Abstract
Background:
Level of education is a well-established risk factor for Alzheimer disease but its relation to cognitive decline, the principal clinical manifestation of the disease, is uncertain.
Methods:
More than 6,000 older residents of a community on the south side of Chicago were interviewed at approximately 3-year intervals for up to 14 years. The interview included administration of four brief tests of cognitive function from which a previously established composite measure of global cognition was derived. We estimated the associations of education with baseline level of cognition and rate of cognitive change in a series of mixed-effects models.
Results:
In an initial analysis, higher level of education was related to higher level of cognition at baseline, but there was no linear association between education and rate of change in cognitive function. In a subsequent analysis with terms to allow for nonlinearity in education and its relation to cognitive decline, rate of cognitive decline at average or high levels of education was slightly increased during earlier years of follow-up but slightly decreased in later years in comparison to low levels of education. Findings were similar among black and white participants. Cognitive performance improved with repeated test administration, but there was no evidence that retest effects were related to education or attenuated education’s association with cognitive change.
Conclusions:
The results suggest that education is robustly associated with level of cognitive function but not with rate of cognitive decline and that the former association primarily accounts for education’s correlation with risk of dementia in old age.
GLOSSARY
- AD
= Alzheimer disease.
Risk of dementia and Alzheimer disease (AD) in old age is reduced in persons with higher levels of educational attainment compared to those with lower levels.1–4 This finding is due in part to the well-established correlation of education with cognitive test performance at all ages. Thus, persons with more schooling, relative to those with less, are likely to begin old age at a higher level of cognitive function and so would need to experience more cognitive decline before reaching a level of impairment meeting dementia criteria. Another way in which education might influence risk of dementia is by a correlation with late life cognitive decline, the primary clinical manifestation of AD. Consistent with this idea, several studies have reported an association of higher educational attainment with reduced cognitive decline.5–23 However, this research is mostly based on change between two measurement points. Although this approach can provide an estimate of rate of change in cognitive function, it has a fundamental limitation: even with statistical adjustments, change in function between two time points is hard to securely distinguish from level of function at either point.24 This limitation is especially telling when the predictor of interest is highly correlated with the outcome as in the present case. Importantly, therefore, such studies are not well positioned to separate the estimate of education’s correlation with cognitive decline from its strong correlation with level of cognition. Assessment of cognition at three or more points in time permits separation of initial level of cognition from rate of change, but fewer of these studies have been published and their findings on the relation of higher educational attainment to cognitive decline have been mixed.12,17,19,21,25–28 Other factors may be contributing to this inconsistency. For example, education has been quantified in different ways (e.g., categorically vs continuously, linearly vs nonlinearly) and its association with cognitive decline may be modified by other variables (e.g., preexisting cognitive impairment, practice effects, race/ethnicity).
In the present study, we test the hypothesis that higher level of education is associated with reduced rate of cognitive decline in old age using data from the Chicago Health and Aging Project, a longitudinal population-based study of aging and AD. Participants are more than 6,000 older African American and white residents of a community on the south side of Chicago. At approximately 3-year intervals for up to 14 years, they completed four brief tests of cognitive performance from which a previously established composite measure of global cognition was derived. We used mixed-effects models to characterize person-specific paths of cognitive change and to test the relation of education to initial level of cognition and annual rate of change. In subsequent analyses, we examined other socioeconomic indicators and tested whether the association of education with change in cognitive function was modified by race, cognitive impairment, or repeated exposure to the cognitive tests.
METHODS
Participants.
To date, 10,186 older community residents have participated in the baseline interview; 118 (1%) were missing data on education or cognitive function. Of the remaining 10,068 people, 1,552 died before the first follow-up interview and 1,580 had not yet reached the date scheduled for the first follow-up. Among the 6,936 eligible for follow-up, 6,533 (94%) completed at least one follow-up interview. Analyses are based on this group. They completed a mean of 3.0 (SD = 1.1) interviews during a mean of 6.5 (SD = 3.6) years of observation. They had a mean age at baseline of 72.2 years (SD = 6.1) and a mean of 12.2 years of formal education (SD = 3.6); 61% were women and 67% were African American.
Assessment of cognitive function.
