A 37-year-old HIV-positive African woman developed severe chronic diarrhea. Her CD4+ T cell count was 25 cells/mm3. Within 1 month of initiation of highly active antiretroviral therapy (HAART) her plasma HIV viral load became undetectable and CD4+ T cell count rose to 96 cells/mm3, and continued to rise over the following months.
Two months after the initiation of HAART she developed vertigo, loss of balance, incoordination, slurred speech, and tremor of the neck and limbs. Neurological examination revealed ocular abnormalities, dysarthria, and monotonic speech. She had bilateral limb dysmetria, past-pointing and endpoint tremor, impaired heel-knee-shin testing, head tremor, and a wide based, ataxic gait.
Initial brain MRI revealed a confluent, nonenhancing area of signal abnormality predominantly involving the inferior right cerebellar hemisphere and extending to the posterior vermis, right cerebellar peduncle, and inferomedial aspect of the left cerebellar hemisphere. Six months later, MRI revealed progression of the cerebellar lesion, with nodular enhancement along the inferomedial aspect of the right cerebellar hemisphere. The patient remained clinically stable. MRI 8 months later revealed a large cystic ring-enhancing lesion in the location of the previously noted high signal intensity lesions of the cerebellum, with compression of the posterior fourth ventricle (figure, A).
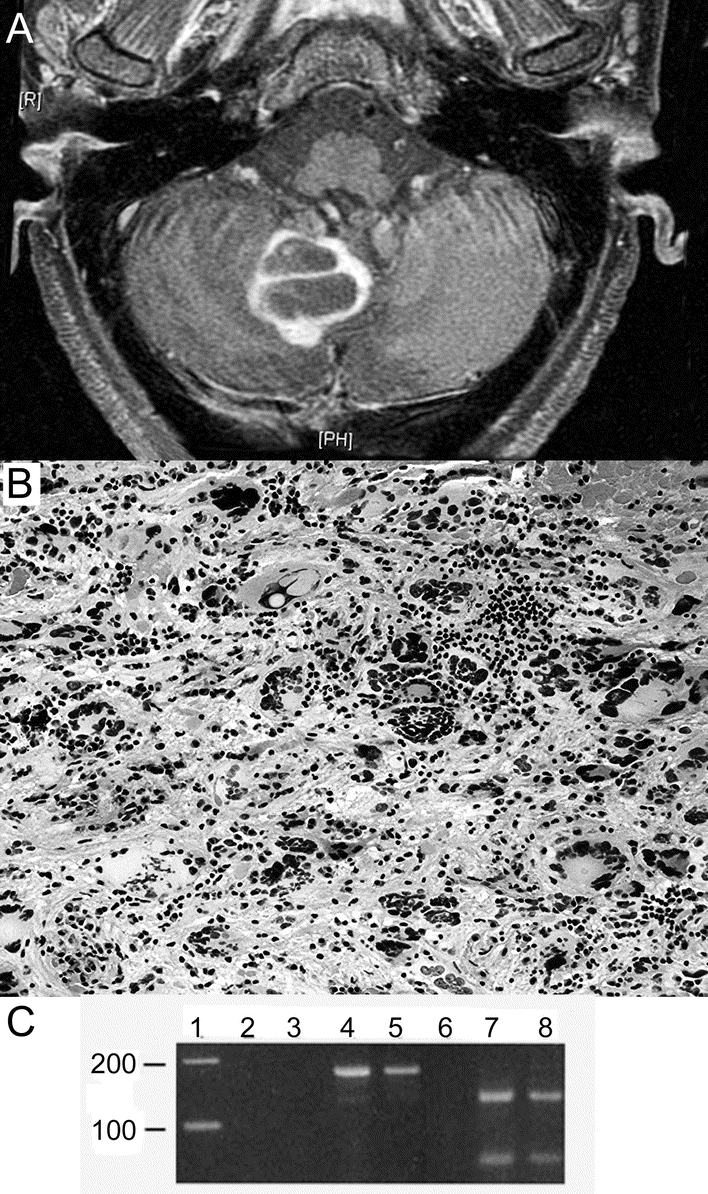
Figure MRI and histologic examination of the cerebellum
(A) Gadolinium-enhanced T1 FLAIR MR image demonstrating a large cystic ring-enhancing lesion in the context of high signal intensity lesions of the cerebellum. (B) Excisional biopsy of the cerebellum demonstrating bizarre multinucleated giant cells surrounded by an inflammatory cell infiltrate (hematoxylin-eosin, original magnification 100×). (C) Ethidium-bromide stained gel demonstrating presence of JCV in the patient’s pseudotumor. Formalin-fixed, paraffin-embedded sections of the patient’s lesion (lanes 4 and 7) and autopsy-derived progressive multifocal leukoencephalopathy (PML) (lanes 5 and 8) and normal brain (lanes 3 and 6) were used to extract DNA for PCR. Lanes 2, 3, 4, and 5 show the results of PCR amplification of a 173 base pair segment of polyoma virus in a reaction run without template DNA (lane 2), with normal brain DNA (lane 3), DNA from the patient’s lesion (lane 4), and from an unrelated case of PML (lane 5). Lanes 6, 7, and 8 display BamHI digests of the PCR products run in lanes 3, 4, and 5, respectively. Both the patient’s lesion and the case of PML show specific, 120 and 53 base pair fragments that occur only with JCV, which has a BamHI restriction site in the amplicon (SV40 and BKV do not share this restriction site). Lane 1 contains a 100 bp DNA ladder.
