Abstract
Background:
Leukoaraiosis (LA) is closely associated with aging, a major determinant of clinical outcome after ischemic stroke. In this study we sought to identify whether LA, independent of advancing age, affects outcome after acute ischemic stroke.
Methods:
LA volume was quantified in 240 patients with ischemic stroke and MRI within 24 hours of symptom onset. We explored the relationship between LA volume at admission and clinical outcome at 6 months, as assessed by the modified Rankin Scale (mRS). An ordinal logistic regression model was developed to analyze the independent effect of LA volume on clinical outcome.
Results:
Bivariate analyses showed a significant correlation between LA volume and mRS at 6 months (r = 0.19, p = 0.003). Mean mRS was 1.7 ± 1.8 among those in the lowest (≤1.2 mL) and 2.5 ± 1.9 in the highest (>9.9 mL) quartiles of LA volume (p = 0.01). The unfavorable prognostic effect of LA volume on clinical outcome was retained in the multivariable model (p = 0.002), which included age, gender, stroke risk factors (hypertension, diabetes mellitus, atrial fibrillation), previous history of brain infarction, admission plasma glucose level, admission NIH Stroke Scale score, IV rtPA treatment, and acute infarct volume on MRI as covariates.
Conclusions:
The volume of leukoaraiosis is a predictor of clinical outcome after ischemic stroke and this relationship persists after adjustment for important prognostic factors including age, initial stroke severity, and infarct volume.
GLOSSARY
- CCS
= Causative Classification of Stroke;
- CE
= cardioaortic embolism;
- CI
= confidence interval;
- DWI
= diffusion-weighted imaging;
- FLAIR
= fluid-attenuated inversion recovery;
- IQR
= interquartile range;
- LA
= leukoaraiosis;
- LAA
= large artery atherosclerosis;
- mRS
= modified Rankin Scale;
- NIHSS
= National Institute of Health Stroke Scale;
- OR
= odds ratio;
- rtPA
= recombinant tissue plasminogen activator;
- SAO
= small artery occlusion;
- STOPStroke
= Screening Technology and Outcome Project in Stroke Study;
- UND/UNC
= undetermined/unclassified.
Stroke is a leading cause of major disability worldwide. Only a third of those who survive an ischemic stroke recover with little or no deficit; the majority remain moderately or severely disabled for life.1 Advancing age is not only a risk factor for developing a stroke, it is also a potent predictor of poor clinical outcome after a stroke occurs.2 Following the dramatic increase in life expectancy in the prior century and the widespread rise in the elderly population, poststroke care has become a major social and economic burden.3 This has led to intense efforts to identify outcome predictors that are amenable to treatment. Although age is a potent predictor of outcome, it is clearly a nonmodifiable characteristic. However, it has been postulated that age may act as a surrogate marker for unknown underlying mechanisms.
Leukoaraiosis (LA), a disease of the cerebral small vessels, is strongly associated with age4 and may be partially responsible for the association between age and stroke outcome. LA is common in the elderly, affecting up to 70% of the population with a mean age of 65.5 LA is an independent predictor of risk of symptomatic stroke,6 hemorrhagic transformation after thrombolysis for ischemic stroke,7 stroke recurrence,8 and poststroke dementia.9 In addition, both age10 and LA11 predict cerebral infarct growth early after stroke. Given the strong correlation between advancing age and increasing volume of LA, we hypothesized that LA may be one potential mechanism underlying the role of age in stroke susceptibility and outcome. Therefore, in the current study we sought to determine whether LA mediates the effect of advancing age on poor clinical outcome after acute ischemic stroke.
METHODS
Study population.
