhage in the first 24 hours.3,4 Ongoing hemorrhage expansion is an independent predictor of mortality and poor functional outcome.3,5
Other than warfarin use,6,7 the factors involved in hemorrhage expansion have been difficult to identify. One report found an association of elevated blood pressure with expansion8 but this was not confirmed in another systematic study.9 Shorter time from symptom onset, an irregularly shaped hematoma, depressed level of consciousness, heavy alcohol use, and low fibrinogen levels have been associated with hemorrhage expansion.10 A recent analysis from a trial of recombinant activated Factor VII found shorter time from symptom onset, larger initial volume of hemorrhage, and elevated blood glucose associated with expansion in most of their models and use of study medication associated with less growth.11 Contrast extravasation on CT angiography may predict hemorrhage expansion.12,13
Whether antiplatelet medication use at the time of ICH influences hemorrhage expansion has been controversial. Multiple articles with different study designs, exclusion criteria, and numbers of patients have reported conflicting results.11,14–21 The purpose of this study was to use the data from a large prospective clinical trial with predefined inclusion criteria, standardized timing of imaging acquisition and blinded analysis of volumes, and standardized outcome assessment to analyze the effect of prior antiplatelet medication use on hemorrhage and edema expansion and functional outcome.
METHODS
We analyzed data from the prospective cohort defined by the placebo arm of the Cerebral Hemorrhage and NXY-059 Treatment (CHANT) trial, a randomized, double-blind, placebo-controlled trial testing the safety and tolerability of NXY-049, a potential neuroprotectant, in patients with acute ICH. The study design and primary results have been described previously.22 Briefly, 607 patients over age 18 years with ICH onset within 6 hours were randomized to receive NXY-059 or placebo and followed for 3 months. All patients received a noncontrast head CT scan or MRI at presentation and a follow-up scan at 72 hours. Patients were treated according to the local standard of care and all concomitant medications taken at the time of enrollment and during the study were recorded.
Neuroimaging was analyzed by Perceptive Informatics, Waltham, MA. Scans were digitized and volumes calculated centrally by semiautomatic planimetry and reconstruction using proprietary software (Alice). The reviewer was blinded to clinical information.
Patients were categorized as taking antiplatelet medications if they reported current use of aspirin, clopidogrel, dipyridamole, triflusal, or indobufen at hospital presentation. Patients on oral anticoagulation were excluded from this analysis because of the overwhelming effect of anticoagulation on hemorrhage volume and outcome. Patients who underwent surgical evacuation prior to neuroimaging at 72 hours were not included in the analysis on hemorrhage volume at 72 hours.
Analysis of ICH volumes was planned using nonparametric tests, since ICH volumes were not expected to be normally distributed. We tested and confirmed non-normality prior to analysis. Calculations of power and detectable differences are not readily calculated under this condition, though with one published method23 we had 80% power to detect a 6.5 mL difference in ICH expansion between the groups, using alpha = 0.05 and SD of 16 mL.
Univariate analysis was performed using t tests for normally distributed continuous variables, Wilcoxon rank sum for non-normal continuous variables, and χ2 or Fisher exact tests as appropriate for categorical data. Multivariable analysis was performed to determine factors independently associated with any hemorrhage expansion and hemorrhage expansion greater than 33% using the log-binomial model to estimate relative risks.24 Multivariable analysis to determine factors independently associated with the modified Rankin score at 90 days was performed using ordered logistic regression after confirming the proportional odds assumption was upheld. All variables known to predict outcome and those significantly associated with antiplatelet medication use at p < 0.10 were included in the multivariable models. For outcomes related to hemorrhage size, one-sided tests were performed since the only expected outcome was hemorrhage expansion. For clinical outcomes, two-sided tests were performed since antiplatelet agents could potentially lead to either harm or benefit. All final models used p < 0.05 to determine significance. Statistical analysis was performed using Stata/IC 10 (StataCorp, College Station, TX).
This study was reviewed by the institutional review board of the University of Pennsylvania but as it involved only analysis of an existing data set provided under a data use agreement it was deemed not to require institutional review board approval.
