Abstract
We report here a protease sensing nanoplatform based on semiconductor nanocrystals or quantum dots (QDs) and bioluminescence resonance energy transfer (QD-BRET) to detect the protease activity in complex biological samples. These nanosensors consist of bioluminescent proteins as the BRET donor, quantum dots as the BRET acceptor, and protease substrates sandwiched between the two as a sensing group. An intein-mediated conjugation strategy was developed for site-specific conjugation of proteins to QDs in preparing these QD nanosensors. In this traceless ligation, the intein itself is spliced out and excluded from the final conjugation product. With this method, we have synthesized a series of QD nanosensors for highly sensitive detection of an important class of protease matrix metalloproteinase (MMP) activity. We demonstrated that these nanosensors can detect the MMP activity in buffers and in mouse serum with the sensitivity to a few ng/ml, and secreted proteases by tumor cells. The suitability of these nanosensors for a multiplex protease assay has also been shown.
Keywords: Quantum dot nanosensors, intein splicing, bioluminescence resonance energy transfer, protease detection, matrix metalloproteinase-2
INTRODUCTION
Matrix metalloproteinases (MMPs) are a family of zinc-dependent secreted endopeptidases crucial for regulated degradation and processing of extracellular matrices. Over-expression of MMPs have been observed in almost every type of human cancers, and found to correlate with advanced tumor stage, increased invasion and metastasis, and shortened survival.1–5 Therefore, sensitive detection of MMPs in tumor samples offers a great clinical value to cancer treatment and monitoring. MMPs are first produced as zymogens in latent forms, and are activated by a cascade of signal transduction events. Current clinic detection of MMPs uses the antibody-based ELISA which cannot tell the latent forms from active enzymes. Several methods based on fluorescence and magnetic resonance to detect MMPs activity have been reported.6–13 For example, both small peptide fluorogenic substrates 6–8 and dye-conjugated polymer sensors9–10 have been synthesized for MMP detection. With their superior fluorescent properties in comparison with organic and genetically encoded fluorophores, semiconductor fluorescent nanocrystals (also known as quantum dots or QDs) have become a popular fluorescent label for both imaging and biosensing applications.11–26 A fluorescence resonance energy transfer (FRET) based assay that exploited QDs as the donor and rhodamine as the acceptor has been developed to detect collagenase activity.27 More recently, gold nanoparticles have been applied as an energy acceptor for QDs in a FRET-based protease assay.28 However, fluorescence based methods for detection of analytes in complex biological media generally face the same challenge—the high background signal from interfering species present in the biological samples. On the other hand, bioluminescence based detection offers great sensitivity even in small living animals due to its extremely low background.29–31 The bioluminescence based assay typically uses a bioluminescent protein such as luciferase that can produce light during its catalysis of a biochemical reaction. We have recently discovered that QDs can act as an energy acceptor in the process of bioluminescence resonance energy transfer (BRET) with a bioluminescent protein such as a mutant of Renilla luciferase as the energy donor.32–33 The biochemical energy produced in the oxidation of the luciferase substrate can resonantly excite the QDs to generate fluorescent emissions. This QD-BRET system provides a powerful tool for highly sensitive in vivo imaging, and here we demonstrate that it can be applied to sense the protease activity in complex biological media with high sensitivity.
In designing such QD-BRET based sensors for detecting MMP activity, we envisioned to put the MMP substrate peptide as the linker between the BRET donor—the bioluminescent protein—and the RBET acceptor—QDs, thus the BRET process between the QD and the protein will be modulated by the protease activity (Scheme 1a). The cleavage of the substrate linker by the protease will lead to the disruption of BRET, resulting in the decrease in the BRET emission from QDs. The preparation of this nanosensor would require a site-specific conjugation method. The current methods generally use either random chemical coupling with active amino acids (e.g. –NH2, –COOH, –SH) on the protein surface or noncovalent complexation mediated by forces like electrostatic interaction and ligand recognition, none of which is suitable for the task in this study. A survey of site-specific bioconjugation methods led us to the intein-mediated chemical ligation system. Intein is a polypeptide sequence inside a protein that is able to excise itself and rejoin the remaining portions with a peptide bond.34–36 It catalyzes the splicing reaction through formation of an active thioester intermediate, and has been widely applied to protein conjugation and immobilization in literature. In this work, we have successfully applied this method to synthesize a series of nanosensors for sensitive detection of MMP-2, MMP-7, and urokinase-type plasminogen activator (uPA). These prepared nanosensors can not only detect these proteases in complex biological media such as mouse serum and tumor secretes with a sensitivity of as low as 1 ng/ml, also detect multiple proteases present in one sample.
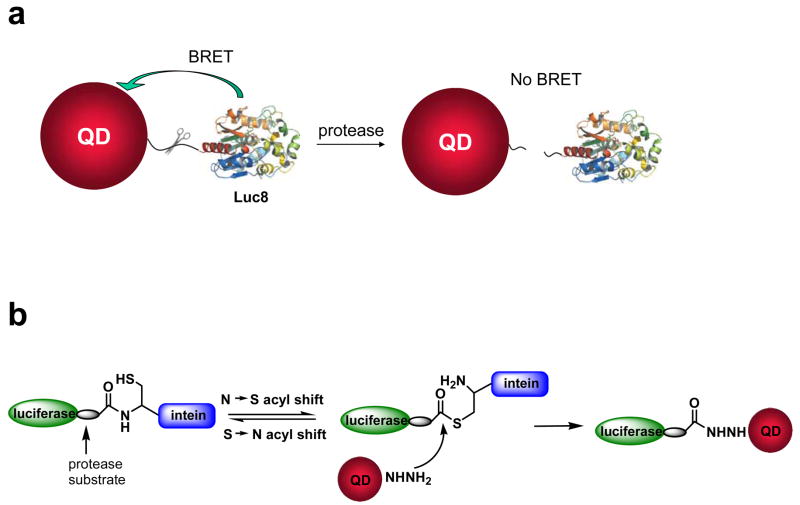
Scheme 1.
