Abstract
Objective:
To investigate transcranial magnetic stimulation (TMS) measures as clinical correlates and longitudinal markers of amyotrophic lateral sclerosis (ALS).
Methods:
We prospectively studied 60 patients with ALS subtypes (sporadic ALS, familial ALS, progressive muscular atrophy, and primary lateral sclerosis) using single pulse TMS, recording from abductor digiti minimi (ADM) and tibialis anterior (TA) muscles. We evaluated three measures: 1) TMS motor response threshold to the ADM, 2) central motor conduction time (CMCT), and 3) motor evoked potential amplitude (correcting for peripheral changes). Patients were evaluated at baseline, compared with controls, and followed every 3 months for up to six visits. Changes were analyzed using generalized estimation equations to test linear trends with time.
Results:
TMS threshold, CMCT, and TMS amplitude correlated (p < 0.05) with clinical upper motor neuron (UMN) signs at baseline and were different (p < 0.05) from normal controls in at least one response. Seventy-eight percent of patients with UMN (41/52) and 50% (4/8) of patients without clinical UMN signs had prolonged CMCT. All three measures revealed significant deterioration over time: TMS amplitude showed the greatest change, decreasing 8% per month; threshold increased 1.8% per month; and CMCT increased by 0.9% per month.
Conclusions:
Transcranial magnetic stimulation (TMS) findings, particularly TMS amplitude, can objectively discriminate corticospinal tract involvement in amyotrophic lateral sclerosis (ALS) from controls and assess the progression of ALS. While central motor conduction time and response threshold worsen by less than 2% per month, TMS amplitude decrease averages 8% per month, and may be a useful objective marker of disease progression.
GLOSSARY
- ADM
= abductor digiti minimi;
- ALS
= amyotrophic lateral sclerosis;
- ANOVA
= analysis of variance;
- CI
= confidence interval;
- CMAP
= compound motor action potential;
- CMCT
= central motor conduction time;
- DTR
= deep tendon stretch reflex;
- fALS
= familial ALS;
- GEE
= generalized estimation equations;
- LMN
= lower motor neuron;
- MEP
= motor evoked potential;
- PLS
= primary lateral sclerosis;
- PMA
= progressive muscular atrophy;
- sALS
= sporadic ALS;
- TA
= tibialis anterior;
- TMS
= transcranial magnetic stimulation;
- UMN
= upper motor neuron.
ALS is diagnosed by finding clinical upper motor neuron (UMN) and lower motor neuron (LMN) signs. LMN dysfunction can be confirmed objectively through electromyography, whereas UMN dysfunction lacks a comparable established marker. Detection of subclinical UMN dysfunction would be helpful for diagnostic purposes, and objective markers of UMN dysfunction may also help clarify the relationship between ALS and its variants, including progressive muscular atrophy (PMA).
PMA has been diagnosed by the absence of clinical UMN signs in the presence of LMN findings for several years. However, autopsies on patients with clinically diagnosed PMA show corticospinal tract degeneration in half of the patients. Therefore, clinical examination alone does not disclose all relevant UMN pathology.1–3
Transcranial magnetic stimulation (TMS) is a neurophysiologic technique used to assess the function of central motor pathways. Standard TMS measures include motor threshold, central motor conduction time (CMCT), and motor evoked potential (MEP) amplitudes. There have been numerous studies of TMS in ALS for over 20 years, investigating a multitude of issues on UMN physiology, cortical excitability and inhibition, the utility of the silent period and other responses to detect early changes in ALS, and the relationship between the UMN and LMN, many of which have been contradictory.4–11 Threshold, CMCT, and MEP are three useful measures of single pulse TMS; however, the diagnostic success of these nonspecific UMN measures varies widely from 16% to 100%.5,9,12–17 Reasons include different muscle recording sites, different methods of calculating CMCT,4,18 and different thresholds for defining diagnostic success (i.e., 1/4 sites prolonged vs 4/4).