Four brief tests of cognitive function were administered as part of the in home interview. Immediate and delayed recall of 12 ideas contained in the East Boston Story were used to assess episodic memory. A modified version of the oral form of the Symbol Digit Modalities Test was used to assess perceptual speed. Global cognition was assessed with the Mini-Mental State Examination, a widely used mental status test. In a principal-components factor analysis of baseline data, these four measures loaded on a single factor that accounted for approximately 74% of the variance in the individual tests.29 Therefore, we formed a composite measure of global cognition by converting raw scores on each test to z scores, using the baseline mean and SD in the population, and then averaging the z scores to get the composite score, as described in previous publications.29–31
Assessment of other covariates.
Educational attainment was expressed as years of formal schooling completed, as reported by the participant. Race was assessed with the US Census questions. Income was assessed by asking participants to choose one of 10 levels of total family income from a “show card.” Lifetime occupation was quantified using the perceived occupational prestige scale of Featherman and Hauser.32 Data on five chronic medical conditions was obtained from self report of heart attack or myocardial infarction, hypertension, stroke, diabetes mellitus, and cancer.
Data analysis.
The statistical analyses were performed by Kenneth Tonnissen under the supervision of Dr. Hebert. We used mixed-effects models to characterize individual paths of change in cognitive function and to test the association of education with baseline level of cognition and rate of change. The initial model had terms for time (in years since baseline) and time squared to allow for nonlinear change. We then added terms for education, education × time, and education × time squared. This and all subsequent models also included terms for age, sex, race, and their interactions with time. We conducted identical analyses substituting first income and then occupation for education. Next, we repeated the analysis adding terms for education squared, education squared × time, and education squared × time squared. We repeated this latter model with terms added for chronic conditions and their interactions with time; with terms added for the interactions among race, education, and time; and with persons with low baseline levels of cognition excluded. To evaluate retest effects, we added indicators for the first, second, third, and fourth retestings (i.e., first, second, third, and fourth follow-ups), as previously described,33,34 and terms for the interaction of education with each indicator to see if retest effects varied by education. We also repeated analyses after excluding the first observation. Models were graphically and analytically validated.
RESULTS
Years of education had a mean of 12.2 (SD = 3.6) and an approximately normal distribution (skewness = 0.0; range: 0 to 30). Higher education was associated with younger age (r = −0.16, p < 0.001) and white race (t[6,532] = 28.9, p < 0.001) but not with sex (t[6,536] = 0.7, p = 0.493).
Change in cognitive function.
At baseline, the composite measure of global cognition ranged from −3.50 to 1.66 (mean = 0.24, SD = 0.74), with higher scores indicating better cognitive functioning. To assess change in cognitive function during the study period, we constructed a mixed-effects model with terms for time (in years since baseline) and time squared to allow for nonlinear change in cognition. The effects of time (estimate = −0.036, SE = 0.009, p < 0.001) and time squared (estimate = −0.001, SE < 0.001, p < 0.001) indicate that on average cognition declined at a gradually accelerating rate. To examine individual differences, we plotted the estimated paths for a 3% random sample of the population (figure 1). Substantial heterogeneity is evident with some persons rapidly declining and others showing little decline or slightly improving.

Figure 1 Predicted individual paths of change in global cognition estimated for a 3% random sample of the population
Education and change in cognitive function.
To test whether education was associated with these individual differences in cognitive decline, we constructed a new model with terms added for education and its interaction with time (table 1, model A). This and all subsequent analyses also included terms to control for the potentially confounding effects of age, sex, and race on initial level of cognition and rate of cognitive change. Higher level of education was associated with higher level of cognition at baseline, as shown by the term for education in table 1. There was no interaction between education and time, however, indicating that people with different levels of education did not differ in rate of cognitive decline. Substituting other socioeconomic indicators, income and lifetime occupation, yielded similar results: they were related to higher level of cognition at baseline but were unrelated to cognitive decline (data not shown).
Table 1 Relation of education to change in cognitive function

The association of education with cognition is probably not linear. Therefore, we repeated the analysis with four additional terms: the interaction of education with time squared, which allows the association of education with change in cognitive function to shift with time, plus a quadratic term for education and terms for its interactions with time and time squared (table 1, model B). It was only after the addition of these terms that education had any association with cognitive change. To better understand the complex interactions between time and education that emerged in this model, we plotted the predicted 12-year cognitive trajectories at three different levels of education (figure 2). Substantial differences are apparent in level of cognitive performance. Rates of cognitive change are broadly similar, but those with low education (8 years, 10th percentile) appear to be declining slightly less than persons with average (12 years, 50th percentile) or high (16 years, 90th percentile) levels of education but only in the early years of follow-up. By contrast, in the later years of follow-up there is a slight benefit of higher education though it is too small to be apparent in the figure. To see if differences in health affected findings, we repeated the analysis with indicators for the presence of five chronic conditions during the study period (heart disease, hypertension, stroke, diabetes, cancer), and results were unchanged (table 1, model C).

