Abstract
Objective:
Tourette syndrome (TS) is a common neurodevelopmental disorder marked by tics and behavioral comorbidities. Clinical pharmacology suggests that dopaminergic signaling abnormalities are part of the pathophysiology of TS. Prior molecular imaging studies of nigrostriatal dopaminergic terminal markers report conflicting results. Our goal was to characterize the distribution of nigrostriatal dopaminergic terminals in subjects with TS.
Methods:
Thirty-three adult subjects with TS were studied with PET using [11C]dihydrotetrabenazine (DTBZ), a ligand for the type 2 vesicular monoamine transporter, and with [11C] methylphenidate (MP), a ligand for the plasmalemmal dopamine transporter. Subjects were characterized with standard rating instruments for tic severity, obsessive-compulsive behaviors, and attentional deficits.
Results:
We found no differences between subjects with TS and control subjects in DTBZ and MP binding in any striatal region. There was no correlation between binding measures and clinical variables. Ventral striatal DTBZ and MP binding distributions in subjects with TS were normal.
Conclusions:
We found no evidence of increased striatal dopaminergic innervation in Tourette syndrome (TS). Discrepancy between our present results and those of other studies may be explained by heterogeneity of TS.
GLOSSARY
- BP
= binding potential;
- CAARS-S:S
= Conners Adult ADHD Rating Scale-Self Report: Short version;
- DAT
= dopamine transporter;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- DTBZ
= [11C]dihydrotetrabenazine;
- DVR
= ratio of volumes of distribution;
- FDR
= false discovery rate;
- MP
= [11C]methylphenidate;
- OCBs
= obsessive-compulsive behaviors;
- TS
= Tourette syndrome;
- VOI
= volume of interest;
- YBOCS
= Yale-Brown Obsessive Compulsive Scale;
- YGTSS
= Yale Global Tic Severity Scale.
Tourette syndrome (TS) is a common disorder marked by the presence of tics (fluctuating repetitive involuntary movements).1–3 TS has a heritable polygenic component.4,5 TS has a distinctive natural history with onset of tics in childhood, common exacerbation of tics before and around the onset of puberty, and frequent remission or moderation of tics as patients with TS enter adulthood. This natural history suggests a disorder of brain development. TS commonly is accompanied by obsessive-compulsive disorder and other psychiatric comorbidities.
Tics are ameliorated by treatment with dopamine D2 receptor antagonists, leading to speculation that dopaminergic signaling mechanisms are involved in the pathophysiology of tics. This clinical pharmacology and other data suggest that TS is a basal ganglia disorder.1 Direct evidence for this inference is modest. TS is not fatal and only a small amount of postmortem material has been analyzed, without definitive conclusions.6,7 PET and SPECT with tracers binding to dopaminergic terminal markers have been employed to search for evidence of striatal dopaminergic abnormalities in TS. These studies return conflicting results.8,9
Our prior studies of striatal [11C]dihydrotetrabenazine (DTBZ) binding, a ligand for the type 2 vesicular monoamine transporter (VMAT2), indicated increased ventral but not dorsal striatal dopaminergic innervation.10,11 Most PET and SPECT imaging studies have been marked by small subject numbers, varying subject populations, use of tracers whose targets may undergo physiologic or pharmacologic regulation of expression, and inclusion of subjects receiving dopaminergic antagonists. In an effort to overcome these deficiencies, we report the largest study to date of striatal dopaminergic terminal markers in TS. We utilized both [11C]DTBZ and [11C]methylphenidate (MP), a ligand for the dopamine transporter (DAT), and studied well-characterized subjects not treated with dopamine antagonists.
METHODS
Subjects.