The TMS MEP is activated through both UMN and LMN pathways after magnetic stimulation of the cortex. In ALS, TMS amplitudes are often attenuated or absent19–21; even patients with pseudobulbar features are prone to have small, desynchronized MEPs.12 Reduced TMS MEP/M-wave amplitude ratio may be more closely correlated with pyramidal tract involvement than prolonged CMCT.21 There are fewer investigations of TMS amplitude compared to CMCT. One study found TMS amplitude abnormalities in only 16 of 54 patients with ALS (30%),5 whereas another found a significant difference between patients and controls at three recording sites, with amplitude reductions in almost all patients.19
The sensitivity of baseline TMS measures in ALS has been studied extensively, with mixed results, but there have been few longitudinal studies of TMS changes in ALS. Most reports showed no change in TMS threshold with time6,22,23 but one noted significant increase.17 CMCT has generally been reported to not increase with time.6,22,24 In a small series, TMS MEP amplitude and MEP/peripheral compound motor action potential (CMAP) ratio did not change with disease progression.22
In this study, we report on TMS threshold, CMCT, and amplitude data that were not included in an overarching report on neurophysiologic, brain imaging, and clinical methods to quantify and track progression in patients with ALS.25
METHODS
Subjects.
Patients with suspected ALS or ALS variants were evaluated for eligibility and enrolled through the Eleanor and Lou Gehrig MDA/ALS Research Center at Columbia University as previously described.25 Qualifying patients included those with a suspected diagnosis according to El Escorial criteria26 of sporadic or familial ALS (sALS and fALS), primary lateral sclerosis (PLS), and PMA. The study enrolled 60 patients, 23 of whom had evaluations spanning a minimum of four visits up to 1.5 years (table 1) and measured clinical variables as described.25 Control data for CMCT baseline measures were from 33 normal subjects (mean ± SD age 44.8 ± 11.8 years) collected previously. All patients provided informed consent, and this study protocol was approved in accordance with Columbia University Medical Center Institutional Review Board guidelines.
Table 1 Demographic and clinical information

Neuromuscular assessment.
Clinical examination relevant to TMS testing at each baseline and follow-up visit consisted of height, forced vital capacity measurements, strength in 36 skeletal muscles including grip and pinch, muscle tone, deep tendon stretch reflexes (DTRs), pathologic responses (Babinski and Hoffmann), and finger and foot tapping speed. Examinations were performed by a senior neurologist and an experienced ALS clinical evaluator.25
Electrophysiologic procedures.
Motor conduction tests using TMS and peripheral spinal root stimulation were obtained at baseline and at 3-month intervals up to six visits. TMS was performed using a cap stimulator (Cadwell MES-10; Cadwell Inc., Kennewick, WA) at Cz recording from bilateral abductor digiti minimi (ADM) and tibialis anterior (TA) muscles in a belly–tendon arrangement with 1-cm disc electrodes at 100% machine output. Electrical spinal root stimulation was performed over C7 and L1 using a high voltage stimulator (Digitimer, Ltd., Hertfordshire, UK) at the same output settings across all visits for each patient. Supramaximal stimulation was not possible with either TMS or spinal nerve root stimulation.
Resting TMS threshold to the ADM was recorded as the minimum stimulus intensity producing consistent MEPs with peak-to-peak amplitudes greater than 50 μV in at least 5 of 10 consecutive stimulations.27 Stimulation was increased in 1% to 2% increments of maximum stimulator output until threshold was obtained.
CMCT was calculated through subtraction of the minimal spinal root stimulation latency from the upper spine (for the arms) and lower spine (for the legs) from the minimal latency achieved by cortical stimulation.
TMS and peripheral MEPs were recorded at an amplifier gain of 5 mV per division with high and low pass filters set at 10 Hz and 10 kHz. Latencies and amplitudes were determined manually at a sensitivity of 200 μV/division with a time base of 10 msec/division. Multiple TMS and electrical spinal stimuli were applied to obtain at least two identical trials at each stimulation site to confirm reproducibility.
Amplitudes were calculated from baseline to highest negative peak of the MEP responses at each visit for both TMS and electrical spinal recordings (figure). When stimulus artifact obscured the signal baseline, the MEP amplitude was measured from the negative peak to an interpolated baseline point directly below. TMS and peripheral amplitudes were collated by a technician blinded to the patient and visit number.