Figure 2 Predicted 12-year paths of change in global cognition in persons with 8 (solid line), 12 (dotted line), or 16 (dashed line) years of education, adjusted for age, sex, and race
Because little is known about the relation of education to cognitive decline in African Americans, we repeated the analysis (model B) with terms to allow for interactions among race, education, and time. The overall goodness of fit for this model was reduced compared to the original analysis, suggesting that the association of education with change in cognitive function did not vary by race.
Impact of cognitive impairment.
Among people with dementia, higher level of education has been associated with more rapid cognitive decline,35,36 suggesting that the presence of individuals with dementia or cognitive impairment in the population might obscure an association of higher education with slower decline in the remainder. To test this possibility, we repeated the analysis (model B) excluding those whose cognitive score at baseline was at or below the 10th percentile, and then conducted similar analyses using the 20th and then 30th percentiles as cutpoints. With these exclusions, which tended to remove people with low education and short follow-up, higher education was associated with slightly less rapid cognitive decline early in follow-up but with slightly more rapid decline later in follow-up.
Impact of retesting.
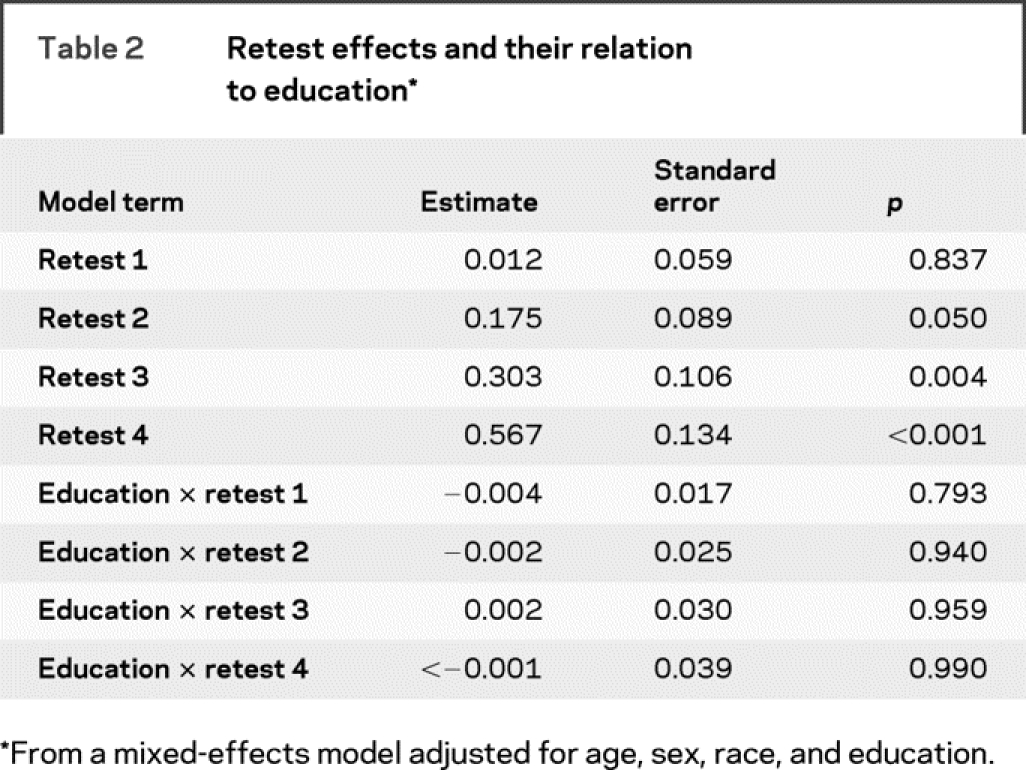
Repeated administration of cognitive tests can improve performance. If such retest effects were related to education, they might obscure an association with change in cognitive function. We investigated this possibility in two ways. First, to evaluate retest effects across the range of follow-up, we added indicators for the first, second, third, and fourth retestings and their interactions with education (table 2). The terms for the second, third, and fourth indicators were positive and significant, meaning that cognitive test performance improved with repeated test administration. These retest effects were not associated with education, however, as shown by the interaction terms in table 2. Second, to test whether education had a disproportionate impact on the initial testing experience, we repeated the primary model with the baseline observation removed. In this analysis, education’s association with change in cognitive function was eliminated.