RESULTS
There were 282 patients included in this analysis, including 70 (24.8%) patients who were taking antiplatelet medications at ICH onset. Of these, 56 patients were taking aspirin alone, 5 patients were taking clopidogrel alone, 3 were taking aspirin and clopidogrel, 2 were taking aspirin and dipyridamole, 2 were taking triflusal, 1 was taking dipyridamole alone, and 1 was taking indobufen. None of the patients on antiplatelet medications received platelet transfusions. All patients except one were functionally independent before the hemorrhage occurred. Five patients had surgical evacuation of their hemorrhages (all supratentorial); one had been on an antiplatelet medication, four were not.
In univariate analysis, older age and male sex were significantly associated with antiplatelet medication use. Patients taking antiplatelet medications were also more likely to have a history of hypertension and prior stroke. The baseline characteristics are shown in table 1.
Table 1 Baseline patient characteristics by antiplatelet medication use
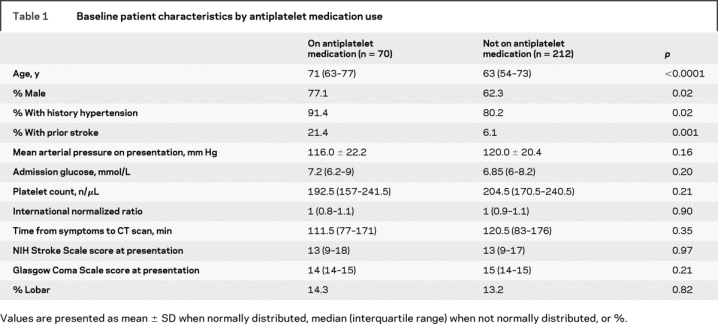
There were 270 patients with complete imaging data. Two additional patients were excluded because of neurosurgical evacuation prior to follow-up imaging, leaving 268 patients in the analysis on hemorrhage expansion. Overall, 26% of patients had greater than 33% increase in hemorrhage volume and 65% of patients had some increase in hemorrhage volume between the initial and follow-up imaging. The overall mean percent change in hemorrhage volume was 29%. Use of antiplatelet medications had no association with initial hemorrhage volume, likelihood to have follow-up imaging, hemorrhage volume at 72 hours, or hemorrhage or edema growth+ table 2). Patients on aspirin, as compared to those on any other antiplatelet medication, had similar hemorrhage volumes (initial hemorrhage volume median 13.3 [interquartile range (IQR), 8.0–32.7] mL) and rates of expansion (hemorrhage volume at 72 hours median 14.0 [IQR 7.7–39.1] mL, 56% of patients had some hemorrhage expansion, and 24% had hemorrhage expansion greater than 33%).
Table 2 Volume and outcome data by use of antiplatelet medications
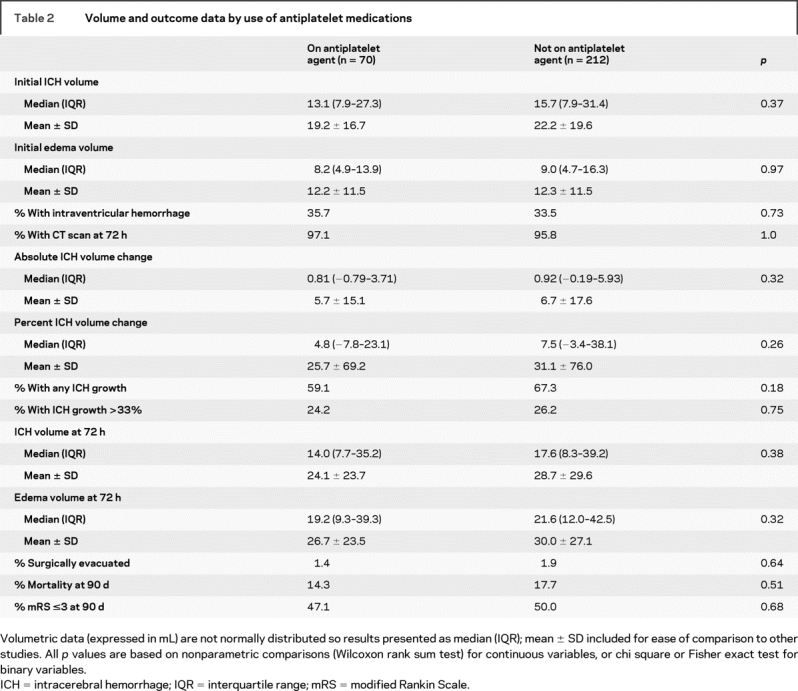
There were 279 patients with clinical outcome assessment (modified Rankin scale [mRS]) and 282 patients with mortality data at 90 days. Use of antiplatelet medications had no association with mRS, mortality, or need for surgical evacuation (table 2 and figure).