We recruited 33 adult individuals (≥18 years) with TS (table 1). All subjects met DSM-IV criteria for TS with the severity criterion relaxed. Subjects taking dopamine antagonist or stimulant preparations within the 6 months before study were excluded. Almost all subjects with TS had not used dopamine antagonists for years or never used dopamine antagonists. There are limited data on the long-term effects of dopamine antagonist treatment. A prior PET study indicated that dopamine D2 receptor occupancy normalizes within weeks of cessation of oral dopamine antagonists.12 Clinical experience with drug-induced parkinsonism indicates persistent pharmacodynamic effects of dopamine antagonists lasting as long as a year.13 Current use of α-adrenergic agonists (clonidine, guanfacine) or serotonin selective reuptake inhibitors was acceptable. Exclusion criteria included presence of another primary neurologic disorder. Control subjects recruited were without neurologic or psychiatric disease. Control subjects were not rated but questioned for clinical histories of tics or comorbid psychiatric disorders. We studied 28 age-comparable controls (mean age = 36 years; SD = 13 years; 14 men, 14 women). All study procedures were approved by the Institutional Review Board at the University of Michigan School of Medicine. Informed consent was obtained from all subjects.
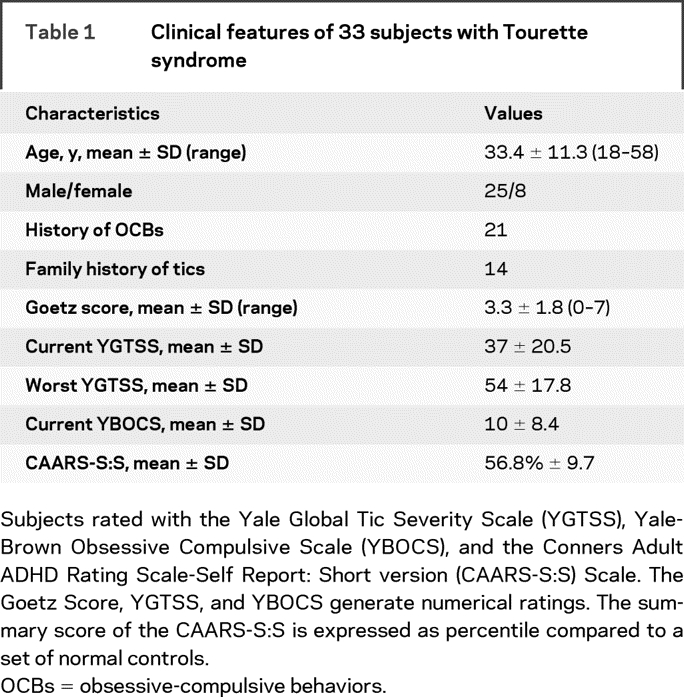
Table 1 Clinical features of 33 subjects with Tourette syndrome
Clinical ratings.
All subjects with TS were evaluated on the study day with a standard general medical and neurologic examination to exclude confounding medical or neurologic disease and administration of standard rating scales. We used the Yale Global Tic Severity Scale (YGTSS), the Yale-Brown Obsessive-Compulsive Scale (YBOCS), and a self-administered attention scale (the Conners Adult ADHD Rating Scale-Self Report: Short version [CAARS-S:S]). Current tic score was assigned with the rating scale of Goetz et al.14 based on observation during the interview and examination. All subjects were evaluated by one experienced rater (R.L.A.). Four subjects with TS participated in our prior study but no data from that study were used.11
[11C]DTBZ and [11C]MP PET imaging.
The [11C]methylphenidate ([11C]MP) PET studies were acquired as 17 scan frames over a total of 80 minutes as follows: four × 30 seconds; three × 1 minute; two × 2.5 minutes; two × 5 minutes; and six × 10 minutes. Radiotracer was administered as a bolus plus constant infusion using 60% as a slow bolus over 30 seconds, followed by constant infusion of the remaining 40% over the 80 minutes study duration. [11C]DTBZ PET studies were performed identically except for the following: 15 frames were acquired over 60 minutes, omitting the last two 10-minute frames, and the bolus:infusion schedule was 55% as a bolus over 30 seconds, followed by infusion of the remaining 45% over 60 minutes. PET studies were acquired in three-dimensional mode with interplane septa retracted on a Siemens ECAT HR+ scanner (Siemens Medical Solutions USA, Malvern, PA). Two-dimensional transmission scans were acquired for measured attenuation correction followed by segmentation and reprojection. Standard corrections were made for dead-time, randoms, radioactive decay, scatter, and attenuation. Image reconstruction consisted of Fourier rebinning (FORE) of three-dimensional data into two-dimensional projections, followed by 2D-OSEM (ordered subsets expectation-maximization); 4 iterations, 16 subsets. No smoothing filters were used during or post reconstruction. Resultant images had both in-plane and axial resolution of approximately 5.0–5.5 mm full-width at half-maximum.