Design and preparation of QD-BRET based nanosensors for MMP sensing. a) Schematic of the nanosensor comprising of a QD and luciferase proteins (Luc8) that are linked to the QD through an MMP peptide substrate. b) Intein-mediated site-specific conjugation of Luc8 fusion proteins to QDs.
EXPERIMENTAL SECTION
Materials
Carboxylated quantum dots were from Invitrogen. 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) was from Fluka. Tris(2-Carboxyethyl) phosphine, hydrochloride (TCEP) was from Pierce. Coelenterazine was from Prolume. MMP-7 and MMP-2 were from Calbiochem, and uPA was from Genescript. Fluorescence and bioluminescence emission spectra were collected on a Fluoro Max-3 (Jobin Yvon Inc.). For bioluminescence measurements, the excitation light was blocked, and emission spectra were corrected with a correction file provided by the company. The collected bioluminescence emission spectra were further corrected for the Luc8 kinetics over the course of data acquisition (~20 s).
Plasmid constructions
For the insertion of intein GyrA into the C-terminus of Luc8, two additional restriction sites EcoR I and Hind III were introduced into pBAD-Luc8 plasmid. The intein GyrA mutant (N198A) gene was amplified from pTWIN-MBP1 (New England Biolabs) with 5′-primer (5′-A ATT GAA TTC TGC ATC ACG GGA GAT GCT) and 3′-primer (5′-A GCT AAG CTT GGT GAG GCC AGT AGC GTG-3′). The PCR product was digested by EcoR I and Hind III, and ligated into the same enzyme-digested pBAD-Luc8 to give pBAD-Luc8-GyrA plasmid. To insert the MMP-7 substrate sequence between Luc8 and GyrA, Luc8 gene was amplified from pBAD-Luc8 with 5′-primer (5′-A TGC CCA TGG CTT CCA AGG TGT AC-3′), and the 3′-primer (5′-ATGC GAA TTC ACC ACC CAT TGT CAG TGA CAG AGG TAC TCC TCC CTG CTC GTT CTT CAG-3′) was designed for MMP-7 substrate sequence (Val-Pro-Leu-Ser-Leu-Thr-Met-Gly)38; The MMP-2 substrate sequence (Ile-Pro-Val-Ser-Leu-Arg-Ser-Gly) was also introduced through 3′ primer by 5′-ATGC GAA TTC ACC ACC GCT ACG CAG ACT TAC AAT ACC ACC CTG CTC GTT CTT CAG-3′; The uPA substrate sequence (Gly-Gly-Ser-Gly-Arg-Ser-Ala-Asn-Ala-Lys-Cys) was inserted by 5′-GAA TTC TGA CCC ACC ACC ACC GCA TTT CGC ATT TGC ACT GCG ACC ACT TCC TCC-3′. The PCR products were digested with Nco I and EcoR I, and ligated into the same enzyme-digested pBAD-Luc8-GyrA to give plasmids containing different protease cleavage sites.
Protein expression and purification
The expression plasmids were transformed into LMG194 cell, an E. coli strain deficient of arabinose. The transformed cells were grown in 1 L of LB media at 37 °C, and induced with 0.2% arabinose at the OD600 of ~0.6. After 4 hours induction at 30 °C, the cells were harvested by centrifugation and frozen at −80 °C. The cells were thawed in 10 ml of 20 mM Tris pH 7.4, 20 mM imidazole, 300 mM NaCl containing 1 mg/ml lysozyme, 5 μg/ml DNAse I, and 10 μg/ml RNAse A. The resuspended cells were incubated for 30 min at room temperature, and sonicated for <1 min. The lysates were clarified by centrifugation at 15,000 rpm for 30 min at 4 °C. The clarified supernatant containing expressed proteins was incubated with 2 ml of Ni-NTA agarose (Qiagen) at 4 °C for 1 h with gently shaking. The Ni-NTA agarose beads were washed with 100 mL of 20 mM Tris pH 7.4, 20 mM imidazole, 300 mM NaCl. His-tagged proteins were eluted with 5 ml of the same buffer containing 250 mM of imidazole. The eluted fusion proteins were further purified using FPLC on Source 15Q anion exchange column. The luciferase activity of the Luc8-GyrA and other fusion proteins containing protease substrates was assessed by a calibrated luminometer with coelenterazine as the substrate.
Conjugation of adipic dihydrazide to QDs
A typical conjugation solution contained 0.1 μM of carboxylate QDs (Invitrogen), 50 μM of adipic dihydrazide (ADH), and 20 μM of 1-ethyl-3(3-dimethylamino-propyl) carbodiimide hydrochloride (EDC) in a volume of 1.2 ml of PBS buffer. The mixture was incubated for 4 h at room temperature. The QD hydrazide was purified by removing excess ADH and EDC with a sephadex G-25 column followed with three times washing by centrifugation with a 100K amicon ultra-4 filter (Millipore).
Intein-mediated conjugation of proteins onto QDs
Luc8 was conjugated to QDs by the addition of 4–40 μM of Luc8-GyrA to 130 nM of QD hydrazide in the presence of TCEP (at the same concentration as the protein) for 2 h at room temperature. The Luc8-QD conjugates were recovered by 4–5 times washings with a 100K amicon ultra-4 filter at 5000 g. For the preparation of protease nanosensors, for example, QD-MMP-7-Luc8, 10 μM of the fusion protein was added to 120 nM of QD hydrazide.