Figure The distribution of modified Rankin Scale scores at 90 days by use of antiplatelet medications at the time of intracerebral hemorrhage
Outcome scores are grouped as 0–1 (no symptoms or minimal symptoms without disability), 2–3 (some symptoms but able to look after own affairs or walk independently), 4–5 (unable to walk or attend to own affairs without assistance to bed bound requiring constant nursing care), and 6 (dead). p = 0.96 By ordered logistic regression.
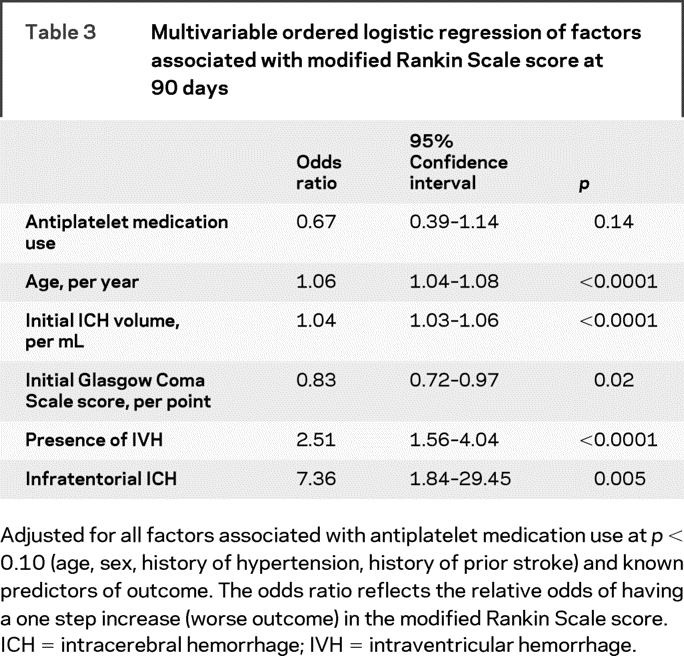
In order to account for the differences in baseline characteristics by antiplatelet medication use, multivariable models were created for any hemorrhage expansion, hemorrhage expansion greater than 33%, and clinical outcome including age, history of hypertension, prior stroke, sex, and use of antiplatelet medications. We also included time from symptom onset to neuroimaging in the models on hemorrhage expansion and variables known to predict outcome after ICH in the model on mRS score at 90 days. The relative risk of antiplatelet medication use for any hemorrhage expansion was 0.85 (upper limit of confidence interval [UCI] 1.03), p = 0.16. The relative risk for hemorrhage expansion greater than 33% was 0.77 (UCI 1.18), p = 0.32. Time from symptoms to CT scan was significantly associated with any hemorrhage expansion (odds ratio, per minute 0.994 [UCI 0.998], p = 0.004). Age, initial volume of hemorrhage, Glasgow Coma Scale score at presentation, presence of intraventricular hemorrhage (IVH), and infratentorial location of hemorrhage were significantly associated with clinical outcome, but antiplatelet medication use was not (table 3).