Parametric imaging.
Standard parametric image calculations were performed using equilibrium analysis.15 This analysis is based on a reference region assumed to be devoid of specific binding to normalize radiotracer binding measures, yielding estimates of the ratio of volumes of distribution (DVR) between any target region (voxel) and the reference region. The binding potential, BP, is DVR-1, effectively subtracting the nonspecific binding assumed to be the same between target and reference tissues. Occipital cortex was used as the reference region for both tracers. Cerebellum has been used previously as the reference region for MP; however, there is DTBZ-specific binding in cerebellum.16 An estimate of relative transport parameters (K1R; K1 ratio) between target and reference tissues was also calculated.
Estimates of the equilibrium ratio of the tracer’s tissue concentrations in a given voxel (vox) and in the reference region (rr) are used to estimate the BP of the given voxel.
where Cvox(eq) and Crr(eq) are the concentrations of tracer in a given voxel and reference region measured at equilibrium. For [11C]MP, data from 50 to 80 minutes postinjection was used to estimate BP. For [11C]DTBZ, data from 30 to 60 minutes was used for BP. K1R for both tracers were estimated by a simple ratio from the early data postinjection (0 to 4 minutes):
Volume of interest analysis.
After calculation of functional parametric images for each subject, these maps were anatomically reoriented to the anterior commissure–posterior commissure line. Linear scaling was followed by nonlinear deformation to the reference coordinate system defined by the anatomic atlas of Talairach and Tournoux.17–19 These anatomic transformations were determined on the basis of each subject’s K1R parametric images, and were then applied to that subject’s DVR images.
Volumes of interest (VOIs) for subregions of the basal ganglia were extracted from the anatomically standardized parametric images. We previously digitized the anatomic atlas of Talairach and Tournoux and created a full set of brain regions including both cerebral cortical and subcortical areas. Binding values corresponding to VOIs from both left and right hemispheres were extracted from caudate nucleus, anterior putamen, posterior putamen, and ventral striatum for each subject.
We also created a ventral striatal volume based on the voxel-wise analysis from our prior study.11 An irregular VOI of ∼3 cm3 was determined from the peak t values for the statistical t test of TS vs control subjects. This volume was created in the right hemisphere (hemisphere of greatest significance in the prior study) and an equivalent VOI created for the left hemisphere by mirroring the volumes across the sagittal midline.
Statistical analysis.
For the VOI analyses, two-sample Student t tests were used for comparison of TS and NC subjects, using two-tailed tests with unequal variances between groups. Significance values are reported of these tests without correction for multiple comparisons.
For voxel-wise analysis, a generous striatal mask was found based on thresholding the average MP or TBZ data, resulting in a 4,275 voxel analysis. SPM2 (Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm) with 5% false discovery rate (FDR) thresholding was used for all analyses.20 In each case, four datasets were considered: DVR and K1R from the MP data, and DVR and K1R from the TBZ data. For the two-group analysis comparison, the TS and NC groups were compared with a two-sample t test. Average striatum VOI intensity was also computed and analyzed with a two-sample t test. In each case, the result was computed for MP and TBZ separately. The regression analysis on the patients with TS consisted of voxel-wise multiple linear regressions using five covariates: Goetz Tic Rating scores, current YGTSS scores, worst YGTSS scores, YBOCS scores, and CAARS scores. These five regressors were assessed with six F-statistic images (5 individual F-tests, as well as one omnibus F-test), resulting in a total of 24 F images over the four datasets.
To assess whether ventral striatal VOI DTBZ and MP binding was distributed normally, binding data for these VOIs were analyzed with the Kolmogorov-Smirnov test. No correction for multiple comparisons was applied.
RESULTS
Subject characteristics.
The mean age of subjects was 33.4 years (SD, 11.3 years; range, 18–58 years). There were 25 men and 8 women. Two thirds described a history of obsessive thinking or compulsive behavior and approximately half had a family history of tics. The mean Goetz score was 3.3 (SD, 1.8; range, 0–7). The mean YGTSS summary score for current tics was 37 (SD, 21) and mean summary score for worst tics was 54 (SD, 18); p < 0.001, Wilcoxon signed-rank test. The mean YBOCS was 10.2 (SD, 8.4) and the mean CAARS-S:S was 15.2 (SD, 5.1; mean cumulative percentile, 56.8). Subject characteristics are summarized in table 1.
