Abstract
Background:
Long QT syndrome (LQTS) typically presents with syncope, seizures, or sudden death. Patients with LQTS have been misdiagnosed with a seizure disorder or epilepsy and treated with antiepileptic drug (AED) medication. The gene, KCNH2, responsible for type 2 LQTS (LQT2), was cloned originally from the hippocampus and encodes a potassium channel active in hippocampal astrocytes. We sought to test the hypothesis that a “seizure phenotype” was ascribed more commonly to patients with LQT2.
Methods:
Charts were reviewed for 343 consecutive, unrelated patients (232 females, average age at diagnosis 27 ± 18 years, QTc 471 ± 57 msec) clinically evaluated and genetically tested for LQTS from 1998 to 2006 at two large LQTS referral centers. A positive seizure phenotype was defined as the presence of either a personal or family history of seizures or history of AED therapy.
Results:
A seizure phenotype was recorded in 98/343 (29%) probands. A seizure phenotype was more common in LQT2 (36/77, 47%) than LQT1 (16/72, 22%, p < 0.002) and LQT3 (7/28, 25%, p < 0.05, NS). LQT1 and LQT3 combined cohorts did not differ significantly from expected, background rates of a seizure phenotype. A personal history of seizures was more common in LQT2 (30/77, 39%) than all other subtypes of LQTS (11/106, 10%, p < 0.001).
Conclusions:
A diagnostic consideration of epilepsy and treatment with antiepileptic drug medications was more common in patients with LQT2. Like noncardiac organ phenotypes observed in other LQTS-susceptibility genes such as KCNQ1/deafness and SCN5A/gastrointestinal symptoms, this novel LQT2-epilepsy association raises the possibility that LQT2-causing perturbations in the KCNH2-encoded potassium channel may confer susceptibility for recurrent seizure activity.
GLOSSARY
- AED
= antiepileptic drug;
- LQT1
= type 1 LQTS;
- LQT2
= type 2 LQTS;
- LQTS
= long QT syndrome;
- TdP
= torsades de pointes.
Congenital long QT syndrome (LQTS) was first described as Jervell and Lange-Nielsen syndrome and Romano Ward syndrome in the late 1950s and early 1960s.1-3 LQTS is now understood as a collection of genetically distinct arrhythmogenic disorders resulting from genetic mutations in cardiac potassium and sodium ion channels, thus termed cardiac channelopathies.4 Recent investigations have shown that mutations in non-channel proteins can also cause LQTS.5,6 The trademark event for the patient with symptomatic LQTS is the potentially lethal ventricular dysrhythmia known as torsades de pointes (TdP).7 TdP can precipitate syncope, seizures, or sudden death, depending on whether the heart rhythm spontaneously reverts to normal rhythm or if the patient is defibrillated back to normal rhythm before death occurs.8,9
LQTS affects approximately 1 in 2,500 persons and patients with LQTS are often diagnosed with a seizure disorder, being fainters, or having “spells.”10 Numerous LQTS genotype-specific arrhythmogenic triggers have been identified.11,12 For example, swimming is relatively gene-specific for type 1 LQTS (LQT1)13,14 while events that occur in women during the postpartum period, as well as those triggered by auditory stimuli, often indicate the presence of type 2 LQTS (LQT2).12,15-18 The onset of TdP has been shown recently to be gene-specific as well, with LQT2 patients preferentially having pauses in cardiac rhythm prior to the onset of TdP.19 Besides the long appreciated sensorineural hearing loss observed in patients with bi-allelic KCNQ1 mutations (i.e., Jervell and Lange-Nielsen syndrome),5 the search for noncardiac phenotypic expression involving other organs continues. For example, patients with LQT3-causing SCN5A mutations have been demonstrated to have an increased prevalence of gastrointestinal symptoms.20
In 1995, loss-of-function mutations in the 1,159 amino acid-containing alpha subunit of the rapidly activating delayed rectifying potassium channel, IKr, encoded by the human ether a go-go related gene, HERG, were discovered as the cause for LQT2.21 In addition, the Drosophila homolog of the HERG gene was found to be encoded by the aptly named seizure locus. Mutations in the seizure locus caused temperature-induced hyperactivity followed by paralysis in Drosophila.22 Notably, the HERG gene, now annotated as KCNH2, was discovered originally in a hippocampal cDNA library in 1994, although murine ERG is expressed in other regions of the brain as well.23 Further studies reported that hippocampal expression of the ERG family of potassium channels is distributed preferentially to hippocampal astrocytes24 that may regulate neuronal excitability.24-27
Considering that the LQT2-associated HERG potassium channel is present and active in significant quantity in glial cells, and recognizing that blockage of potassium homeostasis in glial cells can be epileptogenic,24-26 it is tempting to speculate that patients with LQTS, particularly type 2 LQTS, may in fact exhibit neurally mediated seizures or epilepsy rather than simply a ventricular arrhythmia with subsequent collapse and seizure activity (i.e., torsadogenic seizures). Accordingly, we set out to test the hypothesis that either a prior personal or family history of seizure activity or treatment with antiepileptic drug (AED) medication were more common among patients with LQT2.