Table 2 Retest effects and their relation to education
DISCUSSION
In more than 6,000 community dwelling old persons, we examined the relation of education to rate of change in cognitive function during up to 14 years of observation. Level of educational attainment was robustly related to level of cognitive function at study onset. By contrast, we found little evidence that education is associated with cognitive decline. When associations were observed, they were invariably small, with the presence and direction of effects conditional on other variables. In the context of previous work, the findings suggest that education affects risk of late life dementia primarily by virtue of its association with level of cognition and not by an association with rate of cognitive decline.
A simple tally of prior research tends to support an association between higher educational attainment and reduced cognitive decline, with nearly half of published studies reporting the association,5–11,13–15,22,23 and about one quarter finding mixed evidence of it.12,16–21 In the negative studies,25–28,37,38 however, change was more likely to be based on three or more cognitive assessments rather than only two (4/8 vs 2/19, p = 0.059, Fisher exact test) and on 6 or more years of observation rather than less than 6 (5/11 vs 0/14, p = 0.003, Fisher exact test). Further, in each of three projects that published two articles on education and cognitive decline, education was related to cognitive decline in the earlier publication,15,21,22 but not in the later publication despite additional data collection.25,26,28 In research with three or more assessments of cognition, evidence linking higher level of education with reduced cognitive decline has been limited, with four studies reporting no association25–28 and four finding it to be conditional on some other variable (e.g., sex,12 cognitive outcome12,17,19). In short, the studies best equipped to assess change in cognitive function, by virtue of measuring it more often during a longer period of time, were the least likely to find an association with education. This is the opposite of the pattern that one would expect if education were truly related to change in cognitive function. Instead, we propose that education has little or no association with change in cognition and that evidence to the contrary mainly reflects difficulty disentangling level of cognition, which is robustly related to education, from change.
Little is known about education and cognitive decline in black persons.39 In this biracial population, the association of education with cognitive decline was comparable in black and white participants.
Repeated administration of cognitive tests leads to improved performance,33,34 and such retest effects might influence the association of education with cognitive change.27 One prior study did find that lower education was associated with greater improvement from the first to second testing and that after controlling for this apparent retest effect, higher education predicted less cognitive decline during the remainder of the study.21 However, cognitive retest effects are known to persist beyond a single retesting34 and education was not related to retest effects across the range of follow-up in this population, consistent with previous research.34 There was a differential effect of the first observation, but deleting it eliminated education’s association with cognitive change rather than enlarging it.
Because education’s association with change in cognitive function may shift as impairment in cognition begins to emerge,40 the presence of cognitive impairment within a cohort might obscure a protective effect of education. In this population, however, excluding persons with low cognitive function at baseline did not have this effect: although higher education was associated with reduced cognitive decline in the early years of follow-up, it was associated with more rapid decline in later years.
Confidence in these findings is strengthened by several factors. First, they are based on a geographically defined and racially diverse population of older persons. Second, the availability of a psychometrically sound composite measure of cognition administered at regular intervals with high follow-up participation in survivors enhanced our ability to reliably assess individual differences in rate of cognitive change and their relation to education. Finally, the long follow-up period enhanced our ability to distinguish initial level of cognition from rate of change, and the large cohort size made it possible to identify even small effects.
This study also has important limitations. Because results are based on a measure of global cognition, we cannot rule out the possibility that education is related to decline in some cognitive domains but not others. In addition, the measure of educational attainment, years of schooling, assumes an equivalence of educational quality across persons and time that is unlikely to be true. Incorporating data on educational quality (e.g., spending per pupil in county) with amount of schooling might provide further insight into the association of education with age-related cognitive decline.
ACKNOWLEDGMENT
The authors thank the residents of Morgan Park, Washington Heights, and Beverly who participated in the study. They also thank Ann Marie Lane for community development and oversight of project coordination, Michelle Bos, Holly Hadden, Flavio LaMorticella, and Jennifer Tarpey for coordination of the study, Kenneth Tonnissen for analytic programming, and the staff of the Rush Institute for Healthy Aging.
Address correspondence and reprint requests to Dr. Robert S. Wilson, Rush Alzheimer’s Disease Center, Rush University Medical Center, 600 South Paulina Avenue, Suite 1038, Chicago, IL 60612 rwilson@rush.edu
Supported by National Institute on Aging grants AG 11101 and AG10161 and by National Institute of Environmental Health Sciences grant ES 10902.