TEM and dynamic light scattering
Sizing of the nanoparticles was achieved by dynamic light scattering (Brookhaven 90 plus nanosizer) and transmission electron microscopy (TEM). TEM specimens were prepared by pipetting 5 μl of the samples onto ultrathin Carbon Type A 400-mesh copper grids (Tedpella Inc., Redding, CA) that had been glow-discharged. After about five minutes, the grids were rinsed with deionized water to remove any buffer salts that may be present in the samples and wicked to almost dryness using filter paper. Phosphotungstic Acid (PTA) at pH 7.0 was then added onto the grids to negatively stain the samples. After one minute the grids were completely dried using filter paper. All the specimens were analyzed using a Philips CM20 FEG-TEM operated at 200kV at the Stanford Nanocharacterization Laboratory (SNL). The microscope is also equipped with an energy dispersive X-ray spectrometer (EDS) for compositional analysis.
Protease activity assay with QD-BRET sensors
To a 20 μl solution of 0.3 μM QD655-MMP-7-Luc8 conjugates in 20 mM Tris (pH 7.5) was added MMP-7 at a final concentration of 5 ng/ml-10 μg/ml. The mixture was incubated at room temperature for 1 h. MMP-7 activity was measured by the fluorescence and bioluminescence changes that recorded on Fluoro Max-3. For multiplex assay, 0.15 μM of QD655-MMP-2-Luc8 and 0.2 μM of QD705-uPA-Luc8 were mixed in 20 μl Tris buffer as the substrates.
Detection of MMP activity in tumor cells
Two tumor cell lines, HT29 (Human colorectal adenocarcinoma) and HT1080 (human fibrosarcoma), were cultured at 37 °C for 24 h with serum-free culture medium. Media from cells were concentrated by centricon (Millipore, molecular weight cut-off 30K). MMP-2 activity was detected by incubation QD655-MMP-2-Luc8 in the media. The BRET signal changes were monitored by fluorometer. For comparison, the presence of MMP-2 in the cell culture media was examined by zymography using a 10% Zymogram (Gelatin) gel (Invitrogen). The concentrated media with SDS sample buffer were overloaded without reduction on a gel. After electrophoresis, the gel was incubated in zymogram renaturing buffer for 30 m at room temperature to remove SDS and renature the MMP-2. After then the gel was incubated in the developing buffer at 37 °C for overnight to induce gelatin lysis by renatured MMP-2. The gel was stained and destained with Coomassie blue staining kit.
RESULTS AND DISCUSSION
Design of MMP sensors and conjugation chemistry
Our design of the MMP nanosensors is based on the QD-BRET system we have previously developed, shown in Scheme 1a. We have demonstrated that BRET can occur between QDs and a bioluminescent protein such as a mutant of Renilla luciferase (Luc8) with the QD as an acceptor.32 If the QDs and Luc8 proteins are linked by an MMP substrate, BRET should occur due to the close proximity. The energy transfer efficiency can be quantified by the BRET ratio defined by the emission integration from QDs to that from Luc8. The cleavage of the substrate linker by the MMP will release Luc8 from the QDs, break the QD-BRET process and lead to a decreased BRET ratio.
The key to the preparation of such a nanoconjugate is the use of a protein splicing molecule—intein—to site-specifically ligate the MMP peptide substrate and Luc8 to the QDs. With this approach, our construction strategy (Scheme 1b) starts with a genetic fusion of the intein, the MMP substrate and Luc8 into a recombinant protein. We will then couple adipic dihydrazine (ADH) to EDC activated carboxylated QDs to generate hydrazide coated QDs because the hydrazide is an excellent nucleophile to attack the thioester intermediate in the intein fusion protein and form a stable adduct. The final conjugation proceeds by simply mixing the recombinant protein with the hydrazide QDs in mild buffer conditions. The nucleophilic attack by the hydrazide QDs on the thioester formed by the intein catalysis will cleave the intein out and ligate the C–terminus of the recombinant protein to the QDs (Scheme 1b).
