Abstract
OBJECTIVE
Chronic seizures in women can have adverse effects on reproductive function, such as polycystic ovarian syndrome (PCOS), but it has been difficult to dissociate the effects of epilepsy per se from the role of antiepileptic drugs (AEDs). To distinguish the effects of chronic seizures from AEDs, we used the laboratory rat, where an epileptic condition can be induced without concomitant AED treatment.
METHODS
Adult female rats were administered the chemoconvulsant pilocarpine to initiate status epilepticus (SE), which was decreased in severity by the anticonvulsant diazepam. These rats developed spontaneous seizures in the ensuing weeks, and are therefore termed “epileptic.” Controls were saline-treated rats, or animals that were injected with pilocarpine but did not develop SE. Ovarian cyclicity and weight gain were evaluated for 2-3 months. Serum hormone levels were assayed from trunk blood, collected at the time of death. Paraformaldehyde-fixed ovaries were evaluated quantitatively.
RESULTS
Rats that had pilocarpine-induced seizures had an increased incidence of acyclicity by the end of the study, even if SE did not occur. Ovarian cysts and weight gain were significantly greater in epileptic rats than controls, whether rats maintained cyclicity or not. Serum testosterone was elevated in epileptic rats, but estradiol, progesterone and prolactin were not.
INTERPRETATIONS
The results suggest that an epileptic condition in the rat leads to increased body weight, cystic ovaries and elevated testosterone levels. Although caution is required when comparing female rats to women, the data suggest that epilepsy per se may be sufficient to induce abnormalities in the control of the ovary.
Keywords: Estrogen, progesterone, testosterone, prolactin, estrous cycle, ovaries, pilocarpine, status epilepticus, polycystic ovarian syndrome, temporal lobe epilepsy
INTRODUCTION
There have been many reports of impaired endocrine function in women with epilepsy, including loss of the ovarian cycle (amenorrhea) or irregular cycle duration (oligomenorrhea) and anovulatory cycles1,2. In addition, cases of reduced fertility have been documented 3,4, as well as premature menopause2,5,6, and polycystic ovaries (PCO) or polycystic ovarian syndrome (PCOS7,8), although the frequency is controversial9,10. PCO involves benign cysts that develop in the ovaries, and is more common in women with epilepsy than in the general population7. PCOS is a syndrome that has historically been associated with cystic ovaries, weight gain, hirsutism, and insulin resistance10-12. In 1990, a National Institutes of Health consensus definition was established that includes irregular menstrual cycles and hyperandrogenism, and suggests that weight gain and insulin resistance (“metabolic syndrome”) only be considered as symptoms that are ssociated with PCOS13. In 2003, the European Society of Human Reproduction and Embryology and the American Society for Reproductive Medicine consortium (the Rotterdam ESHRE/ASRM consortium) suggested that cystic ovaries be included in the definition14,15.
Based on the clinical data, it is unclear whether chronic seizures or anticonvulsant use is responsible for endocrinological changes in patients with epilepsy. For PCOS, this has been a subject of considerable debate16-20. Valproic acid appears to consistently increase the risk of ovarian cysts in women with epilepsy, and some other anticonvulsants also do so16,18-20. One way to address the contributions of chronic seizures vs. pharmacotherapy is to use an animal model of epilepsy, because the effects of seizures can be examined independent of anticonvulsant treatment. In addition, the effects of age and environment can be controlled, while genetic variation is reduced. Although it is difficult to generalize information from the rodent to humans, several parallels in data from past comparisons suggest it is useful, if approached with caution. Importantly, it seems reasonable to evaluate reproductive disorders like cystic ovaries, because valproic acid appears to induce cystic ovaries in normal female rats21, suggesting that some common ovarian syndromes may develop across mammals.
The goal of this study was to test the hypothesis that chronic seizures would lead to reproductive dysfunction without any history of antiepileptic (AED) treatment, using an animal model of epilepsy. We focused on an animal model of limbic seizures because it has been suggested that seizure activity in limbic structures might be particularly likely to influence the reproductive system, due to the interconnectivity of limbic nuclei with the hypothalamus2,7,22.
Testing this hypothesis is not simple, however, because most studies of female rodents with limbic seizures suggest that the experimental procedures to induce an epileptic state are accompanied by a loss of reproductive function. This problem precludes the goal, i.e., to study a reproductively normal animal with epilepsy for a long period of time, and determine if reproductive function ultimately becomes abnormal or not. For example, kindling the amygdala in the female rat causes cessation of the ovarian cycle (the “estrous cycle” in rodents) before the the epileptic state is reached23. Loss of the estrous cycle may be due to amygdala damage, because experimental lesions to the amygdala can lead to cessation of the estrous cycle in the rodent24,25.
Other methods to induce epilepsy, besides kindling, also are associated with reproductive abnormalities. For example, after intrahippocampal kainic acid injection in the anesthetized female rat, it was reported that the animals developed spontaneous seizures, but it was also mentioned that they had irregular estrous cycles26. Systemic pilocarpine administration has been used in female Wistar rats to induce SE, followed by an epileptic state after a delay of a few weeks27,28. These animals also demonstrated irregular estrous cycles27,28, although not universally. Indeed, some animals appeared to sustain many important components of reproductive function, because some could become pregnant27. Hormonal changes were reported27,28, but all animals were pooled, both those with apparently normal estrous cycles (cyclic) and irregular cycles (acyclic). Therefore, it is currently unclear how a state of epilepsy in the reproductively normal female rodent might ultimately lead to changes in endocrinological measures such as cyclicity, serum hormone levels, or pathology such as PCOS.
Here we used the pilocarpine model of temporal lobe epilepsy (TLE) to achieve these goals. Intervention with diazepam shortly after the onset of epilepsy was able to modify the procedure enough so that animals with regular estrous cycles were possible to study after SE. We also studied animals that were treated with pilocarpine, but did not develop SE. Some of these animals had a few limbic seizures immediately after pilocarpine administration, instead of SE. These animals are usually not studied at all, but were of interest because they allowed us to compare the effects of a single cluster of seizures in a normal rat, relative to an epileptic state.
METHODS
Subjects
Adult Sprague-Dawley female rats were obtained from Taconic farms (Germantown, NY). Rats were housed 2-3/cage at 64-75°F and 40-75% relative humidity, in standard opaque cages with corn cob bedding and a 12 hr light:dark cycle (lights on, 7:00 a.m.). Food (Purina 5001 chow; WF Fisher & Son, Somerville, NJ) and water were provided ad libitum. Cages with 1-2 adult male rats were placed between cages of female rats, to facilitate regular estrous cycles29.
All procedures met the guidelines of the New York State Department of Health and National Institutes of Health guidelines. Chemicals were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise specified.
Vaginal cytology and terminology associated with estrous cyclicity
Starting at 50 days of age, female rats were examined daily between approximately 9:00 a.m. and 12:00 p.m. to assess the relative frequency of cell types of the vaginal epithelium in a 30 μl sample of vaginal fluid23. For the purposes of the present study, nomenclature for the days of the estrous cycle are as follows: proestrus, estrus, diestrus 1 and diestrus 2. On proestrus, estradiol surges during the mid-morning, followed by a rise in progesterone that begins at approximately mid-day and ovulation in the evening (if the light cycle begins at 7:00 a.m.). By estrous morning, both estradiol and progesterone values have returned to baseline levels. During diestrus (diestrus 1 and diestrus 2), values of estradiol and progesterone are low relative to their peak values on proestrus day and the ensuing night25. We have previously shown that vaginal cytologic examination is predictive of cycle stage, confirmed by evaluating serum hormone levels of estradiol and progesterone30, which matched the values of the normal Sprague-Dawley rat31. Animals that failed to show three consecutive four-day patterns before treatment were excluded.