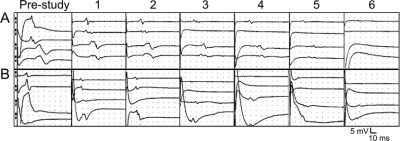
Figure Longitudinal tracings from a sample patient with sporadic amyotrophic lateral sclerosis from prestudy through visits 1 to 6
Amplitude and time are standardized across all visits. (A) Transcranial magnetic stimulation (TMS) motor evoked potential (MEP) responses from cortical stimulation over the vertex. Traces are from the right and left abductor digiti minimi (ADM) (A1 and A2), right and left tibialis anterior (TA) (A3 and A4). (B) Peripheral MEP responses stimulating over the cervical spine to the right and left ADM (B5 and B6), and lumbar spine to the right and left TA (B7 and B8).
Data analysis.
Baseline analyses included all patients (n = 60). One-way analyses of variance (ANOVAs) were performed comparing the four diagnostic groups (sALS, PLS, PMA, and fALS). In the case of a significant ANOVA result, Tukey post hoc analyses were performed to determine which groups differed. Additionally, sALS, PLS, and fALS were collapsed into a single UMN group. t Tests were used to compare UMN vs PMA groups, and bulbar vs limb onset groups. Pearson product-moment correlation coefficients were used to correlate CMCT and threshold stimulation value, and clinical variables at baseline. Partial correlations were performed to determine the relationship between the TMS amplitudes and clinical variables, controlling for peripheral MEP amplitudes.
CMCT was dichotomized into normal and abnormal categories for the purpose of assessing diagnostic sensitivity. CMCT was rated as abnormal when two SD greater than the average of the control group. The CMCT in the control group was 7.8 ± 1.5 msec to the ADM, 13.5 ± 1.6 msec to the TA. Amplitudes were not dichotomized. The diagnostic category fALS was excluded from amplitude analyses due to missing data and low sample size (n = 5).
Longitudinal change in TMS measures were analyzed using generalized estimation equations (GEE) to test linear trends as the percent change with time. Each patient’s data were treated as a cluster with repeated measurements at different visits, and used in an exchangeable working correlation matrix.28,29 Peripheral MEP amplitude measures were included as covariates when assessing change in TMS amplitudes over time. Interactions of time with diagnostic group (UMN vs PMA) and site of onset (bulbar vs limb) also were investigated.
RESULTS
Thirty-six of the 60 patients completed two or more follow-up visits, and 11 finished all six follow-up visits spanning a period of 1.5 years, including prestudy baseline visits (table 1).
Threshold baseline.
Baseline TMS thresholds were greater in UMN than in PMA, but did reach significance (38 ± 9% vs 31 ± 6%, p = 0.058). There was no difference in TMS threshold by site of disease onset. When compared with clinical and physiologic variables, ADM threshold values correlated significantly with finger tapping rate, the brachioradialis DTR, ipsilateral CMCT, and TMS amplitudes (table 2).
Table 2 Clinical and physiologic correlations

Threshold change over time.
TMS thresholds increased among all cases and UMN cases by 1.8% per month (p < 0.0001). Among the eight PMA cases available for analysis, threshold did not change significantly, nor were any trends apparent in that group.
CMCT baseline.
CMCT at baseline did not correlate with patient age or duration of disease. CMCT to the TA was prolonged relative to controls among patients with sALS (mean = 24.0 msec, 95% confidence interval [CI] = 19.7–28.3 msec; p < 0.0005) and patients with fALS (mean = 23 msec, 95% CI = 17.7–28.3 msec; p = 0.01). TA CMCT also was prolonged in the combined UMN group (sALS, fALS, and PLS, p < 0.0005) compared to control values to the TA (mean = 13.5 msec, 95% CI = 12.0–15.0 msec). CMCT to the ADM showed no difference between patients and controls.