Intein-mediated ligation of Luc8 to QDs
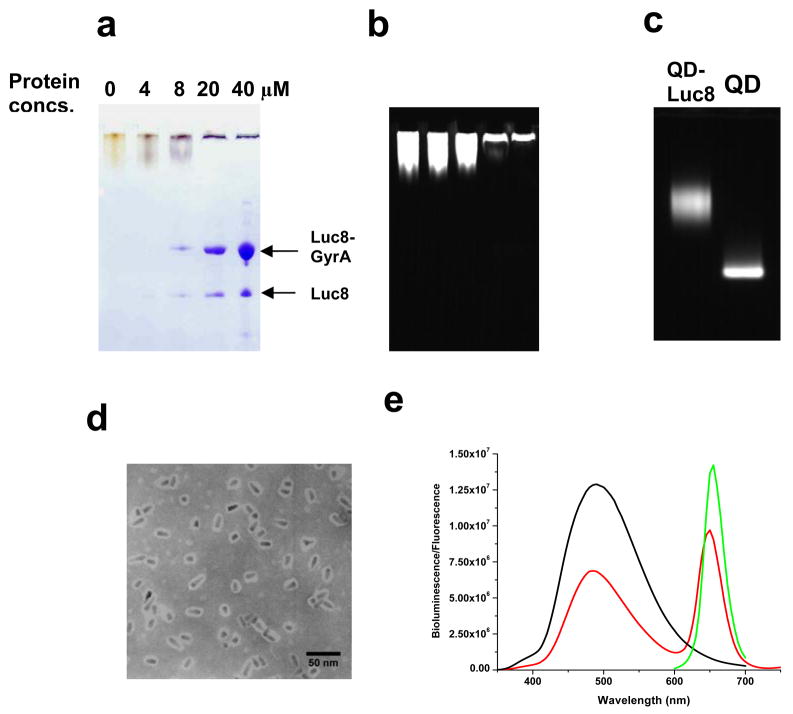
To establish the intein-mediated nanoconjugation strategy, we first prepared the recombinant protein with a Mex GyrA intein (Mycobacterium xenopi gyrase A intein, a 198-aa natural mini intein, which lacks a central intein endonuclease domain) 39 fused to the C-terminus of Luc8. Carboxylate coated QD655s were coupled to ADH to generate the hydrazide coated QD655s. The conjugation of this fusion protein with the hydrazide QD655s was carried out with different protein/QDs ratios at room temperature for 2 hours, and the progress of conjugation was monitored by the SDS polyacrylamide gel electrophoresis. The unconjugated QD655s appeared yellow on the gel after the Coomassie Blue staining while the conjugates became blue due to the staining of the attached proteins (Figure 1a). At 4 μM of the fusion protein concentration, little staining was observed on the gel at the fusion protein size (indicated by the arrow in Figure 1a) since they were conjugated to the QDs. When the protein was in excess, all the QD-Luc8 conjugates remained in the gel wells, probably due to the increased size upon the protein conjugation to QD655s (Figure 1a, b). The fusion protein could also have spontaneous hydrolysis during the reaction, which afforded the free Luc8 that was removed during centrifugation. The size difference between the conjugates and QD655s were further analyzed on the agarose gel, revealing a slower mobility of the QD conjugates in comparison to the unconjugated QD655s (Figure 1c). This conjugation was robust since there were no free QDs left in the reaction mixture (Figure 1c), confirming that the QD hydrazides were an efficient nucleophile. 40
Figure 1.
Characterization of intein-mediated conjugation of Luc8 to QDs. The reactions were carried out with 4–40 μM of Luc8-GyrA fusion protein and 130 nM of QD655 hydrazide in 20 mM Tris buffer, pH 7.5 at room temperature for 2 h. a) SDS-PAGE gel analysis of conjugation reaction. b) Fluorescence image of the SDS-PAGE gel in a (excited at 302 nm). c) Fluorescence image of agarose gel of the unconjugated QDs and the conjugation reaction mixture. d) TEM micrograph of the prepared QD655-Luc8 conjugates (Note that the white halos around the particles represent proteins). e) Fluorescence emission spectrum of QD655 (λex = 480 nm; green), bioluminescent spectrum of Luc8 in the oxidation of coelenterazine (black), and bioluminescence emission spectrum of ligation product QD655-Luc8 in Tris buffer (red).
The Luc8-QD conjugates were recovered by removing the excess proteins with filters, and were further characterized by dynamic light scattering and TEM. Dynamic light scattering measurement afforded an average hydrodynamic size of 48 nm, which was corroborated by the TEM result (Figure 1d).
The BRET efficiency of the conjugates was also evaluated. The fluorescence of QDs was observed upon the addition of coelenterazine, the substrate of Luc8, confirming the bioluminescence transfer from luciferase-catalyzed oxidation to QDs (Figure 1e). The BRET ratio (ratio of emission at 655 nm/480 nm) was calculated as 0.66. The smaller BRET ratio in comparison to QB-Luc8 conjugates prepared by EDC-mediated direct coupling may be due to the increased distance between QD and Luc8 and also the different orientation of the Luc8 protein on the QD. Indeed, the effect of both on the BRET efficiency has been previously observed; for example, the increased distance in the biotin-streptavidin mediated QD-Luc8 conjugate led to a BRET ratio of 0.37,32 and in the QB-Luc8 conjugates prepared by the EDC-mediated direct coupling, different pH and buffers may lead to the coupling at different amino groups of Luc8 with the QD, resulting in a different BRET ratio.33
QD nanosensor for MMP-7 detection in buffer and serum
After establishing the intein-mediated nanoconjugation method, we then applied it to prepare the nanosensor for MMP-7 detection. Matrilysin MMP-7 cleaves a peptide substrate VPLS-LTMG efficiently between the serine and leucine residues.38 This substrate sequence was genetically inserted into the recombinant protein between the Luc8 and GyrA. In a similar procedure, the intein-mediated conjugation of the fusion protein with the hydrazide coated QDs afforded the MMP-7 nanosensor QD655-MMP-7-Luc8.