When estrous cycles became irregular in the course of this study, two patterns were evident. Both patterns reflect acyclicity, but suggest differences in the associated endocrine state32,33. One pattern, termed “irregular” was defined by vaginal cytology that lacked any pattern. From one day to the next, samples demonstrated leukocytes primarily, or the major cell type was an epithelial cell or cornified epithelial cell. There was no predominance of any one cell type for any 2 day period, which occurs during the normal estrous cycle, i.e., when leukocytes dominate for 2 days of diestrus, followed by a 2 day period when samples primarily include epithelial and cornified epithelial cells, signalling proestrus and estrus. In the acyclic rats that were irregular, ovulation occurred intermittently, indicated by a vaginal sample dominated by cornified epithelial cells, but there was no consistent diestrous period before or afterwards.
The second pattern of acyclicity was associated with persistent vaginal cornification, which reflects the endocrine state that is common in the aged rat, “persistent estrus.” Persistent estrus is accompanied by elevated serum estradiol levels and the predominance of cornified epithelial cells in the daily vaginal sample (i.e., an absence of leukocytes)24,33. Persistent estrus is also associated with cystic follicles24. Because of the differences in a rat that is an intermittent ovulator (irregular) and a rat in persistent estrus (no ovulation, chronic elevation in estradiol without diestrus), animals with persistent estrus are referred to differently than animals with intermittent ovulation.
Assays of steroid hormones
Trunk blood was collected at the time of death, and centrifuged to separate serum. Serum samples were frozen at -20°C until use. Radioimmunoassay (RIA) was conducted for estradiol as previously described,30,34 and enzyme-linked immunoabsorbent assay (ELISA) for progesterone and testosterone (ELISA kits 582701and 582901 from Cayman Chemicals, Ann Arbor, MI). Assays were conducted in triplicate, and averaged values used for statistical comparisons. The investigator who conducted the assays was blinded to the history of the animal, i.e., saline vs. pilocarpine treatment, epileptic or not.
Histology
Following deep anesthesia by urethane (2.5 g/kg, i.p.), animals were perfusion-fixed through the aorta with 0.9% NaCl for 3 minutes by gravity feed, followed by 4% paraformaldehyde (in 0.1 M phosphate buffer, pH 7.4) for 5 min. One ovary/animal was transferred to 30% sucrose solution after at least 3 days. Sections (35 μm) were cut using a cryostat (IEC Minotome; Global Medical Instrumentation, Inc.; Ramsey, MN) and consecutive sections were mounted on subbed slides. After drying overnight, sections were stained using cresyl violet. Slides were transferred through a graded series of alchohol (70%, 90%, 95%, 100%, 100%, 3 min each), cleared in xylene (5 min), rehydrated, rinsed in distilled water, and then incubated in 1% cresyl violet acetate for approximately 1 min. Sections were then rinsed in distilled water, dehydrated, cleared in xylene, and coverslipped in Permount (Fisher Scientific; Pittsburgh, PA).
To evaluate ovarian morphology more closely, the second ovary was embedded in paraffin. 8 μm sections from the ovary were cut with a microtome (Ultracut; Leica Microsystems, Inc.; Bannockburn, IL), deparaffinized, and alternate sections were mounted on subbed slides and stained with hematoxylin and eosin (Hematoxylin counterstain kit; Vector Laboratories; Burlingame, CA). Slides were incubated in hematoxylin solution for 1 min, rinsed in distilled water, immersed in acid rinse solution, rinsed in distilled water, incubated in bluing solution for 1 min, rinsed again, and mounted using Permount.
A blinded investigator examined sections at 2-100x using an Olympus BX51 microscope (Olympus of America, Center Valley, PA) and Insight Spot camera (Spectra Services Inc., Ontario, NY) to quantify the number of cysts per ovary and obtain photomicrographs. Cysts were defined as large fluid-filled cavities that differed from normal follicles in having only a thin granulosa cell layer23 (Figures 2,4). Consecutive sections were evaluated because cysts were large enough that they were present in more than one section. To be sure that each cyst was only counted once, serial sections were examined. There was no evidence of asymmetry in ovarian pathology, so the results report the number of cysts for only one of the ovaries.
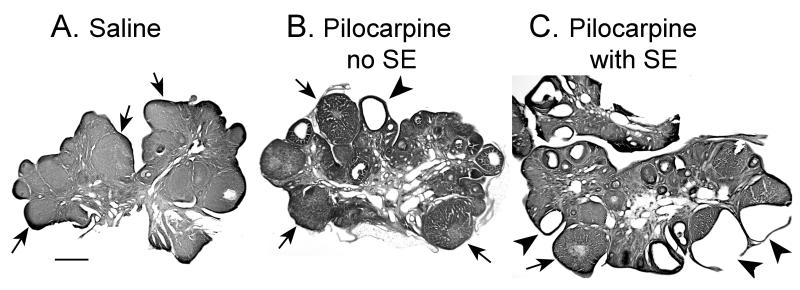
Figure 2. Epileptic rats exhibited greater ovarian pathology than control rats.
A. A micrograph demonstrates normal ovarian morphology in a saline control rat that was euthanized on first morning of diestrus, 3 months after treatment with saline. The ovary is dominated by corpora lutea (arrows) and there is no evidence of cystic follicles. Calibration = 500 μm.
B. A micrograph of an ovary from a different rat that was treated with pilocarpine but did not experience more than 1 stage 5 seizure. This rat demonstrated regular estrous cycles and was euthanized 2.5 months after pilocarpine treatment, on the morning of diestrus 1. There are normal corpora lutea (arrows) and a cyst (arrowhead).
C. A micrograph of an ovary from a rat that had pilocarpine-induced SE and maintained regular estrous cycles for 2.5 months thereafter, the length of the study. There are many cystic follicles (arrowheads), and some have a broken wall (arrowheads at lower right). The ovary exhibits only a few normal corpora lutea (arrows), despite the fact that the animal was euthanized on the first morning of diestrus, when corpora lutea should be abundant.
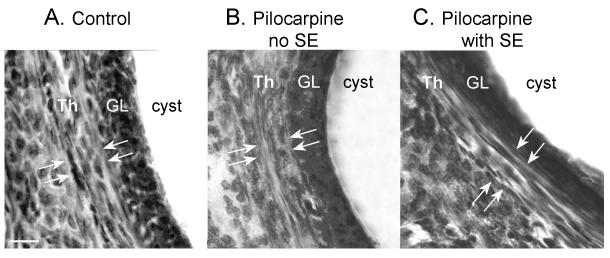
Figure 4. Lack of thecal cell hyperplasia in ovarian cysts of pilocarpine-treated rats.
Cystic follicles from a saline control (A), pilocarpine control (B), and epileptic rat (C). Hyperplasia of the thecal cell layer (Th) surrounding the granulosa cell layer (GL) was not detectable. Arrows demarcate the borders of the thecal cell layer. Calibration = 50 μm.
The pilocarpine model
Atropine methylbromide (1 mg/kg, s.c.) was injected, followed 30 min later by pilocarpine hydrochloride (350 mg/kg, s.c.). After 1 hr of SE had passed, diazepam (5 mg/kg, i.p.; Henry Schein, Inc.; Melville, NY) was injected. Saline controls received all drugs except pilocarpine, which was replaced by an equivalent volume of 0.9% saline. Diazepam was administered to saline controls and to pilocarpine-treated rats that did not enter SE. Diazepam was administered in these rats after approximately the same delay following atropine injection as the animals that had SE, i.e., approximately 1.5 hrs after injection of atropine.