We retrospectively analyzed the clinical and imaging data collected at a single center, as part of a prospective multicenter study evaluating the utility of new CT-based neuroimaging technology to improve prediction of stroke subtype and outcome (Screening Technology and Outcome Project in Stroke [STOPStroke] Study). The STOPStroke Study enrolled consecutive patients who were evaluated by multimodal CT (noncontrast CT, CT angiography, CT perfusion) within 24 hours of symptom onset for symptoms consistent with an acute ischemic stroke. Clinical outcome status was determined using the modified Rankin Scale (mRS) obtained by telephone interview at 6 months. The study started in March 2003 and follow-up was completed in July 2006. For the present analysis, patients were eligible if 1) they had a brain MRI with diffusion-weighted imaging (DWI) performed within the first 24 hours of symptom onset confirming a clinically relevant infarct and 2) they did not receive investigational treatments. Patients with prestroke disability (mRS ≥2) were excluded. All patients underwent a standardized evaluation of rehabilitation needs by the same team of physical, occupational, and speech therapists, and were given an individualized rehabilitation treatment plan before discharge. The local institutional review board approved all aspects of the study.
Data collection.
Admission National Institute of Health Stroke Scale (NIHSS) score, prestroke disability, and clinical outcome at 6 months as defined by mRS were collected prospectively. Clinical characteristics including time of stroke onset (defined as last time the patient was known to be well), time to radiographic examinations, and demographic and medical information were ascertained for each patient through retrospective chart review. We classified stroke etiology using the Causative Classification of Stroke (CCS) system.12
Image acquisition and analysis.
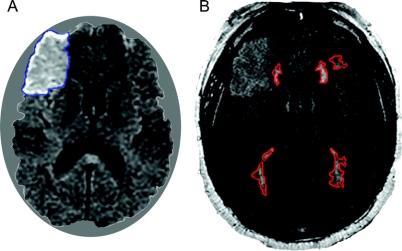
MRI was performed using 1.5-T scanners (GE Signa, GE Medical Systems, Milwaukee, WI; or Siemens Sonata, Siemens Medical Solutions, Erlangen, Germany). The image acquisition parameters were summarized in detail elsewhere.11 We used MRIcro software (University of Nottingham, UK) to create region of interest maps of supratentorial LA by signal intensity thresholding followed by manual editing as necessary.11,13 LA was measured from fluid-attenuated inversion recovery (FLAIR) images obtained ≤24 hours from stroke onset in order to minimize obscuring of the boundaries of LA by signal changes secondary to the acute ischemic lesion (figure 1). In patients with prominent signal increase on FLAIR, we used DWI for visual guidance to distinguish the borders of ischemic lesion and LA. LA was identified as any white matter hyperintensity within the region starting at the lateral ventricular border and extending up to the cortico-medullary junction on acute FLAIR images.14 Hyperintense lesions involving the convolutional white matter, U-fibers, corpus callosum, internal capsule, and anterior commissure were not regarded as LA.15 Also excluded from LA outlines were chronic infarcts that clearly corresponded to a vascular territory according to previously published templates.16 In order to normalize LA volumes to head size, we used mid-sagittal cross-sectional intracranial area (ICA) as a surrogate measure of the intracranial volume.13,17 In addition to LA volume, we manually outlined acute ischemic lesions on admission DWI and final infarcts on follow-up FLAIR or CT when available. Follow-up images were obtained >14 days after stroke onset in order to minimize the confounding effect of brain edema and hemorrhagic conversion on final infarct volume measurements. The calculated acute and final infarct volumes were then normalized with respect to ICA. The intraclass correlation coefficient for the method used for lesion outlining was reported to be 0.9813 for LA, 0.9717 for ICA, 0.9918 for DWI, and 0.9918 for final infarct volume outlines. All MRI measurements were performed by readers blinded to clinical data, including clinical outcome.
Figure 1 Example of infarct and leukoaraiosis (LA) volume assessment
Diffusion-weighted imaging (DWI) (A) obtained at 18 hours after symptom onset shows an acute ischemic lesion consistent with right middle cerebral artery branch occlusion. The signal in regions corresponding to the DWI lesion appears to be increased on fluid-attenuated inversion recovery images as well (B), acquired at the same session with DWI. Despite partial obscuration of the boundaries of LA by the ischemic lesion signal, it is still possible to distinguish them by differences in signal intensities and with guidance by DWI.