METHODS
Chart review.
In this IRB-approved study, the medical records were reviewed for all unrelated patients (n = 343) clinically evaluated and genetically tested for LQTS between 1998 and 2006 at two LQTS referral centers: 1) Mayo Clinic's Long QT Syndrome Clinic in Rochester, MN (n = 208) and 2) the Academic Medical Center, Amsterdam, The Netherlands (n = 135). The reviewers (J.N.J., N.H.) were blinded at all times to the patients' genotypes.
Classifications of patients were given based on genotype by a third author (C.M.H.) independent of the reviewers. Patients were classified as genotype positive LQTS if they had a clinically relevant genetic mutation in one of the known LQTS-susceptibility genes. Patients were classified as genotype negative LQTS if they had a clinical diagnosis of LQTS given by a physician specializing in LQTS (M.J.A., A.A.M.W.), but in whom genetic testing of the known LQTS-susceptibility genes was negative. A classification of normal was given if the patient had a negative genetic test as well as a negative clinical history for LQTS as determined by a physician specializing in LQTS (M.J.A., A.A.M.W.).
A positive “seizure phenotype” was defined as the presence of either a personal or family history of seizures or epilepsy or a history of AED therapy. A family history of seizures was considered positive if the patient could name the affected family member, and if the family member shared genetic material with the proband. Family members with a history of seizures but who had married into the family were excluded. Vague histories of seizures in distant family members not clearly known by the proband and their immediate family were excluded. Only unrelated patients were reviewed to prevent the presence of a few large families from skewing the family history data. A patient was considered to have a positive treatment history if they had been placed on an AED medication for greater than 1 day, and if the medical treatment was specifically prescribed for a presumed seizure disorder. AED treatment for nonepileptic conditions (e.g., chronic pain) was not included. Given that the current standard of care for the evaluation of LQTS does not include an EEG, documented EEG evidence of epileptiform activity was not a requirement for inclusion in this study.
We attempted to account for and exclude all acquired causes of seizures in the cohorts. Patients with a history of having a single seizure with documented fever were excluded. Also, patients with a history of seizures within months following a traumatic head injury (or patient's family members with a similar history) were excluded. All other seizure histories were classified as a positive seizure phenotype.
Genetic testing.
Patients in both the Mayo Clinic and Amsterdam cohorts had genetic testing performed using denaturing high performance liquid chromatography and direct DNA sequencing. For the Mayo cohort, comprehensive genetic testing for additional mutations in the five most common LQTS-susceptibility genes was performed routinely even after the first mutation was found, given that multiple mutations occur at a frequency of 5–10%. For the Amsterdam cohort, further genetic testing after first mutation identification was per protocol and was continued only if there was a severely prolonged QT interval or if the patient was symptomatic. The vast majority of the patients in the Amsterdam cohort of this study, however, were symptomatic, and thus had comprehensive assessment of at least KCNQ1 (LQT1), KCNH2 (LQT2), and SCN5A (LQT3), which accounts for approximately 75% of all LQTS and over 95% of the patients with genetically identifiable LQT1-12 genotypes.
Statistical analysis.
Statistical analyses were performed with the assistance of the Mayo Clinic CTSA Service Center, Rochester, MN. All continuous variables were reported as the mean ± SD. Proportions were analyzed and compared using a two-tailed Fisher exact test. Means were analyzed using the independent groups t test for means. QTc values were measured manually and calculated using the standard Bazett formula.28 A p value < 0.05 was considered to be significant.