Approximately 4 hrs after diazepam injection, dextrose-lactate Ringer’s solution (Henry Schein) was injected s.c. (2.5 ml), at the time when diazepam-induced sedation was maximal. For the subsequent 7 days, an apple that was cut open was left at the base of the cage. Food pellets were also placed at the base of the cage. Procedures for controls were the same as those animals that had SE.
Seizure monitoring
Monitoring was conducted to confirm that animals which had experienced SE developed a state of epilepsy. An epileptic state was defined as recurrent stage 3-5 seizures that were unprovoked and were observed sporadically over the months of the study. Intermittent observations captured multiple spontaneous seizures in all animals that had SE, so the use of intermittent observation did not appear to limit the ability to define an epileptic state. Daily behavioral observations were made for 10-30 min periods, chosen at random between 8:30 a.m. and 6:30 p.m., starting immediately after treatment with pilocarpine. Observations were made of rats that had SE, and pilocarpine-treated rats that did not have SE. Behaviors associated with seizures s (stages 1-5) were only observed in the animals that had SE. For the epileptic rats in this study, the first observed convulsion (stage 3 -5) occurred 5-10 days after pilocarpine treatment.
Data analysis
Statistical comparisons were made using Origin (v.7.0; OriginLabs, Northampton, MA), Simple Interactive Statistical Analysis (SISA; www.quantitativeskills.com) or Microsoft Excel (Microsoft Corp., Redmond, WA). Means ± standard errors are reported in the Results. Student’s t-tests assumed unequal variance and were two-tailed. The criterion for statistical significance (p) was 0.05. Micrographs were assembled using Adobe Photoshop CS (Adobe Systems Inc., San Jose, CA).
RESULTS
I. Experimental groups
Adult female rats were administered saline (n=14) or pilocarpine (n=74) on the morning (10:00-11:00 a.m.) of proestrus, estrus, diestrus 1, or diestrus 2, at approximately 2-3 months of age. In the text that follows, rats that were injected with saline instead of pilocarpine are “saline controls,” and pilocarpine-treated rats that did not have SE are referred to as “pilocarpine controls.” Pilocarpine-treated rats that had SE are referred to as “epileptic,” because they developed an epileptic state after SE, as defined above (see Methods). There was no significant difference in the mean age at the time of treatment (saline controls, 2.65 ± 0.17 months, n=14; pilocarpine controls, 3.11 ± 0.24 months, n=40; epileptic rats, 2.62 ± 0.36 months, n=9; one-way ANOVA: F= 0.922549; p = 0.4029).
An additional group of age-matched, untreated animals (n=14) was pooled with saline controls for some comparisons. In these instances, the pooled group is referred to as “controls” in these instances, rather than “saline controls.” The groups were pooled because their serum hormone values were not significantly different, nor was their incidence of cysts and acyclicity, or increase in body weight (see below).
Animals were euthanized at approximately 5 months of age during the mid-morning (10:00-11:30 a.m.). The age at the time of euthanasia for all animals was not different (controls: 5.92 ± 0.35 months, n=28; pilocarpine controls: 6.22 ± 0.30 months, n=40; epileptic rats: 5.69 ± 0.25 months, n=9; one-way ANOVA: F=0.444369; p=0.6429).
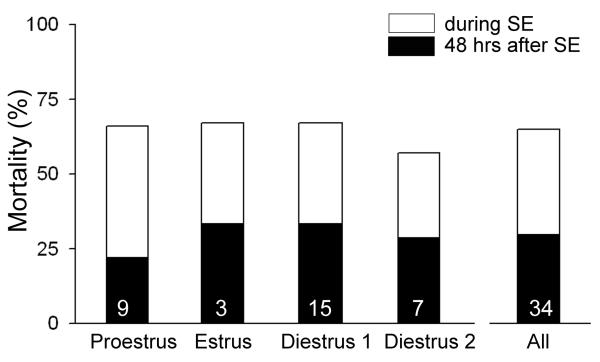
For those animals that had pilocarpine-induced SE (n=34), there was mortality in the 48 hrs after SE, despite the use of diazepam to reduce the severity of SE (Figure 1). This was surprising because male rats that were treated with pilocarpine to induce SE, and were similar in age to the females (i.e., the age range for males was 2.2-3.5 months old) exhibited significantly lower mortality, even with a higher dose of pilocarpine is required to initiate SE (380 mg/kg instead of 350 mg/kg; 4/21 or 19 % mortality within 48 hrs for males compared to 22/34 or 65% for females; χ2-test, p<0.05). Mortality in females did not appear to vary with cycle stage (Figure 1). Note that the lack of variation with cycle stage suggested that animals which received pilocarpine on different days of the estrous cycle could be pooled for further analysis, and this was done for the results described below. It is notable that few animals were injected with pilocarpine on the morning of estrus (3/34), because a previous study showed that SE rarely occurs when pilocarpine is injected at this cycle stage, using the pilocarpine dose employed here35.
Figure 1. Mortality after pilocarpine-induced SE in female rats.
Mortality associated with pilocarpine-induced SE is listed as a function of the day of the estrous cycle when pilocarpine was administered. Each animal was injected with pilocarpine in the morning (see Methods). The mortality in each group was defined either as death during SE (white portion of each bar) or within 48 hrs of SE (black portion of each bar). The total mortality is indicated by the top of each bar (sum of mortality during and after SE). There were no statistically significant differences in total mortality in the 4 groups (proestrus, estrus, diestrus 1 or diestrus 2; Fisher’s Exact test 2*4, p = 0.9502), suggesting that mortality was not influenced by the cycle stage when pilocarpine was administered. Only 3 animals were treated with pilocarpine on estrous morning, because previous studies showed that SE rarely occurs in animals treated on estrous morning using the dose in this study (350 mg/kg;27).
II. Estrous cyclicity
A. Effects of SE on estrous cyclicity
In epileptic rats, acyclicity was common. Of the 9 rats that survived SE, 3 rats stopped cycling immediately, and the remaining 6 that demonstrated regular estrous cycles initially after SE did not necessarily continue. Three of the 6 eventually developed irregular estrous cycles.
B. Estrous cyclicity in control rats
Even in some control animals, estrous cycles became irregular during the course of the study, which is typical of laboratory rats32,33. In our control group, 1/14 saline controls and 1/14 untreated controls (2/28; 7.1%) developed irregular cycles. In the pilocarpine control group (pilocarpine treatment but no SE), 15/29 rats (51.7%) became irregular in their estrous cycles before euthanasia. This difference in the incidence of acyclicity was significantly different (Fisher’s Exact test, p =0.000203). The pilocarpine controls that developed irregular estrous cycles were not statistically different in age from the controls that ultimately exhibited acyclicity, so age was unlikely to be a contributing factor (controls were followed until 5.93 ± 0.35 months of age, n=28; pilocarpine controls were monitored until they were 5.44 ± 0.20 months old, n=29; Student’s t-test, t statistic=1.1892; p=0.2409).
The acute effects of pilocarpine seemed unlikely to be a factor, since animals only developed acyclicity several weeks after pilocarpine was administered (> 20 days after pilocarpine administration). Therefore, even an acute period of stage 3-5 seizures that developed immediately after pilocarpine treatment, without SE, appears to ultimately influence estrous cycles adversely.