Table 3 Multivariable ordered logistic regression of factors associated with modified Rankin Scale score at 90 days
DISCUSSION
CHANT was the largest published study of patients with ICH followed with prospective imaging and long-term functional assessments. In the placebo-treated group, we found no association between use of antiplatelet medication at the time of ICH and initial hemorrhage or edema volumes, hemorrhage or edema expansion at 72 hours, or functional outcome at 90 days. We defined growth from baseline to 72 hours in four ways: absolute change in ICH volume, percent change in ICH volume (absolute change divided by initial volume), any increase in ICH volume, and 33% or greater increase in ICH volume. Multiple measures were presented to allow for comparisons with other studies and to ensure that both small (any change on a continuous scale) and large (>33% categorical change) effects were considered. In the multivariable models the point estimates for risk of any hemorrhage expansion, hemorrhage expansion greater than 33%, and higher (worse) mRS scores at 90 days were all less than one. If one considers the highest potential risk of harm as the UCIs on hemorrhage expansion, one would conclude the upper boundary of potential risk is 3% for any hemorrhage expansion and 18% for hemorrhage expansion greater than 33%, while conceding that the majority of the CIs for both outcomes were below one. Thus we conclude there was no significant association between clinical or radiologic outcome and antiplatelet medication use, and potential harm due to antiplatelet medication use is very small.
Our results confirm other findings. The most recent study reported factors associated with hemorrhage growth in the phase II trial of recombinant factor VIIa in acute ICH, another randomized clinical trial with prespecified inclusion criteria and standardized timing of neuroimaging.11 The authors constructed five models of hemorrhage expansion (absolute ICH volume change, percent volume change, percent of patients with volume change >33%, absolute ICH + IVH volume change, and percent ICH + IVH volume change) and found that antiplatelet medication use had no association with hemorrhage growth in four of the five models, but was associated with a higher percentage of patients with >33% growth. We could not replicate this finding. However, the trial of recombinant factor VIIa enrolled patients presenting within 3 hours of symptom onset, while CHANT enrolled within 6 hours. Therefore, the mean time from symptom onset to CT scan was 114 ± 35 minutes in the recombinant factor VIIa trial4 compared to 134 ± 66 minutes in CHANT. Although the data were not normally distributed, we presented the mean volumes to allow comparison of the studies and the results are quite similar. The mean percent change in hemorrhage volume was 29% in both studies.4 The mean absolute change in hemorrhage volume was 6.5 mL in CHANT and the statistically predicted mean was 8.7 mL (95% CI 4.9–12.4) in the factor VIIa trial.4 The ICH volumetric data from the factor VIIa trial was from follow-up scans at 24 hours, but since most hemorrhage expansion occurs within the first 24 hours, the values are likely comparable.
Another large study with similar results is a prospective country-wide registry of 1,691 patients with ICH in Germany.14 There was no association of antiplatelet medication use on in-hospital mortality or discharge mRS when adjusting for age and preexisting disability. In addition, a prospective study performed at 34 hospitals in Asia included 783 patients and found no association of in-hospital mortality and antiplatelet medication use.15 However, only 4.3% were on antiplatelet medications, which may have limited the power of the study. Finally, a prospective study performed at two hospitals including 457 patients reported no difference in the proportion of patients with mRS 4 or 5 at discharge between the antiplatelet users and nonusers.16
In a related study, the pooled analysis of the Chinese Acute Stroke Trial and the International Stroke Trial found that 773 (2%) patients were randomized to aspirin or placebo after an ICH because a CT scan was not required for entry into the trial.25 There was no increased risk of death in these patients.
Other studies have reported conflicting results. A retrospective chart review of 251 patients reported that antiplatelet medication use was associated with greater hemorrhage expansion; however, the volumes of ICH on day two CT scans were not different between the antiplatelet medication users and nonusers.17 This raises the question of whether the antiplatelet medication users presented earlier or if there was another confounding variable associated with early hemorrhage growth. Mortality rate did also not differ between the two groups.