RESULTS
Table 1 summarizes the demographics for the composite cohort and the individual Mayo Clinic and Amsterdam cohorts. Demographically, age was the sole significant difference between the two cohorts, with the Mayo cohort having a younger patient population (24 ± 15 years) than the Amsterdam cohort (32 ± 22 years, p = 0.001). Otherwise, there were no other significant differences found between the cohorts in terms of proportion of each genotype, QTc at diagnosis, or percent of patients with positive or negative genotypes.
Table 1 Demographic and genotype data

Overall, a positive seizure phenotype was recorded in 98/343 (29%) patients including 24% of the Mayo Clinic cohort and 36% of the Amsterdam cohort (table 1, p = 0.023). Twenty percent of the probands either had a personal history of seizures or were diagnosed clinically with epilepsy prior to the rendering of genotype proven LQTS. A personal seizure history was more common among the 135 unrelated cases in the Amsterdam cohort (30%) compared to the 208 unrelated cases in the Mayo Clinic cohort (13%, p < 0.001). In addition, 7% of the patients had received antiepileptic pharmacotherapy including 13% of the Amsterdam cohort compared to 4% of the Mayo Clinic cohort (p = 0.004).
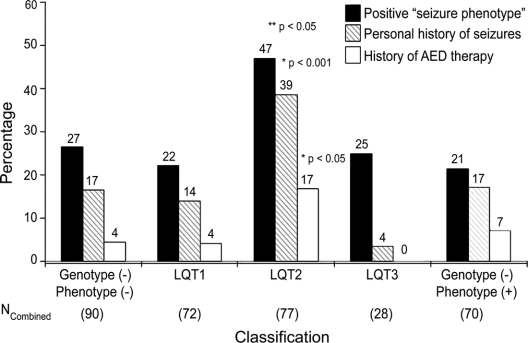
Among the 90 patients ultimately dismissed as normal (genotype negative/LQTS phenotype negative), 27% had a positive seizure phenotype, 17% had been diagnosed as having seizures, and 4% had been treated with AED medications (figure). In comparison, unrelated patients with genetically or clinically diagnosed LQTS had a similar prevalence of a positive seizure phenotype overall (31%, p = NS). However, subset analysis revealed that a positive seizure phenotype, a personal history of seizures, and a personal history of antiepileptic therapy was more common among patients with LQT2 (figure). In fact, a positive seizure phenotype was recorded in nearly half (36/77) of the patients with LQT2 compared to 16/72 (22%, p < 0.002) with LQT1, 7/28 (25%, NS) with LQT3, and 15/70 (21%, p < 0.002) with genotype negative/phenotype positive LQTS. Analysis of the Mayo Clinic and Amsterdam cohorts separately indicate this increased prevalence of positive seizure phenotype in LQT2, 36% in the Mayo Clinic cohort and 58% in the Amsterdam cohort.
Figure Prevalence of “seizure phenotype” in long QT syndrome subtypes
Percentage of patients from the combined cohort for each genotypic classification having a positive seizure phenotype (black bar), personal history of seizures (diagonal line), or history of treatment for seizures with antiepileptic drugs (AEDs, white bar). Not shown are patients with LQT5, LQT6, and LQT7 (n = 6), none of whom had a positive seizure phenotype. *Significance value of combined cohort of LQT2 patients compared to other long QT genotypes. **Significance value of combined cohort of LQT2 patients compared to the other LQTS genotypes (i.e. LQT1 and LQT3) and the genotype negative background rates. When compared to LQT3, LQT2 is more common (47% vs 25%) but did not achieve significance (p = 0.07).
