Abstract
Background:
It is unclear whether the severity of and recovery from the initial demyelinating event (IDE) are recapitulated in subsequent multiple sclerosis (MS) relapses. We sought to identify the factors associated with relapse severity and recovery and to evaluate whether events have inherent severity or recovery.
Methods:
Patients seen at the UCSF MS Clinic within 1 year of disease onset were identified from a prospective database. Ordinal logistic regression was used to analyze predictors of three-level categorizations of event severity and recovery.
Results:
We identified 330 patients with MS or clinically isolated syndrome; 152 had a second event and 63 had a third event. Nonwhite and younger patients were at an increased risk of more severe demyelinating events. A severe prior event predicted a substantial increase in the odds of being above any given severity cutoff for a severe subsequent event (for second event severity, odds ratio [OR] = 5.62, 95% confidence interval [CI] [2.39, 13.26], p < 0.0001; for third event severity, OR = 6.74, 95% CI [1.67, 27.18], p = 0.007). Similarly, poor recovery of the IDE predicted poor second event recovery (OR = 5.28, 95% CI [1.95, 14.25], p = 0.001), while fair or poor second event recovery predicted about a 5- or 13-fold increase in the odds of poor third event recovery. A more severe event also predicted a substantial increase in the odds of poor recovery.
Conclusions:
Patients with severe presentation and poor recovery at disease onset continue on a similar trajectory with subsequent demyelinating events. Whether genetic or other biologic factors are responsible for this pattern remains to be determined.
GLOSSARY
- BS/CE
= brainstem/cerebellum;
- CI
= confidence interval;
- CIS
= clinically isolated syndrome;
- DMT
= disease-modifying therapy;
- EDSS
= Expanded Disability Status Scale;
- FS
= Functional Systems;
- IDE
= initial demyelinating event;
- MS
= multiple sclerosis;
- N/A
= not applicable;
- OR
= odds ratio;
- RRMS
= relapsing-remitting multiple sclerosis;
- VA
= visual acuity.
The significant variability in the severity of and recovery from demyelinating events in relapsing-remitting multiple sclerosis (RRMS) reflects patients' heterogeneity.1,2 The clinical determinants of event severity and recovery are poorly understood, and accumulation of residual disability due to incomplete relapse recovery remains largely unpredictable. Initial demyelinating event (IDE) severity and recovery may be important predictors for both short-and long-term disability (i.e., long-term prognosis may be determined very early in the disease process).1,3–9
A few authors reported that IDE severity is the most significant predictor of IDE recovery, but polyregional or polysymptomatic onset, onset location, and age may also be important.7,10,11 Predictors of event severity in early MS, other predictors of IDE recovery, and predictors of subsequent event recovery have not been studied. Furthermore, it is unknown if a given patient has an inherent tendency to develop clinical demyelinating events of similar severity and recovery.
In this prospective cohort study, we sought to identify clinical factors associated with the severity and recovery of early clinical events in MS. In addition, we evaluated whether an event's severity and recovery are associated with the severity and recovery of subsequent events.
METHODS
This project was approved by the UCSF Committee on Human Research. Clinical and demographic information for all patients seen at the UCSF MS Center is entered into a Microsoft SQL server database (retrospectively at the first visit and prospectively for subsequent visits).10,12 Routine follow-up visits typically occur every 6 months; unscheduled visits occur if a patient has an exacerbation. We queried the database for all patients with clinically isolated syndrome (CIS) and RRMS seen within the first year of disease onset between January 2000 and June 2007.13 We included only patients seen early after disease onset since accumulated disability and frequent symptom fluctuations may preclude accurate relapse characterization later in the disease course.
Patients were divided into two groups to analyze the effects of race/ethnicity: white, non-Hispanic (hereafter referred to as white) and nonwhite (all others). Demyelinating events were new or recurring neurologic symptoms referable to the CNS lasting for at least 48 hours after a remission of 30 days or more since the previous event. Pseudoexacerbations were excluded. Based on clinical history and examination, each patient's relapses were coded as occurring in the spinal cord, brainstem/cerebellum, optic nerve, or cerebrum. An event was considered polyregional if it involved at least two of these locations. A patient was considered as being on disease-modifying therapy (DMT) if he or she had at least 90 days of continuous treatment, since it is thought that there is a lag between initiation of therapy and the onset of therapeutic effectiveness.14,15
The severity of and recovery from the IDE was determined by trained individuals (S.D., E.M.) based on definitions derived from previous publications.10,11,16,17 Mild IDE severity was defined as Functional Systems (FS) scores of 0 to 1 in one to three FSs or visual acuity (VA) better than or equal to 20/40, Expanded Disability Status Scale (EDSS) score range of 0 to 1.5 inclusive; moderate severity was defined as a score of 2 in one or two FSs or four or more scores of 1 or VA of 20/50 to 20/190, EDSS score of 2.0 to 2.5 inclusive; relapses exceeding prior criteria were considered severe. Recovery was scored using the lowest EDSS and FS scores reported between 2 and 12 months after the attack. IDE recovery was considered complete (no residual complaint, normal follow-up examination, all FS scores = 0, follow-up EDSS score = 0), fair (residual subjective complaint that does not impair activity, or at least one FS score of 1 at most or VA better or equal to 20/40, follow-up EDSS score = 1.0 to 1.5), or poor (at least one FS score of 2 or more or VA of 20/50 or worse, follow-up EDSS score 2.0 or greater).