C. Incidence of persistent estrus
The acyclic rats discussed above demonstrated a pattern of vaginal cytology described as irregular. A minority of animals exhibited another pattern, which was reflected by the consistent cornified epithelial cell cytology associated with “persistent estrus,” a common condition in the aged rat (see Methods36,37).
Interestingly, no animal that experienced SE developed persistent estrus. The incidence was 0/9 (0%) for animals that experienced SE compared to 4/28 (14.3%) for controls and 11/40 (27.5%) for pilocarpine controls. The three groups were significantly different (Fisher’s Exact test 2*3, p=0.01342). Therefore, although brief stage 3-5 seizures may increase the risk of persistent estrus, SE appears to decrease it.
The greater incidence of persistent estrus in the pilocarpine-treated group that failed to have SE was surprising, and prompted us examine this group more closely. Pilocarpine controls that developed persistent estrus typically were the ones that exhibited stage 3-5 seizures immediately after pilocarpine treatment. Thus, 10/11 (90.9%) pilocarpine controls that developed persistent estrus had stage 3-5 seizures. Only 1/12 pilocarpine controls without stage 3-5 seizures developed persistent estrus (10/11 or 90.9% vs. 1/12 or 8.3%; Fisher’s Exact test, p=0.000098). Therefore, although pilocarpine itself may not induce persistent estrus, if robust seizures follow pilocarpine administration, there appears to be an increase in the incidence of persistent estrus.
II. Ovarian pathology
Rats that maintained estrous cyclicity throught the study were euthanized on the morning of the first day of diestrus. At this cycle stage, approximately 36 hrs after ovulation, the normal ovary is dominated by corpora lutea. Both saline control and pilocarpine control rats demonstrated ovarian morphology consistent with the normal ovary: a predominance of corpora lutea with very few mature follicles, and almost no evidence of cystic follicles (Figures 2,3). If cysts were present, they were small (less than 300 μm at their widest extent; Figure 2). In contrast, epileptic rats had many cystic follicles, and cysts were often large (> 500 μm; Figure 2). The thin cyst wall was often severed during the sectioning procedure (Figure 2), precluding quantitative comparisons of cyst size across groups.
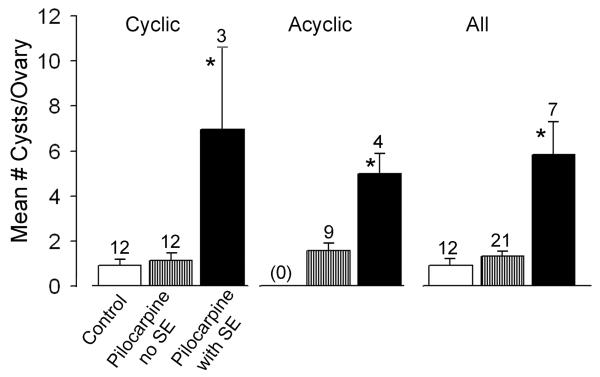
Figure 3. Ovarian cysts in control and epileptic rats.
Ovarian morphology was examined in animals that were administered saline or pilocarpine and were euthanized approximately 2-3 months after treatment. There were significantly more ovarian cysts in animals that had SE, whether they maintained estrous cyclicity throughout the study or not. Animals were similar in age at the time of treatment, and age at the time of death (see text), so age was unlikely to be a factor. In this figure and others, sample sizes are listed over the bar and significance is indicated by the asterisk. Means and statistical comparisons are listed in the text.
The gross morphology of cystic follicles was similar in each group. Thus, the wall was composed of a dense granulosa cell layer (Figures 2, 4), which tended to be thin and fragile in the epileptic animals, as mentioned above. There was no evidence of thecal cell hyperplasia around the cystic follicles in pilocarpine-treated as compared to control rats (Figure 4), in contrast to previous reports in kindled rats23. However, there was a different characteristic of the ovary of epileptic rats that appeared distinct from saline control rats. The ovary of the cyclic epileptic rats was not dominated by corpora lutea, despite the fact that animals were euthanized during diestrus (Figure 2). Instead, numerous follicles at various stages of maturity were present, in addition to some corpora lutea. This mixture of cystic, maturing and degenerating follicles, as well as corpora lutea, contrasted with the ovaries of cyclic control animals, which were dominated by corpora lutea, as would be expected for this stage of the estrous cycle (Figure 2).
Only cyclic animals are discussed above. However, even when acyclic rats were evaluated, epileptic rats developed statistically greater numbers of cysts per ovary than pilocarpine controls (Figure 3). Therefore, independent of the regularity of the estrous cycle, epileptic rats demonstrated significantly more cystic follicles per ovary.
III. Weight gain
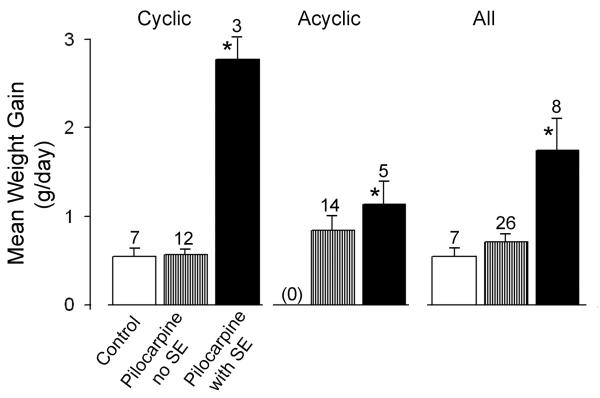
As shown in Figure 5, epileptic rats that maintained estrous cycles gained more weight than controls. For cyclic rats, epileptic rats gained 2.77 ± 0.36 g/day (n=3) whereas controls and pilocarpine controls gained 0.64 ± 0.11 (n=5) and 0.57 ± 0.056 (n=12), respectively (note that weight was not followed in every animal, so the sample size is lower than in other parts of the Results section). These differences were significant (one-way ANOVA: F=72.44715; p<0.0000001). Tukey-Kramer post-hoc analysis demonstrated no differences between controls and pilocarpine controls (p>0.05), but they were each statistically different from epileptic rats (p<0.05).
Figure 5. Weight gain in control and epileptic rats.
In animals that were treated with saline or pilocarpine, the body weight change in g/day was calculated from the time of treatment to the time of death. Animals that experienced SE gained more weight/day than controls, whether they maintained estrous cycles throughout the study or not. Animals that developed persistent estrus were excluded because this condition is accompanied by chronic elevation of estradiol, which has an anorectic effect in the rodent. Mean values and statistics are provided in the text.
For all rats (pooled, i.e., both cyclic and acyclic), epileptic animals gained more weight (1.75 ± 0.36, n=8) than controls (0.55 ± 0.098, n=7) and pilocarpine controls (0.71 ± 0.094, n=26; one-way ANOVA; F=10.72397; p=0.000203). Therefore, weight gain was greater in epileptic rats whether animals maintained estrous cycles or not.
IV. Serum hormone levels
A. Cyclic animals
Comparisons were made between cycling animals that were controls (n=21), pilocarpine controls (n=14), or epileptic (n=3). Values for estradiol, progesterone and testosterone in saline (n=9) vs. untreated controls (n=12) were not statistically different (Student’s t-tests, estradiol: t statistic=1.1882, p=0.2545; progesterone: t statistic=1.2480, p=0.2340; testosterone: t statistic =1.293, p=0.2435).