Similarly, patients with LQT2 were far more likely to have a personal history of seizures or a preceding diagnosis of epilepsy (39%) compared to either the genotype negative/phenotype negative patients (17%) or the other subtypes of LQTS (11/106, 10%, p < 0.001, figure). This increased prevalence of personal seizures in LQT2 was evident in both cohorts independently: 26% vs 6% in the Mayo Clinic cohort (p < 0.008) and 53% vs 18% in the Amsterdam cohort (p < 0.001). A previous diagnosis of seizure disorder was more common among LQT2 patients seen in Amsterdam compared to the LQT2 patients evaluated at the Mayo Clinic (p < 0.02). Among the patients with LQT2 and a personal history of seizures (n = 30), 13 (17% of all LQT2 patients) had been treated with antiepileptic pharmacotherapy (9/20 from Amsterdam and 4/10 from Mayo Clinic) compared to only 12/266 (4.5%) non-LQT2 patients (p < 0.001) including 4% with LQT1, 0% with LQT3, 7% with genotype negative/phenotype positive LQTS, and 4% of patients that lacked both genetic and clinical evidence for a diagnosis of LQTS (figure).
A summary of the mutations noted in patients who have both LQT2 and a positive seizure phenotype is shown in table 2. There were no associations seen between patients with a personal or family history of seizures and the location of the mutation in the potassium channel.
Table 2 Mutations in patients with LQT2 and a positive “seizure phenotype”

DISCUSSION
It is well known that the most common symptomatic triad stemming from TdP (the trademark dysrhythmia of LQTS) is syncope, seizures, or sudden death.7 Seizures have been viewed as the sequelae of prolonged cerebral hypoperfusion secondary to the cardiac dysrhythmia (i.e., torsadogenic seizures). Because these patients may be witnessed having an apparent generalized seizure, it is not uncommon for patients with LQTS to be misdiagnosed with epilepsy and treated with AED medication.7,29
Here, we demonstrate that among two independent cohorts of unrelated patients evaluated clinically and genotyped for LQTS, a seizure phenotype was far more commonly ascribed to patients with LQT2 compared to either background rates or to patients with LQT1. A positive seizure phenotype was also more common in LQT2 compared to LQT3, but due to low total numbers of LQT3 patients, significance was not achieved. It is important to note that, while 39% of LQT2 patients had been labeled as having seizures, only a single patient with LQT3 had a personal history of seizures (p < 0.001).
Nearly half of the patients with LQT2 were classified as having either a personal or family history of seizures or history of AED therapy. In fact, patients with LQT2 were three to four times more likely to have been labeled with a personal history of seizures or formally given a diagnosis of epilepsy compared to patients without LQT2. In addition, nearly one out of five patients with LQT2 had been treated with antiepileptic pharmacotherapy compared to less than 1 out of 20 patients with LQT1 and none of the 28 patients with LQT3. Just like previous phenotype-genotype associations that identified auditory triggers as a relatively LQT2-specific arrhythmogenic trigger18 and the postpartum period as a relatively LQT2-specific temporal period for increased risk among women,17,30 seizures/epilepsy can now be added as far more suggestive of LQT2 genotype status than any other genotype.
KCNH2 was isolated originally from a hippocampal cDNA library.23 It is important to note that both the SCNA sodium channel family and KCNQ family of potassium channels have also been cloned in neuronal tissue.31-34 Indeed, it has been speculated that patients with SCN5A mutations could have an increased incidence of seizures.35 Our data only support an increased risk of seizure diagnosis in LQT2 compared to background. Neither LQT1 (KCNQ1) nor LQT3 (SCN5A) patients had a significantly greater seizure diagnosis frequency when compared to normal patients. Thus, even though there is similar neuronal expression of the two other main LQTS-susceptibility genes, LQT2 appears to be the only subtype commonly associated with a clinical expression of seizures.
Intriguingly, KCNH2-encoded potassium channels are instrumental in potassium homeostasis in hippocampal glia.24 Pharmacologic studies have also shown a relationship between preventing voltage-dependent potassium buffering in astrocytes and the development of epileptiform activity in the hippocampus.27,36 Voltage dependent buffering refers to the neurologic process whereby glia control the potassium concentration in the extraneuronal space in order to maintain normal neuronal conduction of action potentials.37 There has to be a constant extraneuronal potassium concentration in order for the action potential to effectively start and stop as needed. If this is perturbed, there is enhanced susceptibility for epileptic activity.36 Interestingly, blocking ERG potassium channels in astrocytes changes potassium concentrations extraneuronally,24 and such changes in potassium concentrations extraneuronally have been shown to be epileptogenic.36
Mechanistically, it is therefore conceivable that patients with LQT2 may have a decreased seizure threshold secondary to cerebral hypoperfusion stemming from a LQT2-precipitated cardiac dysrhythmia of TdP due in part to the perturbation in the neuronal KCNH2-encoded potassium channels that reside in the hippocampus. Alternatively, it is tempting to speculate that perhaps some of the “cardiac events” in patients with LQT2 are actually neurally mediated seizures secondary to the defect in the hippocampal potassium channels encoded by KCNH2. If patients with LQT2 have a tendency for neurally mediated seizures, then KCNH2 may represent a novel candidate gene for certain types of epilepsy, particularly temporal lobe epilepsy.