CSF revealed WBC 1, Prot 64, Gluc 49, and negative cytomegalovirus DNA PCR, Cysticercosis IgG Ab, Epstein-Barr virus DNA PCR, Venereal Disease Research Laboratory, and Cryptococcus Ag. Bacterial, viral, and fungal cultures were negative. JCV PCR was positive. Stereotactic biopsy of the cerebellar lesion, performed 17 months after the onset of neurologic symptoms, revealed giant cells with pleomorphic hyperchromatic nuclei, often multiple, surrounded by a dense infiltrate of lymphocytes and plasma cells (figure, B). The bizarre, pleomorphic cells were GFAP positive, demonstrated diffuse nuclear reactivity for p53 antigen, a high MIB-1 (Ki-67) index, and focal, faint reactivity for polyoma virus T antigen (figure e-1 on the Neurology® Web site at www.neurology.org). Inflammatory infiltrates marked both for T and B cells (CD3, CD43, CD20, CD79a). The adjacent cerebellar folia were atrophic, with total loss of granular cell neurons, preservation of Purkinje cells, and infiltrates of lymphocytes and histiocytes. Four 10-μm-thick sections of the mass were cut and utilized for DNA extraction; PCR was performed and demonstrated a 173 base pair band diagnostic of polyoma virus; its identity as JCV was further confirmed with a BamHI digest which produces 2 DNA fragments of 120 and 53 base pairs (JCV, but not BKV or SV40 has this restriction site in the amplicon)1 (figure, C). A JCV-associated inflammatory pseudotumor was diagnosed. The patient has a stable pancerebellar syndrome 24 months after onset of neurologic symptoms.
Discussion.
The immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients receiving HAART is characterized by paradoxical clinical or radiologic deterioration despite an increasing CD4+ T cell count and decreasing HIV viral load.2 Foreign organisms become unmasked and trigger a disproportionate immune response.2 IRIS has been reported in association with JCV infection, the cause of progressive multifocal leukoencephalopathy (PML).3 The radiologic features of PML are generally characterized by focal high-signal lesions predominantly affecting white matter structures; in the cerebellum, it has been associated with atrophy of the folia, with or without white matter involvement. While PML generally shows minimal contrast enhancement, this is more frequent in IRIS-associated PML. The radiologic features in the current patient, demonstrating a cystic lesion with nodular enhancement were unusual, raised the possibility of a secondary neoplastic or infectious non–JCV-related lesion, and led to the eventual performance of a brain biopsy.
PML is histologically characterized by the triad of oligodendroglial inclusions, demyelination, and bizarre, atypical astrocytes.4 In the cerebellum, selective loss of granular cell neurons, as seen in the present case, is common. PML may be associated with variable host inflammatory response. In the case of IRIS-associated PML, there are appreciable inflammatory infiltrates, with a preponderance of T cells.4,5 Oligodendroglial nuclei with characteristic viral inclusions may be rare or absent, and bizarre pleomorphic or multinucleated cells may have astrocytic or histiocytic origins.6 Pathology in the current patient was unusual, as the combination of dense inflammation and bizarre glial cells resulted in a pseudotumor formation, heretofore unreported in JCV-associated IRIS. In our patient, oligodendroglial inclusions were not evident, although the abnormal morphology of giant cells was typical of JCV-transformed astrocytes, and the granular cell loss was characteristic of PML. The juxtaposition of the intense predominantly lymphoplasmacytic infiltrate surrounding these transformed cells is likely to represent an IRIS-induced inflammatory response.7
This JCV-associated pseudotumor is an unusual manifestation of the spectrum of IRIS neuropathologies. Given its clinical and radiologic overlap with other tumoral and infectious entities, clinicians must be alert to the differential diagnosis.
Supplementary Material
Supplemental data at www.neurology.org
Supported in part by NIH grant R24MH59724.
Disclosure: The authors report no disclosures.
Received December 4, 2007. Accepted in final form August 14, 2008.
Address correspondence and reprint requests to Dr. Alejandra Gonzalez-Duarte, Mount Sinai Medical Center, Annenberg 2nd Floor, Box 1052, New York, NY 10029; gonzalezduarte@aol.com
&NA;
- 1.Arthur RR, Dagostin S, Shah K. Detection of BK virus and JC virus in urine and brain tissue by the polymerase chain reaction. J Clin Microbiol 1989;27:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murdoch DM, Venter WD, Van Rie A, Feldman C. Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options. Aids Res Ther 2007;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts MT. AIDS-associated progressive multifocal leukoencephalopathy: current management strategies. CNS Drugs 2005;19:671–682. [DOI] [PubMed] [Google Scholar]
- 4.Brooks BR, Walker DL. Progressive multifocal leukoencephalopathy. Neurol Clin 1984;2:299–313. [PubMed] [Google Scholar]
- 5.Safdar A, Rubocki RJ, Horvath JA, Narayan KK, Waldron RL. Fatal immune restoration disease in human immunodeficiency virus type 1-infected patients with progressive multifocal leukoencephalopathy: impact of antiretroviral therapy-associated immune reconstitution. Clin Infect Dis 2002;35:1250–1257. [DOI] [PubMed] [Google Scholar]
- 6.Gray F, Bazille C, Adle-Biassette H, Mikol J, Moulignier A, Scaravilli F. Central nervous system immune reconstitution disease in acquired immunodeficiency syndrome patients receiving highly active antiretroviral treatment. J Neurovirol 2005;11 (suppl 3):16–22. [DOI] [PubMed] [Google Scholar]
- 7.Martinez JV, Mazziotti JV, Efron ED, et al. Immune reconstitution inflammatory syndrome associated with PML in AIDS: a treatable disorder. Neurology 2006;67:1692–1694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