Routine VOIs and ventral striatal VOI analysis.
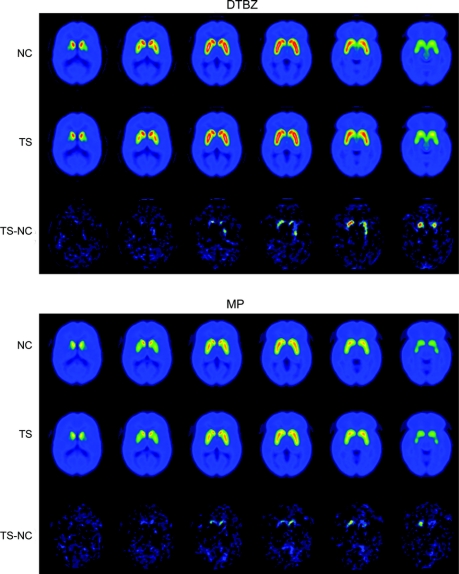
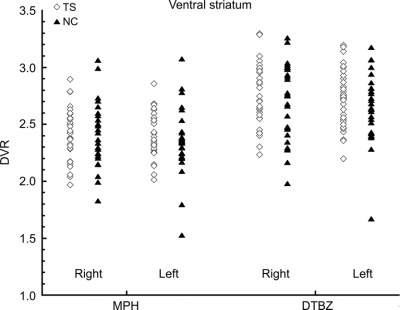
Subtraction of control from TS images suggested increased [11C]DTBZ and [11C]MP binding bilaterally in TS ventral striatum (figure 1). For standard striatal VOIs, however, there were no significant differences between TS and control subjects using standard VOIs (figure 2, table 2). With the ventral striatal VOI derived from our prior study, no significant differences were found between TS and control groups (data not shown).
Figure 1 Average parametric images of [11C]DTBZ and [11C]MP binding in Tourette syndrome (TS) and normal control (NC) subjects
Six brain levels through the striatum for both controls (first and fourth rows) and subjects with TS (second and fifth rows) for DTBZ (top) and MP (bottom). Each slice represents the average DVR of the entire subject group (NC = 28, TS = 33). Subtraction images of TS minus control (third and sixth rows) with modest increases in some voxels in TS ventral striatum.
Figure 2 Scatterplots of [11C]DTBZ and [11C]MP binding in Tourette syndrome and control ventral striatum
DTBZ = [11C]dihydrotetrabenazine; DVR = ratio of volumes of distribution; MP = [11C]methylphenidate.
Table 2 Results of [11C]DTBZ and [11C]MP PET in Tourette syndrome (TS) and normal control (NC) subject striatum
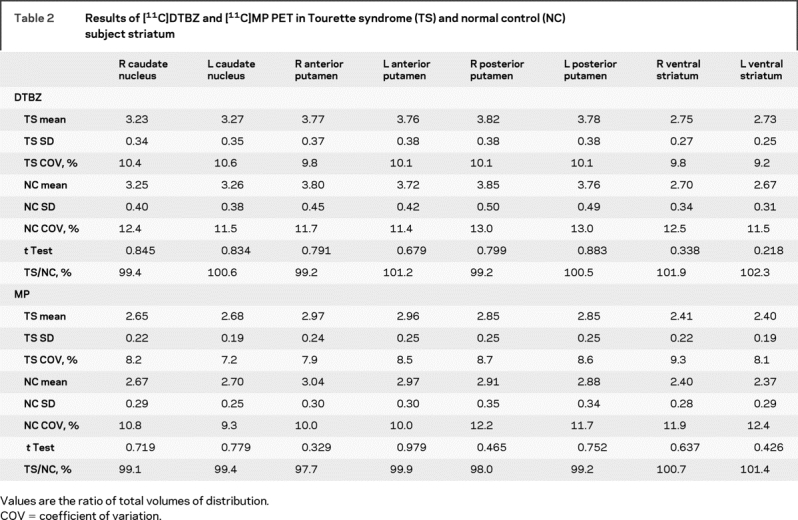
TS and control subjects were comparable in age but not in gender distribution (Pearson χ2 test; p = 0.03). There were no differences between female and male TS subjects in any VOI (data not shown).