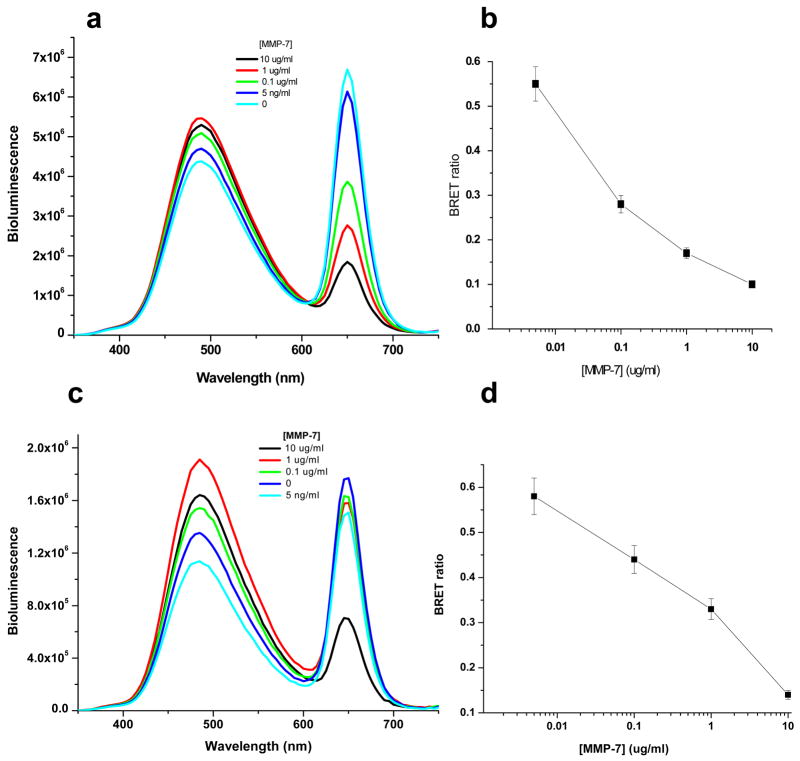
We first evaluated the response of QD655-MMP-7-Luc8 to MMP-7 activity in Tris buffer by measuring the BRET ratio (Figure 2). Before addition of MMP-7, the BRET ratio of the sensor was 0.66. Upon addition of MMP-7, the BRET emission from the QDs at 655 nm decreased, and the bioluminescence signal of Luc8 at 480 nm accordingly increased. Thus, the BRET ratio changes accurately reported the MMP-7 activity: the higher MMP-7 activity, the larger decrease the BRET ratio. With just 1 hour incubation at room temperature, 5 ng/ml of MMP-7 was readily detectable with a decrease in the BRET ratio by more than 17% to 0.55.
Figure 2.
Detection of MMP-7 activity by nanosensor QD655-MMP-7-Luc8 in buffer (a, b) and in mouse serum (c, d). a) Representative emission spectra of QD655-MMP-7-Luc8 (0.3 μM) incubated with indicated MMP-7 concentrations for 1 h incubation in 20 mM Tris buffer (pH 7.5) at room temperature. The emission spectra were measured upon the addition of coelenterazine (1 μg). b) The BRET ratios in a) are plotted versus MMP-7 concentrations. c) Representative bioluminescence spectra of QD655-MMP-7-Luc8 (160 nM) incubated with indicated concentrations of MMP-7 in mouse serum for 1 h at room temperature. d) The BRET ratios in c) are plotted versus MMP-7 concentrations.
We further tested whether this nanosensor could detect the MMP-7 activity in mouse serum. The nanosensor was incubated with varying concentrations of MMP-7 in the mouse serum, and a similar concentration dependent BRET ratio decrease has been observed (Figure 2 c&d). For the range of tested MMP-7 concentration from 5 ng/ml to 10 μg/ml, the BRET ratio was nearly linearly proportional to the logarithmic concentration of MMP-7 for both buffer and serum samples. However, for the same MMP-7 concentration, a smaller decrease in the BRET ratio was observed in serum than in the Tris buffer, suggesting the catalytic efficiency of MMP-7 lower in serum than in the Tris buffer.
It is also important to note that during the measurements, if the amount of the sample was different, the individual emissions from the QDs (at 655 nm) and Luc8 (at 480 nm) would be different, but the BRET ratio canceled the variation in the concentration and thus remained the same. This ratiometric feature makes the assay more reliable and less dependent on the sampling errors in comparison to the intensity-based assays.
Multiplex detection of MMP-2 and uPA
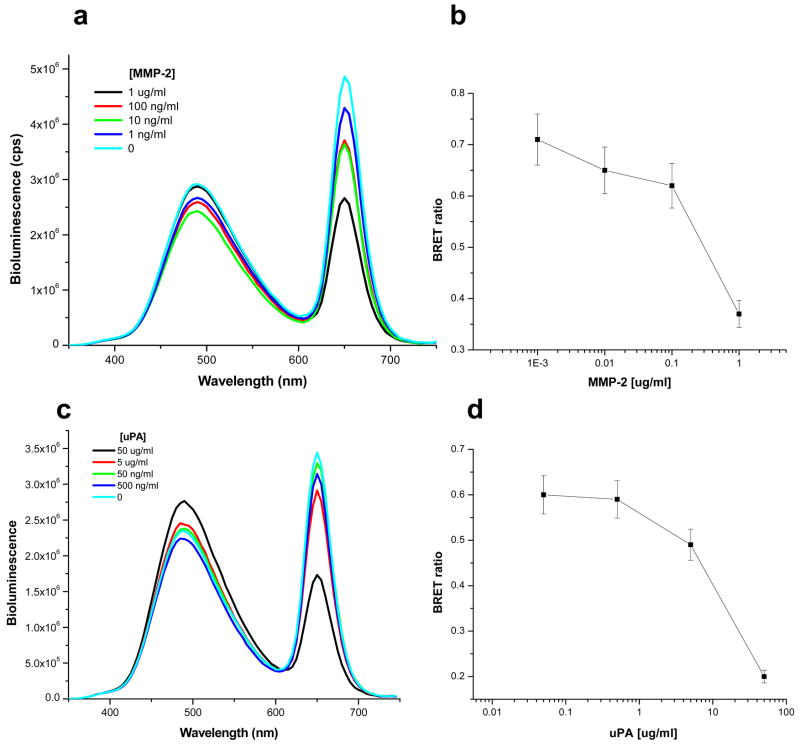
This intein-mediated conjugation method can be easily extended to prepare QD nanosensors for other proteases. By replacing the substrate peptide sequence of MMP-7 with peptide substrates for MMP-2 and urokinase-type plasminogen activator (uPA), we similarly made nanosensors QD655-MMP-2-Luc8 for detecting MMP-2, and QD655-uPA-Luc8 for detecting uPA. The MMP-2 nanosensor can detect as low as 1 ng/ml of MMP-2 (Figure 3a&b) and for the uPA QD nanosensor, the sensitivity was lower with around 500 ng/ml detected with 1 h incubation due to its generally lower catalytic efficiency than MMP-2—its kcat/KM for this peptide substrate is 1200 M−1s−1 (Figure 3c&d).41 This result suggests that our QD-BRET system can serve as a general detection platform for a variety of proteolytic enzymes.