Prolactin is known to increase after seizures in patients and in laboratory animals38-40, but prolactin levels in cyclic epileptic rats were not different from non-epileptic controls (saline controls, 25.5 ± 7.5 ng/ml, n=6; pilocarpine controls, 39.5 ± 16.5 ng/ml, n=4; epileptic, 30.0 ± 7.0 ng/ml, n=3; one-way ANOVA: F=0.04721; p=0.9540). Therefore, based on serum values for prolactin that were measured in a subset of the cohort, it is unlikely that a recent seizure occurred immediately before euthanasia, which could potentially be confounding.
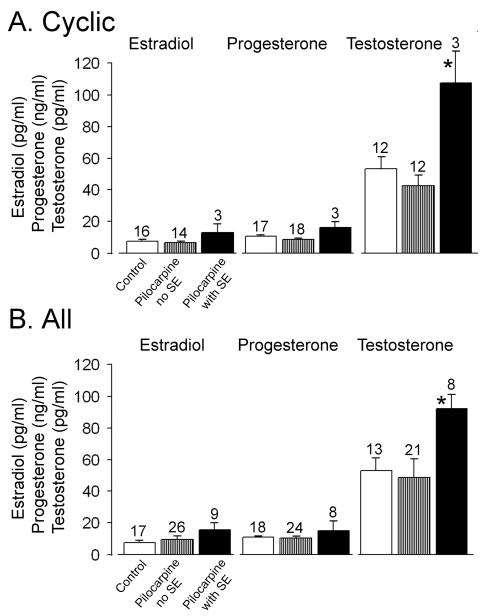
Serum values for estradiol were not statistically different among the three groups (controls: 7.17 ± 0.94 pg/ml, n=17; pilocarpine controls: 6.00 ± 1.12, n=14; epileptic: 12.33 ± 5.21, n=3; Figure 6; one-way ANOVA: F=2.4232; p=0.1052), nor were the values for serum progesterone (controls: 10.12 ± 0.78 ng/ml, n=18; pilocarpine controls, 8.05 ± 1.09, n=13; epileptic, 13.30 ± 3.51, n=3; Figure 6; one-way ANOVA: F=2.6988, p=0.08308). However, testosterone values were elevated in the epileptic rats (controls: 49.92 ± 7.36 pg/ml, n=13; pilocarpine controls 40.17 ± 6.14, n=12; epileptic 101.00 ± 19.22, n=3; Figure 6; one-way ANOVA: F=7.1242; p=0.00356). Post hoc tests revealed that there was no significant difference between controls and pilocarpine controls (Tukey post-hoc test, p<0.05), but they were significantly different from the epileptic group (Tukey post-hoc tests, p<0.05).
Figure 6. Elevated serum testosterone in epileptic rats.
Comparison of serum from control rats and rats that were treated with pilocarpine demonstrates elevated testosterone in rats that had pilocarpine-induced SE, whether they maintained estrous cyclicity throughout the study or did not. Animals were euthanized at the same time of day and time of the estrous cycle (diestrus 1 for cyclic rats, 10:00-11:30 a.m.; diestrus for acyclic rats, 10:00-11:30 a.m.). Mean values and statistics are provided in the text.
B. Acyclic animals
For animals that developed an irregular pattern in their daily vaginal cytology, euthanasia occurred on a day when the vaginal sample was dominated by leukocytes. This choice was made so that the time would approximate diestrus, which would provide a similar endocrine state at the time of death across animals, and maximize the ability to compare data to the cyclic group (euthanized on the first day of diestrus). Controls were not included because only 2 controls developed irregular estrous cycles, precluding statistical comparisons. Serum estradiol levels were not statistically different between pilocarpine controls with irregular cycles (estradiol: 11.96 ± 3.84 pg/ml, n=12), or epileptic rats with irregular cycles (15.83 ± 6.28, n=6; Student’s t-test, t statistic=-0.5263, p=0.6113), and progesterone values were also not different (pilocarpine controls, 12.20 ± 2.26 ng/ml, n=11; epileptic, 13.23 ± 9.41, n=5; Student’s t-test, t statistic=-0.1056; p=0.9201), but testosterone was higher in the epileptic rats (pilocarpine controls, 53.11 ± 23.81 pg/ml, n=9; epileptic, 77.6 ± 6.96, n=5; Student’s t-test, t statistic=-0.9874; p=0.3493; Figure 6). Therefore, whether rats were cyclic or irregular, testosterone was elevated but not estradiol or progesterone.
DISCUSSION
In previous studies of women with epilepsy, it has been difficult to clarify the effects of epilepsy from medications, as well as other factors that are difficult to control in a clinical setting. In laboratory animals, such as rats, the possible confounding contributions of medication, age, environment and genetic variation can be minimized. However, few studies have been conducted because the female rats that have been evaluated, after convulsant procedures that are used to initiate epileptogenesis, rarely exhibit normal estrous cycles23,26-28. For these reasons, there is no information available on the ways that reproductive function in a normal cycling female epileptic rat could be influenced by an extended duration of epilepsy. For example, would a few months of chronic seizures in an epileptic female rat (a relatively long time in the context of the rat lifespan) alter estrous cycles? Would serum hormone levels change? Importantly, there are studies of serum hormone levels in epileptic female rats27,28, but animals were not clearly distinguished between those that were cyclic or not, and as a result, animals with distinct endocrine states were likely to have been pooled. For cyclic animals, it is important to control for estrous cycle stage, given the rapid fluctuations in hormone levels that occur even in 8 hrs in the laboratory rat31,41.
Altered cyclicity in pilocarpine-treated female rats and women with epilepsy
The epileptic rats that maintained estrous cycles after SE usually became acyclic at some point thereafter. It is difficult to ignore the similarity to women with epilepsy, because women with epilepsy often develop menstrual cycle disturbances (amenorrhea, oligomenorrhea, anovulatory cycles8,22). Based on the data provided here, we suggest that a contribution to menstrual cycle changes in women with epilepsy could be the seizures.
There is an aspect of this study that is noteworthy in the context of a discussion concerning women with epilepsy: age. Women who are younger appear more vulnerable to menstrual cycle disturbances than older women4,8. Therefore, it is relevant that the cohort of female rats used in the current study were relatively young; in the female rat, puberty is reached at approximately 1.5 months42, and our animals were treated with pilocarpine at approximately 2-3 months of age. A greater incidence of acyclicity may have developed in the current study than would have been found if older female rats had been used.
The present study may be limited in how much it can tell us about women with epilepsy for several reasons. One that is obvious is that rodents were used. Another issue is that the seizures were limbic. The type of epilepsy may be important to reproductive dysfunction, although this is unclear at the present time. In analyses of patients with distinct types of epilepsy, no evidence was provided that the type of epilepsy was a factor in fertility9 or menstrual disorders 43. However,it has been suggested that limbic seizures are most likely to influence the reproductive system, and laterality plays a role, as well as the seizure focus2,22. In one study that compared patients with TLE and primary generalized epilepsy, it was reported that anovulation was more frequent in the patients with TLE 44. It makes intuitive sense that limbic seizures would be likely to influence reproductive function, given the robust interconnections between limbic nuclei and the hypothalamus, as discussed elsewhere2,3,7,17,22,24. In the pilocarpine model, seizures are considered limbic because the convulsions are consistent with the limbic seizures described by Racine45. The pilocarpine model in the rat is usually considered analogous to TLE,46,47 although this association might be best viewed with caution given that brain damage is typically greater in the pilocarpine model than in patients with TLE48,49, and electrographic seizures in pilocarpine-treated rats may show evidence of seizures or extralimbic origin. For example, seizures in pilocarpine-treated epileptic rats include spike and wave discharges (presumably thalamic in origin)50, which could be due to thalamic pathology in animals with SE51.