Voxel-wise analysis.
The voxel-wise two-group analysis was negative, with no voxels surviving a 5% threshold in any of the four analyses. Likewise, none of the two-group VOI analyses were significant. Of the 24 F images used for regression analysis, only one had any positive voxels (MP K1R had one suprathreshold voxel), though this is roughly consistent with the 1-in-20 false-positive results expected with total noise data. (FDR has weak control of family-wise error, meaning that with a 5% FDR threshold with completely null data, the per-image chance of any false-positive results is 5%.)
To further detect possible subthreshold effects in the regression analysis, an arbitrary α = 0.001 uncorrected threshold was used and the number of suprathreshold voxels were counted. With 4,275 voxels, fraction 4,275 × 0.001 = 4.275 voxels would be expected by chance. Only 2 of the 24 voxel counts exceed the expected count of 4.275 voxels (table e-1 on the Neurology® Web site at www.neurology.org). Owing to the smoothness of the images, p values for these numbers are not available; however, the small number of voxels exceeding the expected count of 4.275 voxels seems consistent with a null hypothesis of no effect. In summary, several voxel-wise tests were completed, both using 5% FDR corrected and 0.1% uncorrected, and in all cases the results were consistent with chance.
Normality of striatal [11C]DTBZ and [11C]MP binding.
Because TS is a syndrome that may result from heterogeneous underlying pathophysiologies, we searched for heterogeneity in ventral striatal TS or control subject DTBZ and MP binding. Analysis of ventral striatal standard VOI data indicated normal distribution of DTBZ and MP binding in these regions with the exception of MP binding in control left ventral striatum (p = 0.042; Kolmogorov-Smirnov test; uncorrected for multiple comparisons).
DISCUSSION
Prior PET or SPECT studies of striatal dopamine terminal markers in TS return conflicting results. Different tracers have been used, including [18F]fluorodopa, PET and SPECT DAT ligands, and the VMAT2 ligand [11C]DTBZ. SPECT imaging studies with DAT ligands have reported diffusely increased or unchanged striatal DAT expression compared with control subjects.21–27 Correlations between striatal DAT binding and some clinical variables have been reported but these findings are not reproducible.22,23,25 One study of untreated children describes diffuse, marked increase in striatal DAT binding.26 A small number of studies report subregional changes in striatal dopamine terminal markers, including increased [18F]fluorodopa uptake within the left caudate and right midbrain.28
Our present data indicate small, nonsignificant differences in dopamine terminal markers in TS striatum. Our prior study, employing comparable methodology, described significantly increased VMAT2 binding within the ventral striatum, on the order of 12–17%.11 Neither dataset shows any change in VMAT2 binding in the dorsal striatum.
Several features distinguish our present study from prior work, including our own prior study. The present study is based on a relatively large subject number and no subjects were receiving treatment with dopamine antagonists or stimulants. Our subjects were all adults (≥18 years), which is true of most other studies, although one [18F]fluorodopa PET study studied 11 untreated adolescents (12–17 years) and a [123I]IPT SPECT study studied untreated children (ages 6–12).26,28 While most symptomatic subjects with TS are children and adolescents, our subject pool has most of the features of TS in typical childhood cases; male predominance, a high percentage of subjects with a family history of tics, and the presence of comorbid obsessive-compulsive behaviors (OCBs). YGTSS ratings revealed that current tic burden is lower than worst-ever (generally during adolescence) tic burden, a result consistent with the known natural history of TS. Despite self-report among our subjects of a high frequency of OCBs, the mean YBOCS score revealed only a mild to moderate burden of OCBs in our subjects. Similarly, the mean CAARS-S:S score was only slightly elevated. It is possible that by excluding subjects treated with dopamine antagonists, we excluded a subgroup with more marked tics and psychiatric comorbidities and more marked changes in striatal dopamine terminal marker binding. The mean Goetz score for this group of subjects with TS was 3.3. This is identical to the mean Goetz score for the subjects with TS in our initial study, despite the fact that approximately one third of those individuals were using a dopamine antagonist.11 The identity of mean Goetz scores between our initial and present study groups suggests that our initial group had greater tic intensity. Our present subjects span a range of individuals with mild to marked tics and there was no evidence of a subgroup with increased striatal dopamine terminal binding. Application of the Kolmogorov-Smirnov test to ventral striatal VOI [11C]DTBZ and [11C]MP binding indicates that binding site density was distributed normally. This is evidence in favor of a relatively uniform population of subjects in this cohort. Reanalysis of ventral striatal VOI DTBZ binding in our original study cohort indicates non-normal distribution of ventral striatal DTBZ binding (data not shown). This finding suggests a heterogeneous group of subjects in our prior study.