Figure 3.
Detection of MMP-2 and uPA activity. a) Representative emission spectra of QD655-MMP-2-Luc8 (0.32 μM) incubated with indicated amount of MMP-2 in 20 mM Tris buffer (pH 7.5) for 1 h at room temperature. b) The BRET ratio in a) is plotted versus the MMP-2 concentrations. c) Representative emission spectra of QD655-uPA-Luc8 (0.32 μM) incubated with indicated amount of uPA in 20 mM Tris Buffer (pH 7.5) for 1 h at room temperature. d) The BRET ratio in c) is plotted versus the uPA concentration. All emission spectra were measured upon the addition of coelenterazine (1 μg).
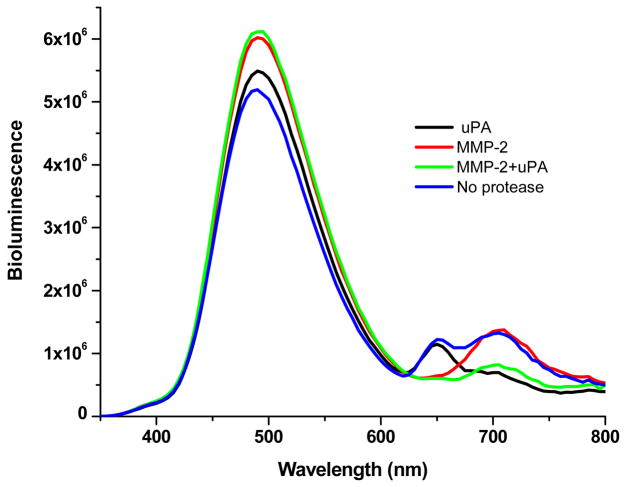
The bioluminescence emission of Luc8 overlaps well with all QD absorption spectra, thus Luc8 may excite several QDs as the BRET donor in a multiplex assay. We used QD705 to prepare the sensor QD705-uPA-Luc8, and mixed it with QD655-MMP-2-Luc8 for simultaneous detection of MMP-2 and uPA. As shown in Figure 4, both BRET emissions from QD655 and QD705 were observed in the spectrum. When MMP-2 was added to the mixture, only the BRET signals at 655 nm decreased; addition of uPA caused the decrease in the BRET signals at 705 nm. When both MMP-2 and uPA were present, the BRET emissions at 655 and 705 nm decreased. This result has demonstrated that the QD-BRET detection platform can simultaneously assay multiple targets.
Figure 4.
Simultaneous detection of MMP-2 and uPA. A mixture of QD655-MMP-2-Luc8 (0.15 μM) and QD705-uPA-Luc8 (0.2 μM) were incubated with MMP-2 (1 μg/ml; red), uPA (10 μg/ml; black), MMP-2 (1 μg/ml) + uPA (10 μg/ml) (green), or no enzyme (blue) at room temperature for 1 h in 20 mM Tris buffer (pH 7.5).
Detection of MMP-2 secreted by tumor cells
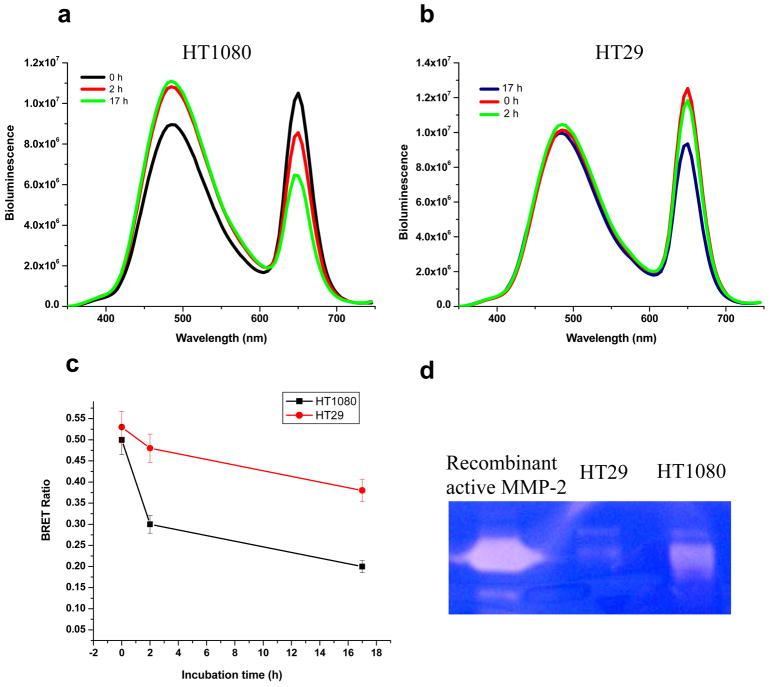
We finally applied the nanosensors to detect the protease activity in tumor cells. Human fibrosarcoma HT1080 cells have been shown to express and secrete high MMP-2 activity 42, and HT29 (Human colon adenocarcinoma cells) expresses low level MMP-2.43 Cell culture media from both cell lines were collected for MMP-2 assay by incubating with nanosensor QD655-MMP-2-Luc8. Within 2 h, HT1080 medium produced a decrease in the BRET ratio from 0.5 to 0.3, but little change in the BRET ratio was detected with HT29 cell medium (from 0.53 to 0.48) (Figure 5 a–c). After 17 h incubation, the BRET ratio for HT1080 dropped to 0.2, indicating near completion of the hydrolysis of the sensor. For HT29, the BRET ratio dropped to just 0.38 after 17 h of incubation. Gelatin zymography independently assayed the MMP-2 level, and confirmed the high activity of MMP-2 in HT1080 and low activity in HT29 cells (Figure 5d). This result shows that our nanosensors can quantify the enzyme activity from tumor samples.