Use of the pilocarpine model in the current study is therefore appropriate, but not without caveats. One problem is that there was substantial mortality after SE and this could have led to a sampling bias. Another caveat is that an animal with limbic seizures may have an increased likelihood of reproductive endocrine dysfunction, for reasons discussed above. Comparisons to other animal models would be useful. However, information is limited. Studies of the kindling model have already shown that evoked stage 5 seizures readily produce reproductive dysfunction, as mentioned above (see Introduction). Therefore, use of kindling as an animal model has its limitations if one is to test the hypothesis that an epileptic state can lead to reproductive dysfunction. In the kindling model, it seems, simply the process of epileptogenesis causes reproductive endocrine abnormalities. Other models that have employed kainic acid or pilocarpine in Wistar rats, a strain that is distinct from the one used in the current study, did not investigate ovarian pathology, but did provide interesting data about pregnancy, and hormone levels. These studies also show that acyclicity develops, but the results are hard to compare with the present study because the animals were not segregated in the same ways26-28. To our knowledge, reproductive function in female rodents using other animal models have not yet been examined, but could help distinguish results specific to models involving kindling or SE from other types of epilepsy.
Acute seizures influence the estrous cycle adversely
Remarkably, rats which did not have SE, and were not epileptic, but exhibited robust (stage 3-5) seizures immediately after pilocarpine administration, still tended to exhibit reproductive abnormalities eventually. This is surprising because most laboratories consider rats treated with pilocarpine to require SE before significant changes develop that have long-term consequences. The data suggest that a brief period of seizures, particularly if they involve limbic structures, will impact the endocrine system. This could be due to changes within the hypothalamus after a brief cluster of seizures, immediately after convulsant administration (in this case, pilocarpine), possibly a consequence glutamate release from neurons projecting to hypothalamus that are involved in seizure activity. Pilocarpine itself is unlikely to have played a role, because only rats with pilocarpine-induced convulsions demonstrated long-term effects.
Polycystic ovaries in female epileptic rats
In this study, female rats with chronic seizures developed more ovarian cysts, greater weight gain, and had elevated serum testosterone levels relative to controls. The most parsimonious explanation is that a condition similar to polycystic ovarian disease developed in the female rat. Inasmuch as a comparison can be made between the rat model and the clinical condition of PCOS, the results suggest that seizures per se can lead to a PCOS like condition and other associated symptoms that are common in women with epilepsy. One cannot conclude that AEDs do not also play a role, in women with PCOS, but the data suggest that seizures themselves may contribute. This is significant because it supports the idea that chronic disturbances in reproductive function develop after a history of seizures, independent of AED use.
Although there are many hypothetical mechanisms to explain cyst formation, one that may be particularly relevant to women with epilepsy is irregular LH secretion, because it has been documented in women with epilepsy 52,53. The hypothesis that abnormal LH contributes to cyst formation was originally suggested from studies of women without epilepsy that had irregular LH secretion54. The cysts in these women can be explained by lack of ovulation due to disrupted neuroendocrine signaling. After ovulation fails, ovarian follicles eventually become cysts. Because LH normally stimulates the ovarian thecal interstitial cells to secrete androgen, hyperandrogenism would result as well. With consequent disruption of gonadal feedback to the hypothalamus, the basis for a chronic condition associated with cystic ovaries could be explained. This hypothesis remains attractive, although there are many alternative explanations for PCO and PCOS 55.
Increased body weight is an important issue in itself, because epilepsy is often associated with weight gain in both men and women. Interestingly, this may be more common in females56. In a review of the clinical literature, it was concluded that AED use can explain weight gain57, and potentially explain insulin resistance that in turn leads to weight gain18. However, data from untreated patients with epilepsy are unavailable. In light of the lack of data from an unmedicated population, it is unclear whether an epileptic condition is associated with changes in satiety, energy metabolism, and other factors that could lead to increased body weight. Interestingly, there is data to support an influence of seizures on body weight in rodents. In female rats, seizure-induction using lithium and pilocarpine was followed by extreme weight gain58. However, this may have developed because reproductive dysfunction occurred, leading to reduced estrogen levels, and a loss of the normal anorectic effect of estrogen59. Consistent with this hypothesis, males that were treated in the same way did not gain weight59. Other studies of epileptic male rats have reported loss of weight 60,61. Importantly, the current study would support the idea that female rats gain weight after pilocarpine simply due to their seizures, because estrogen levels were examined, and not significantly altered. More experiments will be required to prove that recurrent seizures increase the risk of weight gain and metabolic syndrome, and whether females are at higher risk than males, regardless of AED use.
Vulnerability of the female rat to SE
It is clear from the data that address mortality immediately after SE that there is an adverse effect of SE in female rats. The independence of mortality on cycle stage was surprising given the general concept that estrogen and progesterone (or its metabolites) are protective62,63. The lack of a relationship between estrogen or progesterone levels and mortality after SE may be due to the fact that SE is an extreme insult, and the protective effects of reproductive steroids are unable to mitigate such a severe challenge. An important consideration is that the chemoconvulsant used was a muscarinic agonist, and it is possible that another method to induce SE, one that would act by a different mechanism, might be more sensitive to the relative concentrations of serum gonadal hormones at the time of convulsant administration.
It is also interesting that there was a sex difference in vulnerability to pilocarpine-induced SE. In males, mortality was relatively low compared to females. This result could be due to the difference in body weight in the males and females that were tested. Male rats are heavier than female rats after approximately 35 days of age, and therefore at the ages that were used here. However, it is not clear, to our knowledge, that heavier animals have increased mortality after SE. Therefore, it is possible that there is an inherent sex difference in the risk of death after SE. To our knowledge, this is a novel concept, although studies of seizure severity provide some support. Convulsants appear to elicit more severe responses in female rodents 64-67, although this is not always the case68. Whether there is increased risk of mortality after SE in women is not supported by available data 69, although few studies appear to have been conducted. Therefore, additional research will be required to evaluate the possibility of sex differences related to mortality after severe seizures, such as SE.
Significance
The major implication of this study is that the female rodent can be used to examine issues that are relevant to endocrine dysfunction in women with epilepsy. Although epileptic female rats have been studied before, it has not been clear that the cycling female epileptic rat develops similar endocrine changes as women with epilepsy. We observed consequences of seizures that, at least superficially, resemble the symptoms reported in women with epilepsy. While caution clearly has to be exercized in extrapolating from any pharmacologically-manipulated animal model to humans, the data are encouraging because they suggest that it may be possible to use the rat model to dissect the underlying mechanisms that lead to reproductive problems in women with this disease. As a corollary of this hypothesis, one might be able to use the rat model as a basis for developing treatments that will enable woman with epilepsy to better maintain normal reproductive function.
Acknowledgements
NIH NS 37562, NIH HD 47890, NSERC.