It should be noted that ERG potassium channel-encoding genes have been shown to be expressed in numerous locations in mouse brain tissue, not limited to the hippocampus.38 Thus, epileptiform involvement of HERG mutations in humans may not only be limited to the hippocampus or temporal lobe. An alternate plausible explanation is that seizure activity could occur secondary to hypoxic-ischemic injury to the hippocampus from unrecognized arrhythmias. This theory would not however explain the specific predominance of seizure history in LQT2 patients compared to other LQTS patients.
Unfortunately, erroneous treatment of LQTS patients with AEDs may in fact be harmful to the patient. Overall, 7% of patients in this study were treated with AED medications. Prior whole cell-patch clamp studies demonstrated that phenobarbital and phenytoin could block HERG-related currents potentially conferring susceptibility to drug-induced TdP, particularly in predisposed patients.39 While this is intriguing, it is also important to note that several antiepileptic medications, including phenytoin, are in part sodium channel blockers and are potentially anti-arrhythmic. In fact, decades ago, phenytoin was commonly used pharmacotherapy for patients with LQTS. Clearly, the results from this LQT2-seizure genotype/phenotype analysis call for further cardiac and neurologic pharmacology interaction studies.
Due to the retrospective study design, there exists the potential for reporting bias in the data collection process. However, the LQTS physicians (M.J.A., A.A.M.W.) solicited data regarding personal or family history of seizures or history of AED therapy uniformly and systematically in their clinical practices, and although not blinded to the patient's genotype, the genotype was generally not established during the initial LQTS consultation. Regardless of the vantage point, a seizure phenotype was more common among patients with LQT2. However, statistical correction for multiple comparisons was not performed.
In addition, we noted that the prevalence of a seizure phenotype in LQT2 was statistically greater among LQT2 patients evaluated in Amsterdam compared to those evaluated at the Mayo Clinic. It is possible that there could be an additional environmental/ethnic contribution. Considering that the patients in the Amsterdam cohort were significantly older on average than those in the Mayo cohort, it is also possible that the Amsterdam cohort simply had more life-years to have experienced a personal or family history of seizures. Regardless, the greater frequency of a personal history of seizures or prior diagnosis of epilepsy and prior treatment with AEDs among patients with LQT2 was completely concordant between both LQTS centers.
Due to the acute and unpredictable onset of clinical events in patients with LQTS, EEGs during events were unavailable to the authors. A postictal EEG was not available either. This is not surprising, however, as an EEG is not viewed as standard of care in the clinical evaluation of patients with LQTS. Certainly, functional proof of neuronally mediated seizure activity in LQT2 patients while in normal sinus rhythm would lend credence to one of the postulated mechanisms. As stated previously, those labeled with a seizure phenotype in this study, in the absence of EEG evidence, may simply have expressed seizure activity following their cardiac-mediated syncopal episode. Regardless of the underlying mechanism, however, two independent cohorts have evidenced a proclivity for seizure labeling among patients with mutations in the KCNH2-encoded, cardiac/neuronal-expressed potassium channel (LQT2). Just as it is debated whether all patients evaluated for generalized epilepsy should have a screening electrocardiogram, this new genotype–phenotype association will likely prompt a new dialogue as to whether patients with LQTS, particularly LQT2, should have a wake- and sleep-deprived EEG.
ACKNOWLEDGMENT
The investigators thank the patients who sought clinical evaluation at the respective LQTS clinics.
Address correspondence and reprint requests to Dr. Michael J. Ackerman, Director, Long QT Syndrome Clinic and the Mayo Clinic Windland Smith Rice Sudden Death Genomics Laboratory, Mayo Clinic, Guggenheim 501, 200 First Street SW, Rochester, MN 55905 ackerman.michael@mayo.edu.