Another prospective study included 194 patients and found an association of antiplatelet medication use on 30-day mortality in multivariable analysis including age, GCS, admission glucose level, ICH volume, and presence of IVH.18 One study included all patients with ICH admitted to a local hospital plus all autopsy-confirmed cases of ICH in the community.19 Of the 208 patients, only 103 had follow-up CT scanning and all at different times. Aspirin use was associated with hemorrhage expansion and mortality, but not with functional outcome at 3 months. Another post hoc analysis of a trial included patients with traumatic or spontaneous ICH who were not ambulatory or had a contraindication to pneumatic compression devices and found prior antiplatelet therapy was associated with mortality.20 There were no data on ICH volumes. Finally, patients admitted with ICH who were treated with tranexamic acid and nicardipine were reported to have an increased rate of hemorrhage growth (defined as >20%) if on antiplatelet therapy.21 However, only eight patients had hemorrhage expansion and one was on warfarin and aspirin and included in the antiplatelet analysis.
Our study adds a significant amount of information to a topic of considerable uncertainty. The strengths of this analysis include a large number of patients, rigorous baseline data collection, standardized imaging timing, centralized, blinded analysis of both hemorrhage and edema volumes, and standardized outcome assessment at 90 days. In addition, since no patient on antiplatelet medications received a platelet transfusion, there is no chance of obscuration of effect by medication reversal, a potential issue not addressed in most other studies.
There are several limitations to this study. It assumes that all patients who reported taking the antiplatelet medication were actually compliant, since poor compliance would potentially dilute the deleterious effects of the medication on outcome. While we attempted to ascertain if any the factors known to contribute to poor outcome were associated with antiplatelet medication use, there is the possibility of confounding by an unknown and unmeasured factor. The majority of patients had small to moderate hemorrhages, consistent with the fact that unconsciousness at presentation was an exclusion criterion for the CHANT clinical trial. Therefore it is possible that some patients with very large hemorrhages due to or exacerbated by antiplatelet medication use were systematically missed. However, if this were true we would have expected to see larger hemorrhages within the antiplatelet medication group in the trial. We also could not ascertain whether antiplatelet medications had any effect on IVH growth, as there is no validated way to quantify IVH and these volumes were not collected in the CHANT trial. Finally, no comment can be made about hemorrhage expansion and outcome in patients on two antiplatelet agents compared to monotherapy because of the small number of patients on dual therapy in this study.
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by Lauren Sansing, MD, and Scott Kasner, MD, University of Pennsylvania, who had full access to the data.
ACKNOWLEDGMENT
The authors thank AstraZeneca for providing the data for this analysis and Andrew Cucchiara, PhD, for his statistical assistance.
Address correspondence and reprint requests to Dr. Lauren H. Sansing, Hospital of the University of Pennsylvania, 3400 Spruce Street, 3 W Gates, Philadelphia, PA 19104 sansingl@uphs.upenn.edu
Editorial, page 1376
e-Pub ahead of print on January 7, 2009, at www.neurology.org.
Funded by NIH T32 HL007954-07 (L.H.S.).
Disclosure: The authors report no disclosures.
Received February 25, 2008. Accepted in final form October 31, 2008.