Multiple factors might account for apparent discrepancies between various studies. Studies with small subject numbers might be prone to overrepresentation of a TS subtype. Studies to date have used subject populations of varying ages. The natural history of TS indicates some developmentally regulated change in brain function. Differences between studies might reflect also differences in brain development. One SPECT imaging study examined nine untreated children (6–12 years) and described a substantial increase in striatal DAT binding, the largest increase described in any recent study.26 Haycock et al.29 suggested that striatal dopaminergic innervation is developmentally regulated, peaking in preadolescence and declining in the adult years. A difference in the timing or magnitude of the development of the striatal dopaminergic innervation is a plausible component of the pathophysiology of tics. Differences might be present at one age and then disappear with aging and development. Recent analyses suggest as well that TS is a heterogeneous condition with varying underlying pathophysiologies.30
There are other data suggesting abnormal dopamine signaling in TS. A recent study reports enhanced putaminal dopamine release following amphetamine infusion in a small group of subjects with TS.31 This group subsequently reported a larger group of subjects with enhanced ventral striatal dopamine release following amphetamine infusion.32
Clinical data and our understanding of the role of the basal ganglia in natural repetitive movements and habit formation support the hypothesis of a developmental basal ganglia abnormality in TS.1 Direct evidence for this hypothesis is modest. Recent postmortem work suggests a change in the number of striatal interneurons and pallidal projection neurons.8 Like most imaging studies, this work is based on a small N and requires replication. An MRI morphometric study of a substantial number of both pediatric and adult TS and control subjects indicates reduced caudate volume and some changes in cortical volumes.33,34 Longitudinal follow-up of children participating in these studies indicated that reduced caudate volume in childhood predicted persistence and severity of TS and OCBs in early adulthood.34 These studies used observer-specified basal ganglia volumes. Recent, though smaller, morphometric studies using voxel-based morphometry and other methods have returned disparate results, with one study reporting increased midbrain gray matter, another reporting increased ventral putaminal volumes, and another reporting no change in TS basal ganglia volumes.35–37 Another recent MRI study of children with TS (ages 7–18 years) reports widespread changes in cortical thickness.38 Other forebrain regions and neurochemical systems have been implicated in TS.39,40 Our experience with molecular imaging in TS stresses the need for systematic, large studies in well-characterized TS and control subject populations. Large-scale, systematic longitudinal analyses with morphometric and molecular brain imaging in TS are needed to understand the neurobiological bases of TS.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by R.A.K., Department of Radiology; W.Z., Department of Biostatistics; T.N., Department of Biostatistics; and K.A.F., Department of Radiology, University of Michigan.
ACKNOWLEDGMENT
The authors thank the Tourette Syndrome Association for subject recruitment.
Supplementary Material
Address correspondence and reprint requests to Dr. Roger L. Albin, 5023 BSRB, 109 Zina Pitcher Place, Ann Arbor, MI 48109-2200 ralbin@umich.edu
Supplemental data at www.neurology.org
Supported by NS15655, UL1RR024986, and a VA Merit Review Grant (R.L.A.).
Disclosure: The authors report no disclosures.
Received September 18, 2008. Accepted in final form January 13, 2009.