REFERENCES
- 1.Svalheim S, Tauboll E, Bjornenak T, Roste LS, Morland T, Saetre ER, Gjerstad L. Do women with epilepsy have increased frequency of menstrual disturbances? Seizure. 2003;12:529–533. doi: 10.1016/s1059-1311(03)00195-x. [DOI] [PubMed] [Google Scholar]
- 2.Herzog AG. Menstrual disorders in women with epilepsy. Neurology. 2006;66(6 S3):S23–S28. doi: 10.1212/wnl.66.66_suppl_3.s23. [DOI] [PubMed] [Google Scholar]
- 3.Nappi C, Meo R, Di Carlo C, Estraneo A, Bilo L. Reduced fertility and neuroendocrine dysfunction in women with epilepsy. Gynecol Endocrinol. 1994;8:133–145. doi: 10.3109/09513599409058035. [DOI] [PubMed] [Google Scholar]
- 4.Schupf N, Ottman R. Reproduction among individuals with idiopathic/cryptogenic epilepsy: risk factors for reduced fertility in marriage. Epilepsia. 1996;37:8330840. doi: 10.1111/j.1528-1157.1996.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 5.Morrell MJ, Montouris GD. Reproductive disturbances in patients with epilepsy. Cleve Clin J Med. 2004;71(S2):S19–24. doi: 10.3949/ccjm.71.suppl_2.s19. [DOI] [PubMed] [Google Scholar]
- 6.Klein P, Serge A, Pezzullo JC. Premature ovarian failure in women with epilepsy. Epilepsia. 2001;42:1584–1589. doi: 10.1046/j.1528-1157.2001.13701r.x. [DOI] [PubMed] [Google Scholar]
- 7.Harden CL. Polycystic ovaries and polycystic ovary syndrome in epilepsy: evidence for neurogonadal disease. Epilepsy Curr. 2005;5:142–146. doi: 10.1111/j.1535-7511.2005.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isojarvi J. Disorders of reproduction in patients with epilepsy: antiepileptic drug related mechanisms. Seizure. 2008;17:111–119. doi: 10.1016/j.seizure.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Olafsson E, Hauser WA, Gudmundsson G. Fertility in patients with epilepsy: a population-based study. Neurology. 1998;51:71–73. doi: 10.1212/wnl.51.1.71. [DOI] [PubMed] [Google Scholar]
- 10.Polson DW. Polycystic ovary syndrome and epilepsy--a gynaecological perspective. Seizure. 2003;12:397–402. doi: 10.1016/s1059-1311(03)00066-9. [DOI] [PubMed] [Google Scholar]
- 11.Zawadzk JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic Ovary Syndrome. Blackwell Scientific; Boston: 1992. pp. 377–384. [Google Scholar]
- 12.The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 13.The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 14.Duncan S. Polycystic ovarian syndrome in women with epilepsy: a review. Epilepsia. 2001;42:60–65. doi: 10.1046/j.1528-1157.2001.042suppl.3060.x. [DOI] [PubMed] [Google Scholar]
- 15.Bilo L, Meo R. Epilepsy and polycystic ovary syndrome: where is the link? Neurol Sci. 2006;27:221–230. doi: 10.1007/s10072-006-0675-y. [DOI] [PubMed] [Google Scholar]
- 16.Genton P, Bauer J, Duncan S, Taylor AE, Balen AH, Eberle A, Pedersen B, Salas-Puig X, Sauer MV. On the association between valproate and polycystic ovary syndrome. Epilepsia. 2001;42:295–304. doi: 10.1046/j.1528-1157.2001.28899.x. [DOI] [PubMed] [Google Scholar]
- 17.Herzog AG, Schachter SC. Valproate and the polycystic ovarian syndrome: final thoughts. Epilepsia. 2001;42:311–315. doi: 10.1046/j.1528-1157.2001.33500.x. [DOI] [PubMed] [Google Scholar]
- 18.Isojärvi JI, Taubøll E, Tapanainen JS, Pakarinen AJ, Laatikainen TJ, Knip M, Myllylä VV. On the association between valproate and polycystic ovary syndrome: a response and an alternative view. Epilepsia. 2001;42:305–310. doi: 10.1046/j.1528-1157.2001.t01-1-28899.x. [DOI] [PubMed] [Google Scholar]
- 19.TaubŁ llE, Isojärvi JI, Harbo HF, Pakarinen AJ, Gjerstad L. Long-term valproate treatment induces changes in ovarian morphology and serum sex steroid hormone levels in female Wistar rats. Seizure. 1999;8:490–493. doi: 10.1053/seiz.1999.0342. [DOI] [PubMed] [Google Scholar]
- 20.Herzog AG. Disorders of reproduction in patients with epilepsy: primary neurological mechanisms. Seizure. 2008;17:101–110. doi: 10.1016/j.seizure.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Edwards HE, Burnham WM, Mendonca A, Bowlby DA, MacLusky NJ. Steroid hormones affect limbic afterdischarge thresholds and kindling rates in adult female rats. Brain Res. 1999;838:136–150. doi: 10.1016/s0006-8993(99)01619-4. [DOI] [PubMed] [Google Scholar]
- 22.Singh KB. Persistent estrus rat models of polycystic ovary disease: an update. Fertil Steril. 2005;84(S2):1228–1234. doi: 10.1016/j.fertnstert.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Mahajan DK. Polycystic ovarian disease: animal models. Endocrinol Metab Clin North Am. 1988;17:705–732. [PubMed] [Google Scholar]
- 24.Amado D, Verreschi IT, Berzaghi MP, Cavalheiro EA. Effects of intrahippocampal injection of kainic acid on estrous cycle in rats. Braz J Med Biol Res. 1987;20:829–832. [PubMed] [Google Scholar]
- 25.Amado D, Cavalheiro EA. Hormonal and gestational parameters in female rats submitted to the pilocarpine model of epilepsy. Epilepsy Res. 1998:266–274. doi: 10.1016/s0920-1211(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 26.Amado D, Cavalheiro EA, Bentivoglio M. Epilepsy and hormonal regulation: the patterns of GnRH and galanin immunoreactivity in the hypothalamus of epileptic female rats. Epilepsy Res. 1993;14:149–159. doi: 10.1016/0920-1211(93)90019-4. [DOI] [PubMed] [Google Scholar]
- 27.Whitten W. Modification of the oestrus cycle of the mouse by external stimuli associated with the male. J Endocrinol. 1956;13:299–303. doi: 10.1677/joe.0.0130399. [DOI] [PubMed] [Google Scholar]
- 28.Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 30.Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Brazil J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- 31.Mora OA, Cabrera MM. Pheromonal male-induced diestrus and cyclicity in aging intact and young estrogenized female rats. Biol Reprod. 1994;50:603–606. doi: 10.1095/biolreprod50.3.603. [DOI] [PubMed] [Google Scholar]
- 32.Scharfman HE, Hintz TM, Gomez J, Malthankar-Phatak GH, Stormes KA, Luine VN, MacLusky NJ. Changes in hippocampal function of ovariectomized rats in response to estradiol replacement that simulates the preovulatory estrogen surge. Eur J Neurosci. 2007;26:2695–2612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scharfman HE, Goodman JH, Rigoulot M-A, Berger RE, Walling SG, Mercurio TC, Stormes KA, MacLusky NJ. Seizure susceptibility in intact and ovariectomized female rats treated with the convulsant pilocarpine. Exp Neurol. 2005;196:73–86. doi: 10.1016/j.expneurol.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LeFevre J, McClintock MK. Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol Reprod. 1988;38:780–789. doi: 10.1095/biolreprod38.4.780. [DOI] [PubMed] [Google Scholar]