Figure 5.
Detection of MMP-2 activity in tumor cell culture media. a) Representative emission spectra of QD655-MMP-2-Luc8 (220 nM) incubated in HT1080 cell culture media for 0, 2, or 17 h. b) QD655-MMP-2-Luc8 (250 nM) was incubated in HT29 cell culture media for 0, 2, or 17 h. c) The plot of the BRET ratio changes in HT1080 and HT29 cell culture media over time. d) Gelatin zymograph assay of the MMP-2 activity in cell media culturing HT1080 and HT29; commercial recombinant active MMP-2 was used as the control.
CONCLUSIONS
In conclusion, we present a protease sensing system based on the BRET between QDs and bioluminescent proteins. A series of nanosensors based on this platform have been made to detect MMP-2, MMP-7 and uPA activity in buffers and in mouse serum with the sensitivity to a few ng/ml concentration. Secreted proteases by tumor cells can be readily detected with our QD nanosensors. The suitability of these nanosensors for a multiplex protease assay has also been demonstrated. Comparing to fluorescence based QD nanosensors, this system offers a unique feature, which is the elimination of physical light excitation. This feature results in high sensitivity of the sensors and makes it work in complex biological media including serum and tumor samples. Another big advantage of this QD-based BRET assay system is that unlike many other methods, which usually detect the intensity changes, it measures the change of the ratio of two emissions. Since the BRET ratios are independent on the fluorescent intensity, the variation in the sample volume between measurements does not affect accurate detection of protease activity. Both characteristics of our BRET assay make it more reliable and sensitive in comparison with other assays.
In preparing these nanosensors, an intein-mediated conjugation method has been developed to site-specifically conjugate protein molecules to quantum dots. Unlike other receptor-mediated conjugation methods such as streptavidin and HaloTag protein,28,44,45 the intein method is a traceless ligation, that is, the intein itself is spliced out and excluded from the final conjugation product. This feature affords the conjugation product with a smaller size, which may be critical to many applications. This intein-mediated conjugation method should work with other nanoparticles as well.
Acknowledgments
We thank Dr. Xiang-Qin Liu at Dalhousie University for providing Ssp DnaE plasmid, Dr Samira Guccione at Stanford University for the access to the DLS instrument and Dr Yi-Shan Yang for technical assistance with the DLS instrument. This work was supported in part by the Department of Defense Breast Cancer Research Program Concept Award (W81XWH-06-1-0642), the National Cancer Institute (1R01CA135294-01) and the National Cancer Institute Centers of Cancer Nanotechnology Excellence (1U54CA119367-01).
References
- 1.Fingleton B. Front Biosci. 2006;11:479–491. doi: 10.2741/1811. [DOI] [PubMed] [Google Scholar]
- 2.Egeblad M, Werb Z. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 3.Brinckerhoff CE, Matrisian LM. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 4.Bachmeier BE, Iancu CM, Jochum M, Nerlich AG. Expert Rev Anticancer Ther. 2005;5:149–163. doi: 10.1586/14737140.5.1.149. [DOI] [PubMed] [Google Scholar]
- 5.Overall CM, Kleifeld O. Nature Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 6.Murphy G, Nguyen Q, Cockett MI, Atkinson SJ, Allan JA, Knight CG, Willenbrock F, Dochery AJP. J Biol Chem. 1994;269:6632–6636. [PubMed] [Google Scholar]
- 7.Knight CG, Willenbrock F, Murphy G. FEBS Lett. 1992;296:263–266. doi: 10.1016/0014-5793(92)80300-6. [DOI] [PubMed] [Google Scholar]
- 8.Pham W, Choi Y, Weissleder R, Tung CH. Bioconjugate Chem. 2004;15:1403–1407. doi: 10.1021/bc049924s. [DOI] [PubMed] [Google Scholar]
- 9.McIntyre JO, Fingleton B, Wells KS, Piston DW, Lynch CC, Gautam S, Matrisian LM. Biochem J. 2004;377:617–628. doi: 10.1042/BJ20030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bremer C, Tung CH, Weissleder R. Nat Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 11.Zhao M, Josephson L, Tang Y, Weissleder R. Angew Chem Int Ed. 2003;42:1375–1378. doi: 10.1002/anie.200390352. [DOI] [PubMed] [Google Scholar]
- 12.Harris TJ, von Maltzahn G, Derfus AM, Ruoslahti E, Bhatia SN. Angew Chem Intl Ed. 2006;45:3161–3165. doi: 10.1002/anie.200600259. [DOI] [PubMed] [Google Scholar]
- 13.Lepage M, Dow WC, Melchior M, You Y, Fingleton B, Quarles CC, Pepin C, Gore JC, Matrisian LM, McIntyre JO. Mol Imaging. 2007;6:393–403. [PubMed] [Google Scholar]