REFERENCES
- 1.Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends Neurosci 2006;29:175–182. [DOI] [PubMed] [Google Scholar]
- 2.Leckman JF. Tourette’s syndrome. Lancet 2002;360:1577–1586. [DOI] [PubMed] [Google Scholar]
- 3.Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain 2000;123:425–462. [DOI] [PubMed] [Google Scholar]
- 4.Walkup J, LaBuda MC, Singer HS, Brown J, Riddle MA, Hurko O. Family study and segregation analysis of Tourette syndrome: evidence for a mixed model of inheritance. Am J Hum Genet 1996;59:684–693. [PMC free article] [PubMed] [Google Scholar]
- 5.Tourette Syndrome Association International Consortium for Genetics. Genome scan for Tourette disorder in affected-sibling-pair and multigenerational families. Am J Hum Genet 2007;80:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swerdlow NR, Young AB. Neuropathology in Tourette syndrome: an update. Adv Neurol 2001;85:151–161. [PubMed] [Google Scholar]
- 7.Kalanithi PSA, Zheng W, Kataoka Y, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. PNAS 2005;102:13307–13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerard E, Peterson BF. Developmental processes and brain imaging studies in Tourette syndrome. J Psychosom Res 2003;55:13–22. [DOI] [PubMed] [Google Scholar]
- 9.Albin RL, Frey KA. Neuroimaging of Tourette syndrome. J Child Neurol 2006;21:672–677. [DOI] [PubMed] [Google Scholar]
- 10.Meyer P, Bohnen NI, Minoshima S, et al. Striatal presynaptic monoaminergic vesicles are not increased in Tourette’s syndrome. Neurology 1999;53:371–374. [DOI] [PubMed] [Google Scholar]
- 11.Albin RL, Koeppe RA, Bohnen NI, et al. Increased ventral striatal monoaminergic innervation in Tourette syndrome. Neurology 2003;61:310–315. [DOI] [PubMed] [Google Scholar]
- 12.Baron JC, Martinot JL, Cambon H, et al. Striatal dopamine receptor occupancy during and following withdrawal from neuroleptic treatment: correlative evaluation by positron emission tomography and plasma prolactin levels. Psychopharmacology 1989;99:463–472. [DOI] [PubMed] [Google Scholar]
- 13.Esper CD, Factor SA. Failure of recognition of drug-induced parkinsonism in the elderly. Mov Disord 2008;23:401–404. [DOI] [PubMed] [Google Scholar]
- 14.Goetz CG, Pappert EJ, Louis ED, Raman R, Leurgans S. Advantages of a modified scoring method for the Rush Video-Based Tic Rating Scale. Mov Disord 1999;14:502–506. [DOI] [PubMed] [Google Scholar]
- 15.Koeppe RA, Frey KA, Kume A, Albin R, Kilbourn MR, Kuhl DE. Equilibrium versus compartmental analysis for assessment of the vesicular monoamine transporter using (+)-alpha-[11C]dihydrotetrabenazine (DTBZ) and positron emission tomography. J Cereb Blood Flow Metab 1997;17:919–931. [DOI] [PubMed] [Google Scholar]
- 16.Koeppe RA, Frey KA, Kuhl DE, Kilbourn MR. Assessment of extrastriatal vesicular monoamine transporter binding site density using stereoisomers of [11C]dihydrotetrabenazine. J Cereb Blood Flow Metab 1999;19:1376–1384. [DOI] [PubMed] [Google Scholar]
- 17.Minoshima S, Koeppe RA, Fessler JA, et al. Integrated and automated data analysis method for neuronal activation studies using O15 water PET. In: Uemura K, Lassen NA, Jones T, Kanno I, eds. Quantification of Brain Function: Tracer Kinetics and Image Analysis in Brain PET, International Congress Series 1030. Tokyo; Excerpta Medica: 1993;409–418. [Google Scholar]
- 18.Minoshima S, Koeppe RA, Frey KA, Kuhl DE. Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J Nucl Med 1994;35:1528–1537. [PubMed] [Google Scholar]
- 19.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 20.Genovese CR, Lazar N, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 2002;15:870–878. [DOI] [PubMed] [Google Scholar]
- 21.Malison RT, McDougle CJ, van Dyck CH, et al. [123I]beta-CIT SPECT imaging of striatal dopamine transporter binding in Tourette’s disorder. Am J Psychiatry 1995;152:1359–1361. [DOI] [PubMed] [Google Scholar]