Editorial, page 208
e-Pub ahead of print on November 26, 2008, at www.neurology.org.
*These two authors contributed equally to the research and writing of this article.
Dr. Michael Ackerman's research program was supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program. M.J.A. is also an Established Investigator of the AHA and is supported by the NIH (HD42569). Dr. Arthur Wilde's research program was supported by the Netherlands Heart Foundation (NHS 2000.059) and the Foundation Leducq (Grant 05 CVD, Alliance against Sudden Cardiac Death).
Disclosure: Dr. Ackerman is a consultant for PGxHealth, Medtronic, and Pfizer.
Received January 7, 2008. Accepted in final form July 16, 2008.
REFERENCES
- 1.Jervell A, Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J 1957;54:59–68. [DOI] [PubMed] [Google Scholar]
- 2.Romano C, Gemme G, Pongiglione R. Rare cardiac arrhythmias of the pediatric age: II: syncopal attacks due to paroxysmal ventricular fibrillation (presentation of 1st case in Italian pediatric literature) Clin Pediatr (Bologna) 1963;45:656–683. [PubMed] [Google Scholar]
- 3.Ward OC. A new familial cardiac syndrome in children. J Ir Med Assoc 1964;54:103–106. [PubMed] [Google Scholar]
- 4.Ackerman M.J. The long QT syndrome: ion channel diseases of the heart. Mayo Clin Proc 1998;73:250–269. [DOI] [PubMed] [Google Scholar]
- 5.Vatta M, Ackerman MJ, Ye B, et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 2006;114:2104–2112. [DOI] [PubMed] [Google Scholar]
- 6.Mohler PJ, Schott JJ, Gramolini AO, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 2003;421:634–639. [DOI] [PubMed] [Google Scholar]
- 7.Ackerman M.J: Cardiac channelopathies: it's in the genes. Nat Med 2004;10:463–464. [DOI] [PubMed] [Google Scholar]
- 8.Moss AJ, Robinson JL. Clinical aspects of the idiopathic long QT syndrome. Ann NY Acad Sci 1992;644:103–111. [DOI] [PubMed] [Google Scholar]
- 9.Vincent GM, Timothy K, Fox J, Zhang L. The inherited long QT syndrome: from ion channel to bedside. Cardiol Rev 1999;7:44–55. [PubMed] [Google Scholar]
- 10.Vincent GM, Timothy KW, Leppert M, Keating M. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N Engl J Med 1992;327:846–852. [DOI] [PubMed] [Google Scholar]
- 11.Ackerman MJ. Genotype–phenotype relationships in congenital long QT syndrome. J Electrocardiol 2005;38(4 suppl):64–68. [DOI] [PubMed] [Google Scholar]
- 12.Van Langen IM, Birnie E, Alders M, Jongbloed RJ, Le Marec H, Wilde AA. The use of genotype–phenotype correlations in mutation analysis for the long QT syndrome. J Med Genet 2003;40:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi G, Kopplin LJ, Tester DJ, Will ML, Haglund CM, Ackerman MJ. Spectrum and frequency of cardiac channel defects in swimming-triggered arrhythmia syndromes. Circulation 2004;110:2119–2124. [DOI] [PubMed] [Google Scholar]
- 14.Ackerman MJ, Tester DJ, Porter CJ. Swimming, a gene-specific arrhythmogenic trigger for inherited long QT syndrome. Mayo Clin Proc 1999;74:1088–1094. [DOI] [PubMed] [Google Scholar]
- 15.Rashba EJ, Zareba W, Moss AJ, et al. Influence of pregnancy on the risk for cardiac events in patients with hereditary long QT syndrome. LQTS Investigators. Circulation 1998;97:451–456. [DOI] [PubMed] [Google Scholar]
- 16.Tester DJ, Will ML, Haglund CM, Ackerman MJ. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm 2005;2:507–517. [DOI] [PubMed] [Google Scholar]
- 17.Khositseth A, Tester DJ, Will ML, Bell CM, Ackerman MJ. Identification of a common genetic substrate underlying postpartum cardiac events in congenital long QT syndrome. Heart Rhythm 2004;1:60–64. [DOI] [PubMed] [Google Scholar]
- 18.Wilde AA, Jongbloed RJ, Doevendans PA, et al. Auditory stimuli as a trigger for arrhythmic events differentiate HERG-related (LQTS2) patients from KVLQT1-related patients (LQTS1). J Am Coll Cardiol 1999;33:327–332. [DOI] [PubMed] [Google Scholar]