There was no CMCT difference among the four diagnostic groups or between patients with bulbar vs limb onset. The combined UMN patients had significantly longer CMCTs to both arms and legs compared with PMA patients. Dichotomized CMCT values revealed that 78% (41/52) of UMN patients had at least one prolonged central conduction, and 13% (7/52) had CMCT prolongation to all four limbs. Four of the eight PMA cases (50%) had prolonged CMCT to the TA.
CMCT to the ADM correlated significantly with increased DTRs in the brachioradialis, as well as decreased finger dexterity. CMCT to the TA correlated with increased patellar and ankle DTRs. There was no correlation between CMCT and muscle strength measures. Patients with a Babinski sign had longer CMCT to the TA than those without findings (29.5 ± 15.7 msec compared to 20.5 ± 10.2 msec), and those with positive Hoffmann signs had longer CMCT to the ADM (18.2 ± 14.6 msec compared to 7.5 ± 0.7 msec). CMCT and clinical correlations are summarized in table 2.
CMCT change over time.
Averaged across all cases, CMCT to the TA increased by an average of 0.9% per month (p = 0.016). The interaction between upper vs lower MN involvement and time was significant such that UMN cases had greater TA CMCT increase than did PMA cases. The increase in CMCT in PMA cases was not significant when analyzed independently.
CMCT to the ADM did not show significant increase over time. Peripheral conduction times remained unchanged in UMN cases. However, in PMA cases peripheral conduction times for both the ADM and TA increased between 0.5% and 0.6% at each successive visit (p < 0.005).
Amplitude baseline.
A one-way ANOVA revealed significant amplitude differences among the diagnostic groups. Tukey HSD post hoc comparisons indicated that PLS had significantly larger, more polyphasic, and dispersed TMS amplitudes than sALS in the TA. Peripheral amplitudes for PLS, however, were neither polyphasic nor dispersed, and were significantly larger than all other diagnostic groups (sALS, fALS, PMA) in the ADM (table 3). There was no difference in baseline amplitude between bulbar and limb onset groups.
Table 3 Baseline TMS and peripheral MEP amplitudes

Partial correlations between clinical signs and baseline TMS amplitudes, controlling for peripheral amplitudes using the latter as covariates in the GEE model, showed significant positive relationships between baseline TMS amplitudes, dexterity, and strength. There was a negative relationship between the brachioradialis and triceps DTRs and TMS amplitude to the ADM. Similarly, patients with positive Hoffmann signs had lower TMS amplitudes to the ADM (2.4 ± 1.2 mV compared to 1.0 ± 1.3 mV). Partial correlation results of Hoffmann sign presence vs TMS amplitude to the ADM, controlling for peripheral amplitude, was also significant (table 2). In the legs, TMS amplitudes correlated directly with foot tapping as well as dorsiflexion and inversion strength.
Amplitude change over time.
TMS amplitude changes over time were analyzed controlling for the peripheral MEP amplitude at each visit by including peripheral amplitude data as a covariate in the GEE model. Across all cases, there was a decrease in TMS amplitude to the ADM of 2.8% per month (p < 0.0001) as well as decrease in TMS amplitude to the TA of 8.0% per month (p < 0.05) after controlling for the corresponding peripheral amplitude decreases. Amplitude decreases were found in both UMN and PMA cases analyzed separately. TMS amplitude drop to the ADM and TA in PMA was greater than in UMN cases, but the effect did not reach significance.
DISCUSSION
We demonstrate that central motor conduction tests using single pulse TMS can both detect corticospinal tract involvement in ALS and measure disease progression, in agreement with reviews of TMS methods.7,9 Three measures from this study (TMS threshold, CMCT and TMS amplitude) revealed differences at baseline and showed changes in patients with ALS over time. TMS amplitude was abnormal and worsened at a greater rate than either threshold or CMCT.
TMS amplitude change, adjusting for peripheral change, was three to eight times greater per month than CMCT or threshold. Though neither motor cortex nor spinal roots could be stimulated supramaximally, this was corrected using peripheral changes in MEP amplitude in the GEE model, and TMS/peripheral amplitude ratios. The differentially greater changes on TMS amplitude in ALS could be due to diminished number of cortical motor neuron cell bodies, axonal loss, desynchronization, and conduction abnormalities affecting long tract axons.