- 35.vom Saal FS, Finch CE, Nelson JF. Natural history and mechanisms of reproductive aging in humans, laboratory rodents, and other selected vertebrates. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven; New York: 1994. pp. 1213–1314. [Google Scholar]
- 36.Bauer J. Epilepsy and prolactin in adults: a clinical review. Epilepsy Res. 1996;24:1–7. doi: 10.1016/0920-1211(96)00009-5. [DOI] [PubMed] [Google Scholar]
- 37.Lin YY, Yen SH, Pan JT, Su MS, Wu ZA, Chan SH. Transient elevation in plasma prolactin level in rats with temporal lobe status epilepticus. Neurology. 1999;53(4):885–887. doi: 10.1212/wnl.53.4.885. [DOI] [PubMed] [Google Scholar]
- 38.Swartz CM, Dunbar E. Postictal prolactin elevations in rats. Neuropsychobiology. 1983;10:1–6. doi: 10.1159/000117976. [DOI] [PubMed] [Google Scholar]
- 39.Freeman ME. The ovarian cycle of the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press; New York: 1988. pp. 1893–1928. [Google Scholar]
- 40.Sharp P, La Regina MC. The Laboratory Rat. CRC Press; New York: 1998. [Google Scholar]
- 41.Murialdo G, Galimberti CA, Magri F, Sampaolo P, Copello F, Gianelli MV, Gazzerro E, Rollero A, Deagatone C, Manni R, Ferrari E, Polleri A, Tartara A. Menstrual cycle and ovary alterations in women with epilepsy on antiepileptic therapy. J Endocrinol Invest. 1997;20:519–526. doi: 10.1007/BF03348013. [DOI] [PubMed] [Google Scholar]
- 42.Cummings LN, Giudice L, Morrell MJ. Ovulatory function in epilepsy. Epilepsia. 1995;36:355–359. doi: 10.1111/j.1528-1157.1995.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 43.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 44.Cavalheiro EA. The pilocarpine model of epilepsy. Ital J Neurol Sci. 1995;16:33–37. doi: 10.1007/BF02229072. [DOI] [PubMed] [Google Scholar]
- 45.Coulter DA, McIntyre DC, Löscher W. Animal models of epilepsy: what can they tell us? Brain Pathol. 2002;12:240–256. doi: 10.1111/j.1750-3639.2002.tb00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turski L, Cavalehrio DA, Czuczwar SJ, Turski WA, Kleinrok Z. The seizures induced by pilocarpine: behavioral, electroencephalographic and neuropathological studies in rodents. Pol J Pharmacol Pharm. 1987;39:545–555. [PubMed] [Google Scholar]
- 47.Clifford DB, Olney JW, Maniotis A, Collins RC, Zorumski CF. The functional anatomy and pathology of lithium-pilocarpine and high dose pilocarpine seizures. Neuroscience. 1987;23:953–968. doi: 10.1016/0306-4522(87)90171-0. [DOI] [PubMed] [Google Scholar]
- 48.Nehlig A, Dube C, Koning E. Status epilepticus induced by lithium-pilocarpine in the immature rat does not change the long-term susceptibility to seizures. Epilepsy Res. 2002;51:189–197. doi: 10.1016/s0920-1211(02)00125-0. [DOI] [PubMed] [Google Scholar]
- 49.Bertram EH, Zhang D, Williamson JM. Multiple roles of midline dorsal thalamic nuclei in induction and spread of limbic seizures. Epilepsia. 2008;49:256–268. doi: 10.1111/j.1528-1167.2007.01408.x. [DOI] [PubMed] [Google Scholar]
- 50.Bilo L, Meo R, Valentino R, Buscaino GA, Striano S, Nappi C. Abnormal patterns of luteinizing hormone pulsatility in women with epilepsy. Fertil Steril. 1991;55:705–711. doi: 10.1016/s0015-0282(16)54234-4. [DOI] [PubMed] [Google Scholar]
- 51.Drislane FW, Coleman AE, Schomer DL, Ives J, Levesque LA, Seibel MM, Herzog AG. Altered pulsatile secretion of luteinizing hormone in women with epilepsy. Neurology. 1994;44:306–310. doi: 10.1212/wnl.44.2.306. [DOI] [PubMed] [Google Scholar]
- 52.Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12:351–361. doi: 10.1093/humupd/dml017. [DOI] [PubMed] [Google Scholar]
- 53.Matalliotakis I, Kourtis A, Koukoura O, Panidis D. Polycystic ovary syndrome: etiology and pathogenesis. Arch Gynecol Obstet. 2006;274:187–197. doi: 10.1007/s00404-006-0171-x. [DOI] [PubMed] [Google Scholar]
- 54.Hamed SA, Fida NM, Hamed EA. States of serum leptin and insulin in children with epilepsy. Risk predictors of weight gain. Eur J Paediatr Neurol. 2008 doi: 10.1016/j.ejpn.2008.05.005. E pub ahead of print: [DOI] [PubMed] [Google Scholar]
- 55.Ben-Menachem E. Weight issues for people with epilepsy--a review. Epilepsia. 2007;48(S9):42–45. doi: 10.1111/j.1528-1167.2007.01402.x. [DOI] [PubMed] [Google Scholar]
- 56.St-Pierre LS, Persinger MA. Extreme obesity in female rats following prepuberal induction of lithium-pilocarpine seizures and a single injection of acepromazine. Epilepsy Behav. 2005;7:411–418. doi: 10.1016/j.yebeh.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carl GF, Critchfield JW, Thompson JL, Holmes GL, Gallagher BB, Keen CL. Genetically epilepsy-prone rats are characterized by altered tissue trace element concentrations. Epilepsia. 1990;31:247–252. doi: 10.1111/j.1528-1157.1990.tb05372.x. [DOI] [PubMed] [Google Scholar]
- 59.Klitgaard H, Matagne A, Vanneste-Goemaere J, Margineanu DG. Pilocarpine-induced epileptogenesis in the rat: impact of initial duration of status epilepticus on electrophysiological and neuropathological alterations. Epilepsy Res. 2002;51:93–107. doi: 10.1016/s0920-1211(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki SBC, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- 61.Singh M. Progesterone-induced neuroprotection. Endocrine. 2006;29:271–274. doi: 10.1385/ENDO:29:2:271. [DOI] [PubMed] [Google Scholar]
- 62.Uzüm G, Akgün-Dar K, Bahçekapili N, Diler AS, Ziylan YZ. Nitric oxide involvement in seizures elicited by pentylentetrazol and sex dependence. Int J Neurosci. 2005;115:1503–1514. doi: 10.1080/00207450590957782. [DOI] [PubMed] [Google Scholar]
- 63.Thomas J, Yang YC. Allylglycine-induced seizures in male and female rats. Physiol Behav. 1991;49:1181–1183. doi: 10.1016/0031-9384(91)90348-r. [DOI] [PubMed] [Google Scholar]
- 64.Pericić D, Manev H, Bujas M. Gonadal hormones and picrotoxin-induced convulsions in male and female rats. Brain Res. 1996;736:174–179. doi: 10.1016/0006-8993(96)00677-4. [DOI] [PubMed] [Google Scholar]
- 65.Tan M, Tan U. Sex difference in susceptibility to epileptic seizures in rats: importance of estrous cycle. Int J Neurosci. 2001;108:175–191. doi: 10.3109/00207450108986513. [DOI] [PubMed] [Google Scholar]
- 66.Bujas M, Pericić D, Jazvinsćak MI. Influence of gender and gonadectomy on bicuculline-induced convulsions and on GABAA receptors. Brain Res Bull. 1997;43:411–416. doi: 10.1016/s0361-9230(97)00027-0. [DOI] [PubMed] [Google Scholar]
- 67.Rossetti AO, Hurwitz S, Logroscino G, Bromfield EB. Prognosis of status epilepticus: role of aetiology, age, and consciousness impairment at presentation. J Neurol Neurosurg Psychiatry. 2006;77:611–615. doi: 10.1136/jnnp.2005.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]