REFERENCES
- 1.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–993. [DOI] [PubMed] [Google Scholar]
- 2.Flaherty ML, Haverbusch M, Sekar P, et al. Long-term mortality after intracerebral hemorrhage. Neurology 2006;66:1182–1186. [DOI] [PubMed] [Google Scholar]
- 3.Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997;28:1–5. [DOI] [PubMed] [Google Scholar]
- 4.Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–785. [DOI] [PubMed] [Google Scholar]
- 5.Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006;66:1175–1181. [DOI] [PubMed] [Google Scholar]
- 6.Lee SB, Manno EM, Layton KF, Wijdicks EF. Progression of warfarin-associated intracerebral hemorrhage after INR normalization with FFP. Neurology 2006;67:1272–1274. [DOI] [PubMed] [Google Scholar]
- 7.Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004;63:1059–1064. [DOI] [PubMed] [Google Scholar]
- 8.Ohwaki K, Yano E, Nagashima H, Hirata M, Nakagomi T, Tamura A. Blood pressure management in acute intracerebral hemorrhage: Relationship between elevated blood pressure and hematoma enlargement. Stroke 2004;35:1364–1367. [DOI] [PubMed] [Google Scholar]
- 9.Jauch EC, Lindsell CJ, Adeoye O, et al. Lack of evidence for an association between hemodynamic variables and hematoma growth in spontaneous intracerebral hemorrhage. Stroke 2006;37:2061–2065. [DOI] [PubMed] [Google Scholar]
- 10.Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R. Multivariable analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke 1998;29:1160–1166. [DOI] [PubMed] [Google Scholar]
- 11.Broderick JP, Diringer MN, Hill MD, et al. Determinants of intracerebral hemorrhage growth: an exploratory analysis. Stroke 2007;38:1072–1075. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein JN, Fazen LE, Snider R, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology 2007;68:889–894. [DOI] [PubMed] [Google Scholar]
- 13.Wada R, Aviv RI, Fox AJ, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke 2007;38:1257–1262. [DOI] [PubMed] [Google Scholar]
- 14.Foerch C, Sitzer M, Steinmetz H, Neumann-Haefelin T. Pretreatment with antiplatelet agents is not independently associated with unfavorable outcome in intracerebral hemorrhage. Stroke 2006;37:2165–2167. [DOI] [PubMed] [Google Scholar]
- 15.Wong KS. Risk factors for early death in acute ischemic stroke and intracerebral hemorrhage: a prospective hospital-based study in Asia. Stroke 1999;30:2326–2330. [DOI] [PubMed] [Google Scholar]
- 16.Caso V, Paciaroni M, Venti M, et al. Effect of on-admission antiplatelet treatment on patients with cerebral hemorrhage. Cerebrovasc Dis 2007;24:215–218. [DOI] [PubMed] [Google Scholar]
- 17.Toyoda K, Okada Y, Minematsu K, et al. Antiplatelet therapy contributes to acute deterioration of intracerebral hemorrhage. Neurology 2005;65:1000–1004. [DOI] [PubMed] [Google Scholar]
- 18.Roquer J, Rodriguez Campello A, Gomis M, Ois A, Puente V, Munteis E. Previous antiplatelet therapy is an independent predictor of 30-day mortality after spontaneous supratentorial intracerebral hemorrhage. J Neurol 2005;252:412–416. [DOI] [PubMed] [Google Scholar]
- 19.Saloheimo P, Ahonen M, Juvela S, Pyhtinen J, Savolainen ER, Hillbom M. Regular aspirin-use preceding the onset of primary intracerebral hemorrhage is an independent predictor for death. Stroke 2006;37:129–133. [DOI] [PubMed] [Google Scholar]
- 20.Lacut K, Le Gal G, Seizeur R, Prat G, Mottier D, Oger E. Antiplatelet drug use preceding the onset of intracerebral hemorrhage is associated with increased mortality. Fund Clin Pharmacol 2007;21:327–333. [DOI] [PubMed] [Google Scholar]
- 21.Sorimachi T, Fujii Y, Morita K, Tanaka R. Predictors of hematoma enlargement in patients with intracerebral hemorrhage treated with rapid administration of antifibrinolytic agents and strict blood pressure control. J Neurosurg 2007;106:250–254. [DOI] [PubMed] [Google Scholar]
- 22.Lyden PD, Shuaib A, Lees KR, et al. Safety and tolerability of NXY-059 for acute intracerebral hemorrhage: the CHANT trial. Stroke 2007;38:2262–2269. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann EL. Nonparametrics: Statistical Methods Based on Ranks, Revised. Upper Saddle River: Prentice Hall;1998. [Google Scholar]
- 24.McNutt L-A, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 2003;157:940–943. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Sandercock P, Pan H, et al. Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40 000 randomized patients from the Chinese Acute Stroke Trial and the International Stroke Trial. Stroke 2000;31:1240–1249. [DOI] [PubMed] [Google Scholar]