Though other longitudinal studies reported no significant CMCT prolongation over time,6,22,24 we found most patients had a prolonged baseline CMCT that gradually but significantly increased in UMN patients. We believe we were able to demonstrate this effect because our sample size was larger and data collection period was longer than previous studies. PMA patients revealed less prolonged CMCTs at baseline compared to UMN patients, and did not demonstrate longitudinal changes in this measure, possibly because PMA progresses slower than other ALS subtypes.4 Nevertheless, CMCT can be abnormal in purely LMN clinical syndromes14,17 and histopathologic and immunochemical studies reveal UMN pathology in LMN patients.1,2 We found that CMCT was prolonged in 50% of our patients without clinical UMN signs.
CMCT correlated with hyperreflexia and loss of dexterity, presence of Hoffmann and Babinski signs, but not with strength measures. Thus, CMCT abnormalities parallel some clinical UMN findings, and could be used to mark UMN dysfunction.14,24 The lack of correlation between CMCT and strength, however, suggests that CMCT does not measure pathophysiologic mechanisms of strength. LMN dysfunction may be the primary cause of weakness in ALS.30 Motor unit number estimation correlates well with muscle strength, but not with UMN signs such as dexterity.25 We found that TMS amplitude correlated strongly with hyperreflexia, dexterity, and strength. However, TMS amplitude did not correlate with Hoffmann or Babinski signs, suggesting that these release responses are modulated by different UMN pathways than strength and dexterity.
There are several limitations to this study. The average age of our normal controls, used to compare differences in baseline CMCT, was 10 years younger than in our patients. This could diminish the strength of our findings. While we did not find a significant difference between patient age and baseline CMCT, this was likely due to the wide range of abnormal CMCT in ALS. Patient data only were factored into the GEE longitudinal analyses of threshold, CMCT, and TMS amplitude; control data were not obtained over time. We also did not analyze the ADM or TA MEP after peripheral nerve stimulation as another measure of lower motor neuron involvement. These probably would not have changed our results of up to 8% change in TMS amplitude. However, it would be important to determine the sensitivity of TMS amplitude in detecting UMN changes, as well as establish longitudinal findings in age-matched controls.
Other concerns may be that minimal F-wave latency methods may be better at determining peripheral conduction times in comparison to magnetic paravertebral stimulation.4,9 However, our subtraction of method used high voltage electrical root stimulation, which arguably provided a more focused stimulus than a paravertebral magnetic pulse. We did not test bulbar or proximal muscles such as the masseter or biceps brachii where subclinical abnormalities may occur earlier and more reliably in ALS.31,32
Future investigations should study longer ALS disease progression by incorporating patients at the earliest stages of disease using GEE to analyze TMS amplitudes and peripheral changes. It would be important to assess TMS amplitude longitudinally, after controlling for peripheral MEP changes, using GEE in normal controls and patients with peripheral neuropathy. Advanced electrophysiologic methods33–35 might be helpful in parsing out the contributions of central and peripheral motor pathology. Combining TMS with routine EMG and nerve conduction studies, macro-EMG, and motor unit number estimation36 of the same muscles in patients with ALS may determine more precisely the relative contributions of UMN and LMN to TMS findings in ALS over time.
AUTHOR CONTRIBUTIONS
M.X. Tang, Department of Biostatistics and Sergievsky Center, Columbia University, conducted the statistical analyses.
ACKNOWLEDGMENT
The authors thank the following individuals for assisting in study coordination and clinical assessment: Vanessa Batista, RN, Sheila Hayes, RPT, MS, and Jacqueline Montes, RPT, MA, Columbia University. The authors also thank the patients and their families for their participation in this study.
Address correspondence and reprint requests to Dr. Seth L. Pullman, The Neurological Institute, 710 West 168th Street, New York, NY 10032 sp31@columbia.edu
Funded by NIH grant NS41672-01 (H.M.), the Muscular Dystrophy Association, and MDA Wings Over Wall Street.
Disclosure: The authors report no disclosures.
Received August 1, 2008. Accepted in final form October 31, 2008.