Statistical analysis.
All numerical variables are expressed as mean ± SD or median (interquartile range [IQR]). Mann-Whitney U and Kruskal-Wallis tests were used to explore the relationship between categorical variables and mRS at 6 months. Spearman correlation was used to analyze the relationship between continuous variables and mRS at 6 months.
We used ordinal logistic regression analysis to test for independent predictors of mRS at 6 months. Independent variables for this model were chosen using a conservative threshold of p < 0.15 at the bivariate analysis stage. As stroke mortality (mRS = 6) is associated with a wide array of factors not necessarily related to the acute brain pathology,19 we first developed a model to test relationships with clinical outcome in patients who were alive at the time of follow-up (model A, dependent variable: mRS 0–5). We then constructed a second ordinal logistic regression model that also included patients who were dead at 6 months poststroke (model B, dependent variable: mRS 0–6). Patients with mRS of 5 and 6 were collapsed into a single group in order to meet the assumption of proportional odds for ordinal logistic regression analysis in model B. The final regression model (model C, dependent variable: mRS 0–6) was an exploratory step toward elucidating if the mechanism of LA volume–clinical outcome relationship was independent of the effect of LA volume on lesion growth during the acute period.11 Due to the small sample size (n = 57), model C included only LA volume and final infarct volume as independent variables. A two-tailed p value of ≤0.05 was considered significant. All statistical analyses were performed using SPSS 11.5.
RESULTS
The STOPStroke Study enrolled a total of 496 patients during the study period. We excluded patients who did not have brain MRI within the first 24 hours (138 patients) and who were lost to follow-up (62 patients). The reasons for inability to obtain MRI included late scanning in 80 (>24 hours of onset), contraindications in 43 (metal implants/foreign body, cardiac pacemaker, excessive body weight), MRI not deemed to be necessary by the treating physician in 10, and MRI performed at an outside center in 5 patients. We further excluded a total of 56 patients because of prestroke mRS ≥2 (22 patients), the use of investigational treatment (22 patients), and motion artifact on MRI precluding determination of LA volume (12 patients). The remaining 240 patients comprised the final study population.
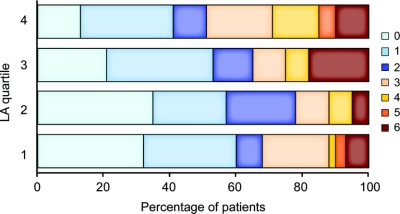
The median time from symptom onset to MRI was 7.5 hours (IQR, 4.7–12.7 hours). LA volume ranged between 0.1 and 57.4 mL, with a median of 3.2 mL (IQR, 1.2–9.9 mL). There was no significant difference between median LA volumes in cerebral hemispheres ipsilateral and contralateral to the site of the acute ischemic lesion (1.7 mL vs 1.5 mL, p = 0.774). In the bivariate analysis, patients with higher LA volumes at the time of their stroke had more severe functional deficits at 6 months poststroke (r = 0.19, p = 0.003) (table 1, figure 2). Mean mRS was 1.7 ± 1.8 among those in the lowest (≤1.2 mL) and 2.5 ± 1.9 in the highest (>9.9 mL) quartiles of LA volume (p = 0.01, n = 60 for each quartile). More patients with LA volume in the highest quartile were discharged to a rehabilitation facility as compared with other quartiles (42% each in the first three quartiles and 65% in the fourth quartile, p = 0.020).