Disclosure: The authors report no disclosures.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Received June 25, 2008. Accepted in final form October 23, 2008.
REFERENCES
- 1.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 1994;271:1004–1010. [PubMed] [Google Scholar]
- 2.Evans DA, Hebert LE, Beckett LA, et al. Education and other measures of socioeconomic status and risk of incident Alzheimer’s disease in a defined population of older persons. Arch Neurol 1997;54:1399–1405. [DOI] [PubMed] [Google Scholar]
- 3.Karp A, Kareholt I, Qiu C, Bellander T, Winblad B, Fratiglioni L. Relation of education and occupation-based socioeconomic status to incident Alzheimer’s disease. Am J Epidemiol 2004;159:175–183. [DOI] [PubMed] [Google Scholar]
- 4.Caamano-Isorna F, Corral M, Montes-Martinez A, Takkouche B. Education and dementia: a meta-analytic study. Neuroepidemiology 2006;26:226–232. [DOI] [PubMed] [Google Scholar]
- 5.Lui L-Y, Stone K, Cauley JA, Hillier T, Yaffe K. Bone loss predicts subsequent cognitive decline in older women: the Study of Osteoporotic Fractures. J Am Geriatr Soc 2003;51:38–43. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Kawachi I, Berkman LF, Grodstein F. Education, other socioeconomic indicators, and cognitive function. Am J Epidemiol 2003;157:712–720. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Buring JE, Cook NR, Grodstein F. The relation of education and income to cognitive function among professional women. Neuroepidemiology 2006;26:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarado BE, Zunzunegui MV, Del Ser T, Beland F. Cognitive decline is related to education and occupation in a Spanish elderly cohort. Aging Clin Exp Res 2002;14:132–142. [DOI] [PubMed] [Google Scholar]
- 9.Lyketsos C, Chen L-S, Anthony JC. Cognitive decline in adulthood: an 11.5 year follow-up of the Baltimore Epidemiologic Catchment Area Study. Am J Psychiatry 1999;156:58–65. [DOI] [PubMed] [Google Scholar]
- 10.Farmer ME, Kittner SJ, Rae DS, Bartko JJ, Regier DA. Education and change in cognitive function: the Epidemiologic Catchment Area Study. Ann Epidemiol 1995;5:1–7. [DOI] [PubMed] [Google Scholar]
- 11.Evans DA, Beckett LA, Albert MS, et al. Level of education and change in cognitive function in a community population of older persons. Ann Epidemiol 1993;3:71–77. [DOI] [PubMed] [Google Scholar]
- 12.Colsher PL, Wallace RB. Longitudinal application of cognitive function measures in a defined population of community-dwelling elders. Ann Epidemiol 1991;1:215–230. [DOI] [PubMed] [Google Scholar]
- 13.Liang J, Borawski-Clark E, Liu X, Sugisawa H. Transitions in cognitive status among the aged in Japan. Soc Sci Med 1996;43:325–337. [DOI] [PubMed] [Google Scholar]
- 14.White L, Katzman R, Losonczy K, et al. Association of education with incidence of cognitive impairment in three established populations for epidemiologic studies of the elderly. J Clin Epidemiol 1994;47:363–374. [DOI] [PubMed] [Google Scholar]
- 15.Christensen H, Korten AE, Jorm AF, Henderson AS, Jacomb PA, Rodgers B. Education and decline in cognitive performance: compensatory but not protective. Int J Geriatr Psychiatry 1997;12:323–330. [DOI] [PubMed] [Google Scholar]
- 16.Butler SM, Ashford JW, Snowdon DA. Age, education, and changes in the Mini-Mental State Exam scores of older women: findings from the Nun Study. J Am Geriatr Soc 1996;44:675–681. [DOI] [PubMed] [Google Scholar]
- 17.Alley D, Suthers K, Crimmins E. Education and cognitive decline in older Americans: results from the AHEAD sample. Res Aging 2007;29:73–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmand B, Smit J, Lindeboom J, et al. Low education is a genuine risk factor for accelerated memory decline and dementia. J Clin Epidemiol 1997;50:1025–1033. [DOI] [PubMed] [Google Scholar]
- 19.Anstey KJ, Hofer SM, Luszcz MA. A latent growth curve analysis of late-life sensory and cognitive function over 8 years: evidence for specific and common factors underlying change. Psychol Aging 2003;18:714–726. [DOI] [PubMed] [Google Scholar]