REFERENCES
- 1.Ince PG, Evans J, Knopp M, et al. Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS. Neurology 2003;60:1252–1258. [DOI] [PubMed] [Google Scholar]
- 2.Iwanaga K, Hayashi S, Oyake M, et al. Neuropathology of sporadic amyotrophic lateral sclerosis of long duration. J Neurol Sci 1997;146:139–143. [DOI] [PubMed] [Google Scholar]
- 3.Leung D, Hays A, Geysu K, DelBene M, Rowland L. Diagnosis of ALS: clinico-pathologic analysis of 76 autopsies. Neurology 1999;52:A164. [Google Scholar]
- 4.Di Lazzaro V, Oliviero A, Profice P, et al. The diagnostic value of motor evoked potentials. Clin Neurophysiol 1999;110:1297–1307. [DOI] [PubMed] [Google Scholar]
- 5.Pouget J, Trefouret S, Attarian S. Transcranial magnetic stimulation (TMS): compared sensitivity of different motor response parameters in ALS. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1 suppl 2:S45–S49. [DOI] [PubMed] [Google Scholar]
- 6.Mills KR. The natural history of central motor abnormalities in amyotrophic lateral sclerosis. Brain 2003;126: 2558–2566. [DOI] [PubMed] [Google Scholar]
- 7.Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology 2007;68:484–488. [DOI] [PubMed] [Google Scholar]
- 8.Zanette G, Tamburin S, Manganotti P, Refatti N, Forgione A, Rizzuto N. Different mechanisms contribute to motor cortex hyperexcitability in amyotrophic lateral sclerosis. Clin Neurophysiol 2002;113:1688–1697. [DOI] [PubMed] [Google Scholar]
- 9.Curra A, Modugno N, Inghilleri M, Manfredi M, Hallett M, Berardelli A. Transcranial magnetic stimulation techniques in clinical investigation. Neurology 2002;59:1851–1859. [DOI] [PubMed] [Google Scholar]
- 10.Wittstock M, Wolters A, Benecke R. Transcallosal inhibition in amyotrophic lateral sclerosis. Clin Neurophysiol 2007;118:301–307. [DOI] [PubMed] [Google Scholar]
- 11.Attarian S, Vedel JP, Pouget J, Schmied A. Progression of cortical and spinal dysfunctions over time in amyotrophic lateral sclerosis. Muscle Nerve 2008;37:364–375. [DOI] [PubMed] [Google Scholar]
- 12.Eisen AA, Shtybel W. AAEM mini-monograph #35: clinical experience with transcranial magnetic stimulation. Muscle Nerve 1990;13:995–1011. [DOI] [PubMed] [Google Scholar]
- 13.Mills KR, Nithi KA. Peripheral and central motor conduction in amyotrophic lateral sclerosis. J Neurol Sci 1998;159:82–87. [DOI] [PubMed] [Google Scholar]
- 14.Miscio G, Pisano F, Mora G, Mazzini L. Motor neuron disease: usefulness of transcranial magnetic stimulation in improving the diagnosis. Clin Neurophysiol 1999;110:975–981. [DOI] [PubMed] [Google Scholar]
- 15.Pohl C, Block W, Traber F, et al. Proton magnetic resonance spectroscopy and transcranial magnetic stimulation for the detection of upper motor neuron degeneration in ALS patients. J Neurol Sci 2001;190:21–27. [DOI] [PubMed] [Google Scholar]
- 16.Schulte-Mattler WJ, Muller T, Zierz S. Transcranial magnetic stimulation compared with upper motor neuron signs in patients with amyotrophic lateral sclerosis. J Neurol Sci 1999;170:51–56. [DOI] [PubMed] [Google Scholar]
- 17.Triggs WJ, Menkes D, Onorato J, et al. Transcranial magnetic stimulation identifies upper motor neuron involvement in motor neuron disease. Neurology 1999;53:605–611. [DOI] [PubMed] [Google Scholar]
- 18.Kimura J, Butzer JF. F-wave conduction velocity in Guillain-Barre syndrome. Assessment of nerve segment between axilla and spinal cord. Arch Neurol 1975;32:524–529. [DOI] [PubMed] [Google Scholar]