Table 1 Bivariate relationships between clinical and imaging characteristics and 6-month modified Rankin Scale score
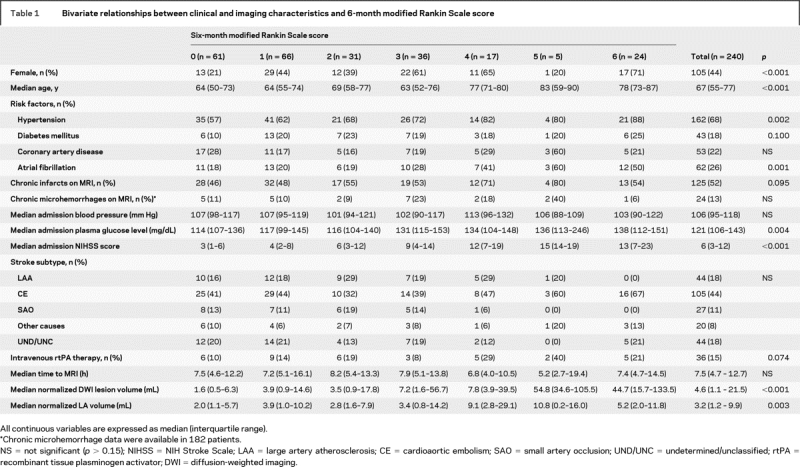
Figure 2 Ranges of modified Rankin Scale are shown for each leukoaraiosis (LA) volume quartile (n = 60)
The results are not adjusted for potential covariates.
All multivariable models fulfilled the proportional odds assumption and were significant at a p value of <0.001 (model A and model B) and 0.003 (model C). There was no collinearity between the covariates. There was an independent effect of LA on clinical outcome in model A (dependent variable: mRS 0–5), with an adjusted odds ratio (OR) of 1.05 (95% confidence interval [CI], 1.02–1.08) for mRS with every 1 mL increase in LA volume (table 2). Additional independent predictors of 6-month mRS were female gender, admission NIHSS score, and admission DWI lesion volume. Age was not an independent predictor in this model. Age, however, became a significant predictor of clinical outcome when LA volume was not included as a covariate in model A. In model B (dependent variable: mRS 0–6), all independent predictors in model A, including LA volume, remained significant. In addition, age was also an independent predictor of clinical outcome in this model. The effect of age (by deciles) on mRS decreased from an OR of 1.31 (95% CI, 1.06–1.62) to 1.17 (95% CI, 0.94–1.47) in model A and from 1.38 (95% CI, 1.13–1.69) to 1.29 (95% CI, 1.04–1.60) in model B, when LA volume was accounted for, suggesting that LA was responsible for a portion of the effect of age on mRS. The results were similar when both models were repeated using contralateral hemisphere LA volume as a covariate.
Table 2 Independent predictors of 6-month modified Rankin Scale (mRS) score
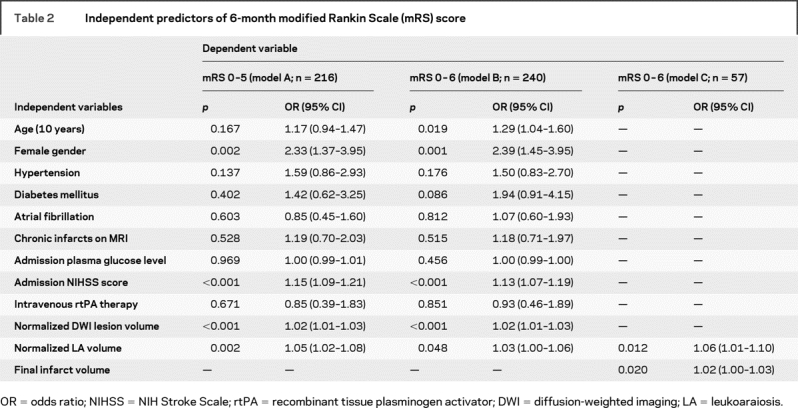
There were 57 patients who had follow-up imaging performed. The median time to follow-up imaging was 91 days (IQR, 47–159 days). The median LA volume and 6-month mRS were not significantly different between the 57 patients with (LA volume = 2.2 mL, mRS = 2) and 183 patients without follow-up imaging (LA volume = 3.8 mL, mRS = 1). The median final infarct volume was 4.7 mL (IQR, 0.6 mL–17.3 mL). The final infarct volume correlated with mRS at 6 months (r = 0.35, p = 0.007). In model C, both LA volume and final infarct volume were independent predictors of 6-month mRS, suggesting that the influence of LA on clinical outcome cannot be only explained by a possible effect on infarct growth (table 2).