- 14.Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP. Science. 1998;281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 15.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Nat Mater. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 17.Burda C, Chen X, Narayanan R, El-Sayed MA. Chem Rev. 2005;105:1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 18.Katz E, Willner I. Angew Chem Int Ed. 2004;43:6042–6108. doi: 10.1002/anie.200400651. [DOI] [PubMed] [Google Scholar]
- 19.Daniel MC, Astruc D. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 20.Alivisatos P. Nat Biotechnol. 2004;22:47–52. doi: 10.1038/nbt927. [DOI] [PubMed] [Google Scholar]
- 21.Medintz IL, Konnert JH, Clapp AR, Stanish I, Twigg ME, Mattoussi H, Mauro JM, Deschamps JR. Proc Natl Acad Sci USA. 2004;101:9612–9617. doi: 10.1073/pnas.0403343101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medinitz IL, Clapp AR, Mattoussi H, Goldman ER, Fisher B, Mauro JM. Nat Mater. 2003;2:630–638. doi: 10.1038/nmat961. [DOI] [PubMed] [Google Scholar]
- 23.Somers RC, Bawendi MG, Nocera DG. Chem Soc Rev. 2007;36:579–591. doi: 10.1039/b517613c. [DOI] [PubMed] [Google Scholar]
- 24.Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. Curr Opin Biotechnol. 2005;16:63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal SJ, Tomlinson I, Adkins EM, Schroeter S, Adams S, Swafford L, McBride J, Wang Y, DeFelice LJ, Blakery RD. J Am Chem Soc. 2008;130:5720–5725. doi: 10.1021/ja003486s. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki M, Husimi Y, Komatsu H, Suzuki K, Douglas KT. J Am Chem Soc. 2008;130:5720–5725. doi: 10.1021/ja710870e. [DOI] [PubMed] [Google Scholar]
- 27.Shi L, De Paoli V, Rosenzweig N, Rosenzweig Z. J Am Chem Soc. 2006;128:10378–10379. doi: 10.1021/ja063509o. [DOI] [PubMed] [Google Scholar]
- 28.Kim YP, Oh YH, oh E, Ko S, Han MK, Kim HS. Anal Chem. 2008;80:4634–4641. doi: 10.1021/ac702416e. [DOI] [PubMed] [Google Scholar]
- 29.Troy T, Jekic-McMullen D, Sambucetti L, Rice B. Mol Imaging. 2004;3:9–23. doi: 10.1162/15353500200403196. [DOI] [PubMed] [Google Scholar]
- 30.Contag PR, Olomu IN, Stevenson DK, Contag CH. Nat Med. 1998;4:245–247. doi: 10.1038/nm0298-245. [DOI] [PubMed] [Google Scholar]
- 31.Contag CH, Bachmann MH. Annu Rev Biomed Eng. 2002;4:35–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 32.So MK, Xu C, Loening AM, Gambhir SS, Rao J. Nat Biotechnol. 2006;24:339–343. doi: 10.1038/nbt1188. [DOI] [PubMed] [Google Scholar]
- 33.So MK, Loening AM, Gambhir SS, Rao J. Nat Protocols. 2006;1:1160–1164. doi: 10.1038/nprot.2006.162. [DOI] [PubMed] [Google Scholar]
- 34.Evans TC, Jr, Xu MQ. Chem Rev. 2002;102:4869–4884. doi: 10.1021/cr9601369. [DOI] [PubMed] [Google Scholar]
- 35.Muralidharan V, Muir TW. Nat Methods. 2006;3:429–438. doi: 10.1038/nmeth886. [DOI] [PubMed] [Google Scholar]
- 36.Muir TW. Annu Rev Biochem. 2003;72:249–289. doi: 10.1146/annurev.biochem.72.121801.161900. [DOI] [PubMed] [Google Scholar]
- 36.Liu XQ. Annu Rev Genetics. 2000;34:61–76. doi: 10.1146/annurev.genet.34.1.61. [DOI] [PubMed] [Google Scholar]
- 37.Loening AM, Fenn TD, Wu AM, Gambhir SS. Protein Eng Des Sel. 2006;19:391–400. doi: 10.1093/protein/gzl023. [DOI] [PubMed] [Google Scholar]
- 38.Turk BE, Huang LL, Piro ET, Cantley LC. Nat Biotechnol. 2001;19:661–667. doi: 10.1038/90273. [DOI] [PubMed] [Google Scholar]
- 39.Telenti A, Southworth M, Alcaide F, Daugelat S, Jacobs WR, Jr, Perler FB. J Bacteriol. 1997;179:6378–6382. doi: 10.1128/jb.179.20.6378-6382.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalia J, Taines T. ChemBioChem. 2006;7:1375–1383. doi: 10.1002/cbic.200600150. [DOI] [PubMed] [Google Scholar]
- 41.Law B, Curino A, Bugge TH, Weissleder R, Tung CH. Chem Biol. 2004;11:99–106. doi: 10.1016/j.chembiol.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 42.Giambernardi TA, Grant GM, Maher VM, McCormick JJ, Taylor GP, Klebe RJ. Matrix Biol. 1998;16:483–496. doi: 10.1016/s0945-053x(98)90019-1. [DOI] [PubMed] [Google Scholar]
- 43.Stahtea XN, Roussidis AE, Kanakis I, Tzanakakis GN, Chalkiadakis G, Mavroudis D, Kletsas D, Nikos K. Int J Cancer. 2007;121:2808–2814. doi: 10.1002/ijc.23029. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, So MK, Rao J. Nano Lett. 2006;6:1988–1992. doi: 10.1021/nl0611586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, So MK, Loening AM, Yao H, Gambhir SS, Rao J. Angew Chem Intl Ed. 2006;45:4936–4940. doi: 10.1002/anie.200601197. [DOI] [PubMed] [Google Scholar]