- 19.Eisen A, Shtybel W, Murphy K, Hoirch M. Cortical magnetic stimulation in amyotrophic lateral sclerosis. Muscle Nerve 1990;13:146–151. [DOI] [PubMed] [Google Scholar]
- 20.Salerno A, Carlander B, Camu W, Georgesco M. Motor evoked potentials (MEPs): evaluation of the different types of responses in amyotrophic lateral sclerosis and primary lateral sclerosis. Electromyogr Clin Neurophysiol 1996;36:361–368. [PubMed] [Google Scholar]
- 21.Uozumi T, Tsuji S, Murai Y. Motor potentials evoked by magnetic stimulation of the motor cortex in normal subjects and patients with motor disorders. Electroencephalogr Clin Neurophysiol 1991;81:251–256. [DOI] [PubMed] [Google Scholar]
- 22.de Carvalho M, Miranda PC, Luis ML, Ducla-Soares E. Cortical muscle representation in amyotrophic lateral sclerosis patients: changes with disease evolution. Muscle Nerve 1999;22:1684–1692. [DOI] [PubMed] [Google Scholar]
- 23.Zanette G, Tamburin S, Manganotti P, Refatti N, Forgione A, Rizzuto N. Changes in motor cortex inhibition over time in patients with amyotrophic lateral sclerosis. J Neurol 2002;249:1723–1728. [DOI] [PubMed] [Google Scholar]
- 24.Claus D, Brunholzl C, Kerling FP, Henschel S. Transcranial magnetic stimulation as a diagnostic and prognostic test in amyotrophic lateral sclerosis. J Neurol Sci 1995;129 suppl:30–34. [DOI] [PubMed] [Google Scholar]
- 25.Mitsumoto H, Ulug AM, Pullman SL, et al. Quantitative objective markers for upper and lower motor neuron dysfunction in ALS. Neurology 2007;68:1402–1410. [DOI] [PubMed] [Google Scholar]
- 26.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis: Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci 1994;124 suppl:96–107. [DOI] [PubMed] [Google Scholar]
- 27.Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application: report of an IFCN committee. Electroencephalogr Clin Neurophysiol 1994;91:79–92. [DOI] [PubMed] [Google Scholar]
- 28.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–130. [PubMed] [Google Scholar]
- 29.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988;44:1049–1060. [PubMed] [Google Scholar]
- 30.Kent-Braun JA, Walker CH, Weiner MW, Miller RG. Functional significance of upper and lower motor neuron impairment in amyotrophic lateral sclerosis. Muscle Nerve 1998;21:762–768. [DOI] [PubMed] [Google Scholar]
- 31.Trompetto C, Caponnetto C, Buccolieri A, Marchese R, Abbruzzese G. Responses of masseter muscles to transcranial magnetic stimulation in patients with amyotrophic lateral sclerosis. Electroencephalogr Clin Neurophysiol 1998;109:309–314. [DOI] [PubMed] [Google Scholar]
- 32.Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Dileone M, Tonali PA. Objective tests for upper motor neuron involvement in amyotrophic lateral sclerosis (ALS). Neurology 2004;63:1988. [DOI] [PubMed] [Google Scholar]
- 33.Komissarow L, Rollnik JD, Bogdanova D, et al. Triple stimulation technique (TST) in amyotrophic lateral sclerosis. Clin Neurophysiol 2004;115:356–360. [DOI] [PubMed] [Google Scholar]
- 34.Triggs WJ, Cros D, Macdonell RA, Chiappa KH, Fang J, Day BJ. Cortical and spinal motor excitability during the transcranial magnetic stimulation silent period in humans. Brain Res 1993;628:39–48. [DOI] [PubMed] [Google Scholar]
- 35.Weber M, Eisen A, Nakajima M. Corticomotoneuronal activity in ALS: changes in the peristimulus time histogram over time. Clin Neurophysiol 2000;111:169– 177. [DOI] [PubMed] [Google Scholar]
- 36.Gooch CL, Harati Y. Motor unit number estimation, ALS and clinical trials. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:71–82. [DOI] [PubMed] [Google Scholar]