DISCUSSION
The results of this study demonstrate that LA influences clinical outcome after ischemic stroke and that this effect appears to be independent of the volume of brain tissue that is irreversibly injured by the acute insult. Our findings also suggest that LA volume may mediate at least a portion of the influence of age on stroke outcome as the effect of age increases when LA is removed as a predictor of clinical outcome.
Despite the fact that LA has consistently been shown to predict development of poststroke dementia,9 prior studies of LA and stroke outcome have been inconclusive; a direct effect of LA on stroke morbidity and mortality has been demonstrated in some20,21 but not other7,22 studies. These discrepancies, in part, resulted from the use of visual scales to quantify LA burden instead of precise volumetric assessment. Prior studies also varied in the scales used to measure stroke outcome. Furthermore, LA burden was often measured using CT rather than MRI, which is far less sensitive to its detection,23 and poses particular challenges in assessing its severity. The present study quantitatively measured LA volume on MRI as opposed to employing a visual rating scale. Our findings demonstrate a small but significant shift in mRS toward less favorable outcomes with increasing LA volume. The relationship between LA volume and outcome persisted after adjustment for a comprehensive list of previously validated predictors of poststroke clinical outcome including age, initial stroke severity, and infarct volume. The small correlation between LA volume and clinical outcome (r = 0.19) probably reflects the fact that individual risk factors in multifactorial conditions such as stroke outcome can only account for a small proportion of the overall variance in the risk. Nevertheless, the highest quartile of LA volume was associated with a 1.5-fold increase in mRS as compared with the lowest quartile. The absolute mean difference in mRS between the lowest and highest LA quartiles was 0.8. This is a robust difference and compares well with the data from several recent therapeutic stroke trials.24,25 For instance, in the National Institute of Neurological Disorders and Stroke tissue plasminogen activator trial, the mean mRS was 2.66 in the treatment and 3.19 in the placebo groups, revealing an absolute difference of only 0.53.26
The relationship between LA and clinical outcome could involve multiple mechanisms. It is well known that LA regions have reduced vascular density27 and cerebral blood flow,28 which may lead to infarct growth in the acute setting by preventing peripheral compensation during ischemic stress.29 Alternately, the presence of dysfunctional neuronal networks in patients with LA may be partially responsible for the association between high LA burden and unfavorable outcome. An intact system of intrahemispheric and interhemispheric connectivity is essential for favorable functional recovery following stroke.30 Neuropathologic and functional neuroimaging studies have shown the presence of decreased neuronal connectivity in patients with LA,31,32 which is most likely due to the demyelination, loss of axons and oligodendrocytes, and astrocytic gliosis.15 This may result in decreased functional connectivity of distant cortical regions,33 which could impair plasticity and inhibit recovery. This mechanism is supported by the observations that LA is associated with poststroke dementia9 and that subcortical lesions can reduce effective interhemispheric interactions in a manner that correlates with a patient’s level of motor impairment.30 Finally, LA is a well-known risk factor for developing poststroke cognitive impairment and depression, which may ultimately adversely affect patients’ compliance with treatment and recovery programs.9,34 Further research using objective measures of connectivity, infarct evolution, and baseline cognitive functioning is needed to elucidate the exact mechanism of the relationship between LA and clinical outcome.