- 20.Arbuckle TY, Maag U, Pushkar D, Chaikelson JS. Individual differences in trajectory of intellectual development over 45 years of adulthood. Psychol Aging 1998;13:663–675. [DOI] [PubMed] [Google Scholar]
- 21.Jacqmin-Gadda H, Fabrigoule C, Commenges D, Dartigues J-F. A 5-year longitudinal study of the Mini-Mental State Examination in normal aging. Am J Epidemiol 1997;145:498–506. [DOI] [PubMed] [Google Scholar]
- 22.Albert MS, Jones K, Savage CR, et al. Predictors of cognitive changes in older persons: MacArthur studies of successful aging. Psychol Aging 2005;10:578–589. [DOI] [PubMed] [Google Scholar]
- 23.Koster A, Penninx BWJH, Bosma H, et al. Socioeconomic differences in cognitive decline and the role of biomedical factors. Ann Epidemiol 2005;15:564–571. [DOI] [PubMed] [Google Scholar]
- 24.Morris MC, Evans DA, Hebert LE, Bienias JL. Methodological issues in the study of cognitive decline. Am J Epidemiol 1999;149:789–793. [DOI] [PubMed] [Google Scholar]
- 25.Christensen H, Hofer SM, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS. Age is no kinder to the better educated: absence of an association investigated using latent growth techniques in a community sample. Psychol Med 2001;31:15–28. [DOI] [PubMed] [Google Scholar]
- 26.Seeman TE, Huang M-H, Bretsky P, Crimmins E, Launer L, Guralnik JM. Education and APOE-ɛ4 in longitudinal cognitive decline: MacArthur studies of successful aging. J Gerontol Psychol Sci 2005;60B:P74–P83. [DOI] [PubMed] [Google Scholar]
- 27.Van Dijk KRA, Van Gerven PWM, Van Boxtel MPJ, Van der Elst W, Jolles J. No protective effects of education during normal cognitive aging: results from the 6-year follow-up of the Maastricht Aging Study. Psychol Aging 2008;23:119–130. [DOI] [PubMed] [Google Scholar]
- 28.Winnock M, Letenneur L, Jacqmin-Gadda H, Dallongeville J, Amouyel P, Dartigues JF. Longitudinal analysis of the effect of apolipoprotein E ε4 and education on cognitive performance in elderly subjects: the PAQUID study. J Neurol Neurosurg Psychiatry 2002;72:794–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol Psychol Sci Soc Sci 1999;54:P155–P160. [DOI] [PubMed] [Google Scholar]
- 30.Wilson RS, Mendes de Leon CF, Bennett DA, Bienias JL, Evans DA. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry 2004;75:126–129. [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson RS, Bennett DA, Mendes de Leon CF, Bienias JL, Morris MC, Evans DA. Distress proneness and cognitive decline in a population of older persons. Psychoneuroendocrinology 2005;30:11–17. [DOI] [PubMed] [Google Scholar]
- 32.Featherman DL, Hauser RM. The measurement of occupation in social surveys. In: Hauser RM, Featherman DL, eds. The Process of Stratification. Academic Press: Orlando; 1977. [Google Scholar]
- 33.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 2002;17:179–193. [PubMed] [Google Scholar]
- 34.Wilson RS, Li Y, Bienias JL, Bennett DA. Cognitive decline in old age: separating retest effects from the effects of growing older. Psychol Aging 2006;21:774–789. [DOI] [PubMed] [Google Scholar]
- 35.Wilson RS, Li Y, Aggarwal NT, et al. Education and the course of cognitive decline in Alzheimer’s disease. Neurology 2004;63:1198–1202. [DOI] [PubMed] [Google Scholar]
- 36.Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2006;77:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shadlen M-F, Larson EB, Wang L, et al. Education modifies the effect of apolipoprotein epsilon 4 on cognitive decline. Neurobiol Aging 2005;26:17–24. [DOI] [PubMed] [Google Scholar]
- 38.Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging 1999;14:245–263. [DOI] [PubMed] [Google Scholar]
- 39.Callahan CM, Hall KS, Hui SL, Musick BS, Unverzagt FW, Hendrie HC. Relationship of age, education, and occupation with dementia among a community based sample of African Americans. Arch Neurol 1996;53:134–140. [DOI] [PubMed] [Google Scholar]
- 40.Hall CB, Derby C, LeVally A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology 2007;69:1657–1664. [DOI] [PubMed] [Google Scholar]