- 19.Tan HL, Bardai A, Shimizu W, et al. Genotype-specific onset of arrhythmias in congenital long-QT syndrome: possible therapy implications. Circulation 2006;114:2096–2103. [DOI] [PubMed] [Google Scholar]
- 20.Locke GR, 3rd, Ackerman MJ, Zinsmeister AR, Thapa P, Farrugia G. Gastrointestinal symptoms in families of patients with an SCN5A-encoded cardiac channelopathy: evidence of an intestinal channelopathy. Am J Gastroenterol 2006;101:1299–1304. [DOI] [PubMed] [Google Scholar]
- 21.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 1995;80:795–803. [DOI] [PubMed] [Google Scholar]
- 22.Titus SA, Warmke JW, Ganetzky B. The Drosophila erg K+ channel polypeptide is encoded by the seizure locus. J Neurosci 1997;17:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warmke JW, Ganetzky B. A family of potassium channel genes related to EAG in Drosophila and mammals. Proc Natl Acad Sci USA 1994;91:3438–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emmi A, Wenzel HJ, Schwartzkroin PA, et al. Do glia have heart? Expression and functional role for ether-a-go-go currents in hippocampal astrocytes. J Neurosci 2000;20:3915–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linden DJ. Long-term potentiation of glial synaptic currents in cerebellar culture. Neuron 1997;18:983–994. [DOI] [PubMed] [Google Scholar]
- 26.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 1999;22:208–215. [DOI] [PubMed] [Google Scholar]
- 27.D'Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Impaired K(+) homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci 1999;19:8152–8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazett H. An analysis of the time-relations of electrocardiograms. Heart 1920; 7:353–370. [Google Scholar]
- 29.Horn CA, Beekman RH, Dick M 2nd, Lacina SJ. The congenital long QT syndrome: an unusual cause of childhood seizures. Am J Dis Child 1986;140:659–661. [DOI] [PubMed] [Google Scholar]
- 30.Seth R, Moss AJ, McNitt S, et al. Long QT syndrome and pregnancy. J Am Coll Cardiol 2007;49:1092–1098. [DOI] [PubMed] [Google Scholar]
- 31.Donahue LM, Coates PW, Lee VH, Ippensen DC, Arze SE, Poduslo SE. The cardiac sodium channel mRNA is expressed in the developing and adult rat and human brain. Brain Res 2000;887:335–343. [DOI] [PubMed] [Google Scholar]
- 32.Biervert C, Schroeder BC, Kubisch C, et al. A potassium channel mutation in neonatal human epilepsy. Science 1998;279:403–6. [DOI] [PubMed] [Google Scholar]
- 33.Charlier C, Singh NA, Ryan SG, et al. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet 1998;18:53–5. [DOI] [PubMed] [Google Scholar]
- 34.Singh NA, Charlier C, Stauffer D, et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet 1998;18:25–29. [DOI] [PubMed] [Google Scholar]
- 35.Noebels JL. Sodium channel gene expression and epilepsy. Novartis Found Symp 2002; 241:109–120; discussion 120–123, 226–232. [PubMed]
- 36.Janigro D, Gasparini S, D'Ambrosio R, McKhann G, 2nd, DiFrancesco D. Reduction of K+ uptake in glia prevents long-term depression maintenance and causes epileptiform activity. J Neurosci 1997;17:2813–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience 2004;129:1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papa M, Boscia F, Canitano A, et al. Expression pattern of the ether-a-gogo-related (ERG) K+ channel-encoding genes ERG1, ERG2, and ERG3 in the adult rat central nervous system. J Comp Neurol 2003;466:119–135. [DOI] [PubMed] [Google Scholar]
- 39.Danielsson BR, Lansdell K, Patmore L, Tomson T. Phenytoin and phenobarbital inhibit human HERG potassium channels. Epilepsy Res 2003;55:147–157. [DOI] [PubMed] [Google Scholar]