Age is one of the most consistent clinical variables associated with clinical outcome in the literature; however, most published studies supporting the role of age on stroke outcome included mortality as part of the outcome measure.2,35 Our findings demonstrate that the effect of age on outcome is of varying degrees and is dependent on the inclusion of mortality as an outcome measure. The deleterious effect of age on clinical outcome appears to be mediated in part by LA volume; age is not an independent predictor of clinical outcome when LA volume is accounted for. In contrast, age is a strong predictor of outcome regardless of LA volume if the outcome measure includes mortality. There may be multiple reasons for this finding. First, the strong correlation between LA volume and age might have decreased the statistical power of the regression models. An alternative explanation could be that age has a differential influence on recovery vs mortality following stroke, especially given the fact that a significant portion of deaths in patients with stroke are not attributed to the stroke but to age-related comorbid conditions.19
The present study has a number of strengths and limitations. The strengths include a relatively large sample size, blind assessment of DWI and LA volumes with respect to clinical outcome, volumetric assessment of LA burden, rigorous adjustment for potential confounders, and the use of mRS as an ordinal variable to prevent the loss of information that occurs during dichotomization.36
An important limitation of our study is its observational prospective nature that resulted in several inevitable dropouts; patients did not consent or could not be scanned because of MRI contraindications or were not available for follow-up assessment. The consent and follow-up bias might have resulted in a systematic error toward selection of a more favorable outcome population37,38; the present cohort was considerably skewed toward milder strokes at baseline (median admission NIHSS score: 6) and more favorable clinical outcome at 6 months (median mRS: 1) as compared with other published consecutive stroke cohorts.1 Nevertheless, because LA volume correlates with stroke severity, our inability to include severe strokes likely resulted in an underestimation of the observed magnitude of relationship between LA volume and clinical outcome. The current study was restricted to patients who had DWI and FLAIR obtained within 24 hours of symptom onset. As a result, patients with severe strokes who were not clinically stable enough for an early MRI, patients with mild stroke with delayed hospital admission, patients with grave short-term prognosis, and patients with contraindications for MRI were missed. However, neither baseline stroke severity nor age, historically the two strongest predictors of stroke outcome, differed among patients with or without admission MRI, arguing against a significant bias in patient selection.
It has long been known that advanced age hinders recovery from acute ischemic stroke. While it is generally assumed that the mechanisms underlying the effect of advanced age on stroke recovery are associated with age-related comorbidities such as osteoporosis, osteoarthritis, and coronary artery disease, it is clear that changes within the brain itself contribute substantially to the brain’s response to injury. LA is emerging as a potent manifestation of the cerebrovascular aging process and a marker of risk for stroke as well as age-related cognitive and gait deterioration.9,39 Our data suggest that the conditions that manifest as LA also contribute to the brain’s recovery from stroke and, because of their relationship to the aging process, underlie a portion of the effect of age on stroke outcome. These observations suggest that therapies that slow the progression of LA hold promise not only for reducing the incidence of stroke, but also reducing stroke severity and improving clinical outcome when it occurs. Indeed, as LA volume appears to be determined only partially by the same vascular risk factors that predispose to symptomatic stroke, such therapies could well involve novel mechanisms currently not exploited in stroke prevention. Given the prevalence of LA among our aging population, such therapies may transform the aging process for future generations.
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by Dr. Arsava, AA Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Address correspondence and reprint requests to Dr. Hakan Ay, AA Martinos Center for Biomedical Imaging and Stroke Service, Departments of Neurology and Radiology, Massachusetts General Hospital, Harvard Medical School, 149 13th Street, Room 2301, Charlestown, MA 02129 hay@partners.org
*These authors contributed equally and share first authorship.
Supported by NIH grants R01-NS059727 (J.R.), R01-NS051412 and P50-NS051343 (A.B.S.), P50-NS051343 (M.H.L.), R01-HS011392 and P50-NS051343 (K.L.F.), and R01-NS038477 (A.G.S.).
Disclosure: M.H.L.: GE Medical Services—research support, advisory board, and speaker fees; Bracco Diagnostics—advisory board and speaker fees; Coaxia—advisory board; K.L.F.: consultant for GE Healthcare; A.G.S.: full disclosures are listed in http://www.biomarkers.org/NewFiles/disclosures.html.
Received October 6, 2008. Accepted in final form January 20, 2009.