- 22.Heinz A, Knable MB, Wolf SS, et al. Tourette’s syndrome: [I-123]beta-CIT SPECT correlates of vocal tic severity. Neurology 1998;51:1069–1074. [DOI] [PubMed] [Google Scholar]
- 23.Muller-Vahl KR, Berding G, Brucke T, et al. Dopamine transporter binding in Gilles de la Tourette syndrome. J Neurol 2000;247:514–520. [DOI] [PubMed] [Google Scholar]
- 24.Stamenkovic M, Schindler SD, Asenbaum S, et al. No change in striatal dopamine re-uptake site density in psychotropic drug naive and in currently treated Tourette’s disorder patients: a [(123)I]-beta-CIT SPECT-study. Eur Neuropsychopharmacol 2001;11:69–74. [DOI] [PubMed] [Google Scholar]
- 25.Serra-Mestres J, Ring HA, Costa DC, et al. Dopamine transporter binding in Gilles de la Tourette syndrome: a [123I]FP-CIT/SPECT study. Acta Psychiatr Scand 2004;109:140–146. [DOI] [PubMed] [Google Scholar]
- 26.Cheon KA, Ryu YH, Namkoong K, Kim CH, Kim JJ, Lee JD. Dopamine transporter density of the basal ganglia assessed with [123I]IPT SPECT in drug-naive children with Tourette’s disorder. Psychiatry Res 2004;130:85–95. [DOI] [PubMed] [Google Scholar]
- 27.Yeh CB, Lee CH, Chou YH, Chang CJ, Ma KH, Huang WS. Evaluating dopamine transporter activity with 99mTc-TRODAT-1 SPECT in drug-naive Tourette’s adults. Nucl Med Commun 2006;27:779–784. [DOI] [PubMed] [Google Scholar]
- 28.Ernst M, Zametkin AJ, Jons PH, Matochik JA, Pascualvaca D, Cohen RM. High presynaptic dopaminergic activity in children with Tourette’s disorder. J Am Acad Child Adolesc Psychiatry 1988;38:86–94. [DOI] [PubMed] [Google Scholar]
- 29.Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J Neurochem 2003;87:574–585. [DOI] [PubMed] [Google Scholar]
- 30.Robertson MM, Althoff RR, Hafez A, Pauls DL. Principal components analysis of a large cohort with Tourette syndrome. Br J Psychiatry 2008;193:31–36. [DOI] [PubMed] [Google Scholar]
- 31.Singer HS, Szymanski S, Giuliano J, et al. Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PET. Am J Psychiatry 2002;159:1329–1336. [DOI] [PubMed] [Google Scholar]
- 32.Wong DF, Brasic J, Singer HS, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology 2008;33:1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson BS, Staib L, Scahill L, et al. Regional brain and ventricular volumes in Tourette syndrome. Arch Gen Psychiatry 2001;58:427–440. [DOI] [PubMed] [Google Scholar]
- 34.Bloch MH, Leckman JF, Zhu H, Peterson BH. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology 2005;65:1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garraux G, Goldfine A, Bohlhalter S, Lerner A, Hanakawa T, Hallett M. Increased midbrain gray matter in Tourette’s syndrome. Ann Neurol 2006;59:381–385. [DOI] [PubMed] [Google Scholar]
- 36.Ludolph AG, Juengling FD, Libal G, Ludolph AC, Fegert JM, Kassubek J. Grey-matter abnormalities in boys with Tourette syndrome: magnetic resonance imaging study using optimized voxel-based morphometry. Br J Psychiatry 2006;188:484–485. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Lee DY, Bailey E, et al. Validity of large-deformation high dimensional brain mapping of the basal ganglia in adults with Tourette syndrome. Psychiatr Res 2007;154:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sowell ER, Kan E, Yoshii Y, et al. Thinning of sensorimotor cortices in children with Tourette syndrome. Nat Neurosci 2008;11:637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Behen M, Chugani HT, Juhasz J, et al. Abnormal brain tryptophan metabolism and clinical correlates in Tourette syndrome. Mov Disord 2007;22:2256–2262. [DOI] [PubMed] [Google Scholar]
- 40.Peterson BS, Choi HA, Hao X, et al. Morphologic features of the amygdala and hippocampus in children and adults with Tourette syndrome. Arch Gen Psychiatry 2007;64:1281–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.