For second and third events, severity was scored the same way as for the IDE if the pre-event EDSS was 0; if the pre-event EDSS was >0, the severity was defined as mild (EDSS increase by 0.5 point, or 1 point change in up to three FS scores), moderate (EDSS increase by 1 or 2 points, or 2 points change in up to two FS, or 1 point change in four or more FS), or severe (exceeding prior criteria).
Recovery from the second or third event was defined as complete if no residual signs or symptoms remained above those present before the attack, fair if EDSS increased by up to 1 point or if there was an increase of 1 point on one or two FS (residual subjective complaint or new residual finding compared to baseline that does not impair activity), or poor if exceeding prior criteria.
Potential predictors of severity and recovery of all events included sex, race/ethnicity, age at onset, event location, and abnormal (at least one T2-weighted hyperintensity) vs normal baseline brain MRI. There were not enough cerebral second or third events to provide useful analyses. Monoregional vs polyregional event status was evaluated for an association with event recovery. The severity of the preceding event was used to predict that event's recovery as well as the subsequent event's severity. Preceding event recovery was used to predict subsequent event recovery. DMT use was added as a predictor of severity and recovery of second and third events. Multivariate models were generated to evaluate potential confounding. Because event severity is to some extent collinear by definition with monoregional vs polyregional status, the multivariate models for recovery included only severity as a predictor.
Statistics.
Statistical analyses were performed by Barbara Grimes, PhD, and Peter Bacchetti, PhD, University of California, San Francisco. Appropriate summary statistics were used to describe categorical and continuous variables. Since severity and recovery were measured on an ordered, three-level scale, ordinal logistic regression was used. This method assumes a common odds ratio (OR) for each predictor's association with both severe vs mild or moderate severity and severe or moderate vs mild severity; similar assumptions were made for recovery (the proportional odds assumption). When there was evidence against this assumption, we dichotomized the outcome and performed logistic regression. For the severity analyses, the dichotomized outcome was severe/moderate vs mild events. Violations occurred for univariate predictors of IDE (brainstem/cerebellar or optic nerve involvement), second event (spinal cord symptoms), and third event (nonwhite race/ethnicity, brainstem/cerebellar or optic nerve symptoms) severity as well as for the IDE and third event multivariate models. For the recovery analyses, the dichotomized outcome was poor/fair vs complete recovery. It was required for univariate analyses for IDE (gender, IDE severity, brainstem/cerebellar, or spinal cord symptoms) and second event (disease-modifying therapy) recovery as well as for the multivariate model of IDE recovery.
RESULTS
Patient and event characteristics.
We identified 330 patients (224 women) seen at the UCSF MS Center within a year of the first MS symptoms; mean follow-up was 759 ± 575 days (median 633 days, range 23–2,692 days). The mean age at IDE onset was 34 ± 12 years. A total of 267 (81%) patients were white; the remaining patients were African American (21), Asian (15), Hispanic or Latino (14), Native American (1), or unknown (12). At onset, 301 (93%) of the 323 patients who had available imaging had an abnormal brain MRI.
A total of 153 (46%) patients received high dose IV steroids for the IDE, and DMT was initiated in 54% of patients (n = 178) during the entire follow-up period (Avonex 29%, Rebif 10%, Copaxone 8%, Betaseron 6%, other 1%). Only 36 patients (24%) who had a second event initiated DMT before it occurred; 35 patients (56%) who had a third event initiated DMT beforehand. A second clinical event was experienced by 46% (n = 152); 19% (n = 63) experienced a third. The severity, recovery, and locations of events are presented in table 1. Events were polyregional in 48 (15%) of first, 20 (13%) of second, and 13 (21%) of third relapses.
Table 1 Locations, severity, and recovery of first, second, and third demyelinating events
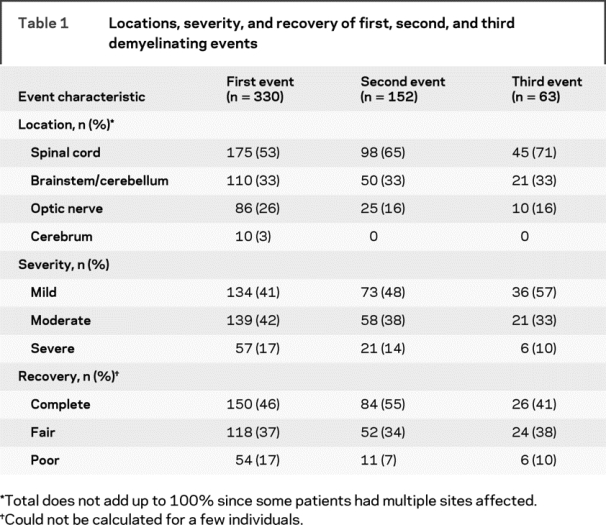
Factors associated with event severity.