REFERENCES
- 1.Slot KB, Berge E, Dorman P, Lewis S, Dennis M, Sandercock P. Oxfordshire Community Stroke Project, the International Stroke Trial (UK): Lothian Stroke Register: Impact of functional status at six months on long term survival in patients with ischaemic stroke: prospective cohort studies. BMJ 2008;336:376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weimar C, König IR, Kraywinkel K, Ziegler A, Diener HC. German Stroke Study Collaboration: Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke 2004;35:158–162. [DOI] [PubMed] [Google Scholar]
- 3.Dombovy ML, Sandok BA, Basford JR. Rehabilitation for stroke: a review. Stroke 1986;17:363–369. [DOI] [PubMed] [Google Scholar]
- 4.Basile AM, Pantoni L, Pracucci G, et al., LADIS Study Group. Age, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes: The LADIS (Leukoaraiosis and Disability in the Elderly) Study. Cerebrovasc Dis 2006;21:315–322. [DOI] [PubMed] [Google Scholar]
- 5.Turner ST, Jack CR, Fornage M, Mosley TH, Boerwinkle E, de Andrade M. Heritability of leukoaraiosis in hypertensive sibships. Hypertension 2004;43:483–487. [DOI] [PubMed] [Google Scholar]
- 6.Kuller LH, Longstreth WT Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ Jr. Cardiovascular Health Study Collaborative Research Group. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke 2004;35:1821–1825. [DOI] [PubMed] [Google Scholar]
- 7.Palumbo V, Boulanger JM, Hill MD, Inzitari D, Buchan AM, CASES Investigators. Leukoaraiosis and intracerebral hemorrhage after thrombolysis in acute stroke. Neurology 2007;68:1020–1024. [DOI] [PubMed] [Google Scholar]
- 8.Hénon H, Vroylandt P, Durieu I, Pasquier F, Leys D. Leukoaraiosis more than dementia is a predictor of stroke recurrence. Stroke 2003;34:2935–2940. [DOI] [PubMed] [Google Scholar]
- 9.De Groot JC, De Leeuw FE, Oudkerk M, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 2002;52:335–341. [DOI] [PubMed] [Google Scholar]
- 10.Ay H, Koroshetz WJ, Vangel M, et al. Conversion of ischemic brain tissue into infarction increases with age. Stroke 2005;36:2632–2636. [DOI] [PubMed] [Google Scholar]
- 11.Ay H, Arsava EM, Rosand J, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke 2008;39:1409–1413. [DOI] [PubMed] [Google Scholar]
- 12.Ay H, Benner T, Arsava EM, et al. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke 2007;38:2979–2984. [DOI] [PubMed] [Google Scholar]
- 13.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology 2006;66:23–29. [DOI] [PubMed] [Google Scholar]
- 14.Fazekas F, Barkhof F, Wahlund LO, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis 2002;13 suppl 2:31–36. [DOI] [PubMed] [Google Scholar]
- 15.Fisher CM. Binswanger’s encephalopathy: a review. J Neurol 1989;236:65–79. [DOI] [PubMed] [Google Scholar]
- 16.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: cerebral hemispheres. Neurology 1998;50:1699–1708. [DOI] [PubMed] [Google Scholar]
- 17.Nandigam RN, Chen YW, Gurol ME, Rosand J, Greenberg SM, Smith EE. Validation of intracranial area as a surrogate measure of intracranial volume when using clinical MRI. J Neuroimaging 2007;17:74–77. [DOI] [PubMed] [Google Scholar]
- 18.Ay H, Arsava EM, Vangel M, et al. Interexaminer difference in infarct volume measurements on MRI: a source of variance in stroke research. Stroke 2008;39:1171–1176. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann A, Rundek T, Mast H, et al. Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study. Neurology 2001;57:2000–2005. [DOI] [PubMed] [Google Scholar]
- 20.Streifler JY, Eliasziw M, Benavente OR, et al., North American Symptomatic Carotid Endarterectomy Trial Group. Prognostic importance of leukoaraiosis in patients with symptomatic internal carotid artery stenosis. Stroke 2002;33:1651–1655. [DOI] [PubMed] [Google Scholar]
- 21.Leys D, Englund E, Del Ser T, et al. White matter changes in stroke patients: relationship with stroke subtype and outcome. Eur Neurol 1999;42:67–75. [DOI] [PubMed] [Google Scholar]
- 22.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Leukoaraiosis in stroke patients: The Copenhagen Stroke Study. Stroke 1995;26:588–592. [DOI] [PubMed] [Google Scholar]
- 23.Wahlund LO, Barkhof F, Fazekas F, et al., European Task Force on Age-Related White Matter Changes. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001;32:1318–1322. [DOI] [PubMed] [Google Scholar]
- 24.Saver JL. Number needed to treat estimates incorporating effects over the entire range of clinical outcomes: novel derivation method and application to thrombolytic therapy for acute stroke. Arch Neurol 2004;61:1066–1070. [DOI] [PubMed] [Google Scholar]
- 25.Abciximab Emergent Stroke Treatment Trial (AbESTT) Investigators. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of a randomized phase 2 trial. Stroke 2005;36:880–890. [DOI] [PubMed] [Google Scholar]
- 26.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 27.Moody DM, Thore CR, Anstrom JA, Challa VR, Langefeld CD, Brown WR. Quantification of afferent vessels shows reduced brain vascular density in subjects with leukoaraiosis. Radiology 2004;233:883–890. [DOI] [PubMed] [Google Scholar]
- 28.O’Sullivan M, Lythgoe DJ, Pereira AC, et al. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology 2002;59:321–326. [DOI] [PubMed] [Google Scholar]
- 29.Heistad DD, Mayhan WG, Coyle P, Baumbach GL. Impaired dilatation of cerebral arterioles in chronic hypertension. Blood Vessels 1990;27:258–262. [DOI] [PubMed] [Google Scholar]
- 30.Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol 2008;63:236–246. [DOI] [PubMed] [Google Scholar]
- 31.Yamanouchi H, Sugiura S, Tomonaga M. Decrease in nerve fibres in cerebral white matter in progressive subcortical vascular encephalopathy of Binswanger type: an electron microscopic study. J Neurol 1989;236:382–387. [DOI] [PubMed] [Google Scholar]
- 32.Ishihara M, Kumita S, Hayashi H, Kumazaki T. Loss of interhemispheric connectivity in patients with lacunar infarction reflected by diffusion-weighted MR imaging and single-photon emission CT. AJNR Am J Neuroradiol 1999;20:991–998. [PMC free article] [PubMed] [Google Scholar]
- 33.Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci 2006;18:418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinyor D, Amato P, Kaloupek DG, Becker R, Goldenberg M, Coopersmith H. Post-stroke depression: relationships to functional impairment, coping strategies, and rehabilitation outcome. Stroke 1986;17:1102–1107. [DOI] [PubMed] [Google Scholar]
- 35.Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999;53:126–131. [DOI] [PubMed] [Google Scholar]
- 36.Bath PM, Gray LJ, Collier T, Pocock S, Carpenter J, Optimising Analysis of Stroke Trials (OAST) Collaboration. Can we improve the statistical analysis of stroke trials? Statistical reanalysis of functional outcomes in stroke trials. Stroke 2007;38:1911–1915. [DOI] [PubMed] [Google Scholar]
- 37.Buckley B, Murphy AW, Byrne M, Glynn L. Selection bias resulting from the requirement for prior consent in observational research: a community cohort of people with ischaemic heart disease. Heart 2007;93:1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kempen GI, van Sonderen E. Psychological attributes and changes in disability among low-functioning older persons: does attrition affect the outcomes? J Clin Epidemiol 2002;55:224–229. [DOI] [PubMed] [Google Scholar]
- 39.Briley DP, Wasay M, Sergent S, Thomas S. Cerebral white matter changes (leukoaraiosis), stroke, and gait disturbance. J Am Geriatr Soc 1997;45:1434–1438. [DOI] [PubMed] [Google Scholar]