Univariate analyses.
Nonwhite and younger patients had a higher odds of experiencing more severe first, second, and third events (table 2). The associations of race and age with third event severity had wide confidence intervals, reflecting the smaller number of subjects. Treatment with DMT prior to the second or third event was not meaningfully associated with severity (table 2).
Table 2 Predictors (univariate) of increased first, second, and third event severity
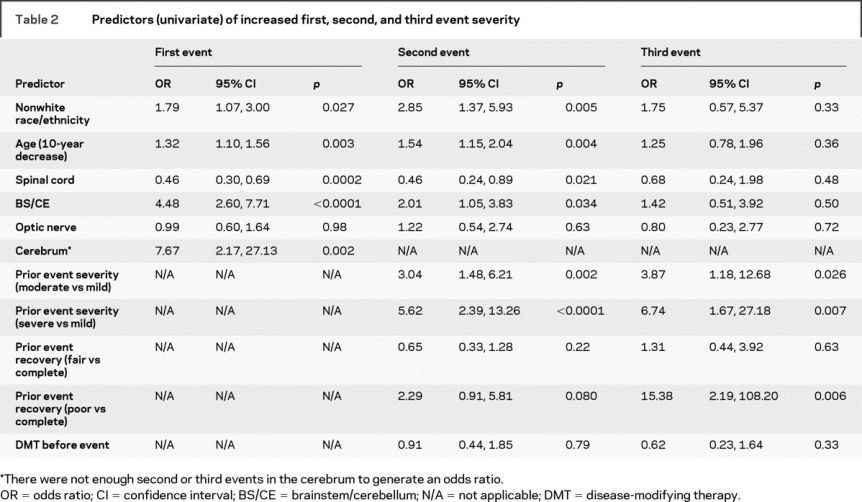
A more severe preceding event was associated with a substantial increase in the odds of a more severe second or third event, with a more than threefold increase in the odds if the first event was moderate compared to mild and a greater than five- to sixfold increase in the odds if the preceding event was severe compared to mild (table 2). Poor recovery of the prior event predicted substantially increased odds of a more severe subsequent event (table 2).
Multivariate analyses.
In the multivariate analysis evaluating predictors of IDE severity, which included age, location, and race, there were not substantial changes from the univariate analyses except that optic neuritis was more likely to be associated with IDE severity, whereas spinal cord onset did not seem to meaningfully predict severity (table 3).
Table 3 Multivariate predictors of increased first, second, and third event severity
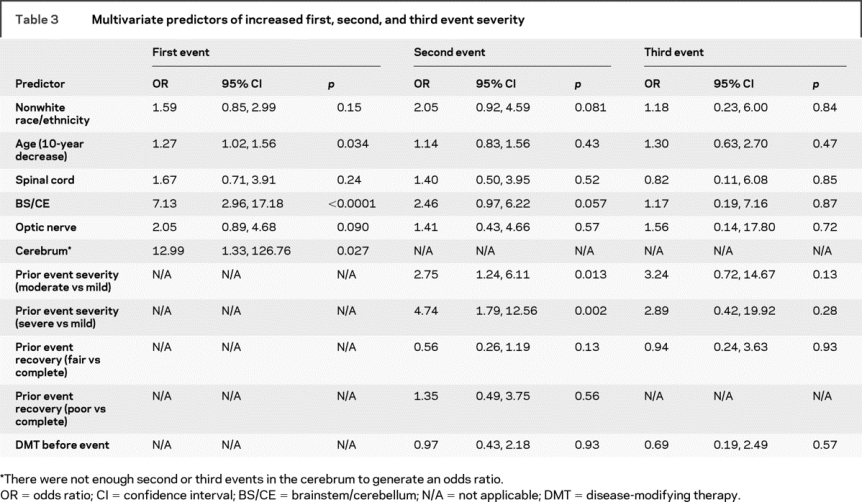
The multivariate model for second event severity included age, race, location, IDE severity and recovery, and DMT. The results were not meaningfully different than in the univariate analyses for nonwhite race, optic nerve or brainstem/cerebellar involvement, IDE severity, fair vs complete IDE recovery, and DMT (table 3), but there was an attenuation of the association of age and of poor vs complete IDE recovery with second event severity. Spinal cord involvement of the second event did not appear to be meaningfully associated with the event's severity (table 3).
The multivariate model for third event severity included age, race, location, second event severity and recovery, and DMT. The measures of association for race, age, location, and DMT were similar to those in the univariate analyses. There was still a large OR for a more severe third event if the prior event was moderate (OR = 3.24, 95% confidence interval [CI] [0.72, 14.67], p = 0.13) or severe (OR = 2.89, 95% CI [0.42, 19.92], p = 0.28) vs mild. The OR associated with fair vs complete second event recovery was not meaningfully different than in the univariate analysis but due to small numbers, an estimate could not be obtained for poor vs complete second event recovery.
Factors associated with event recovery.
Univariate analyses.
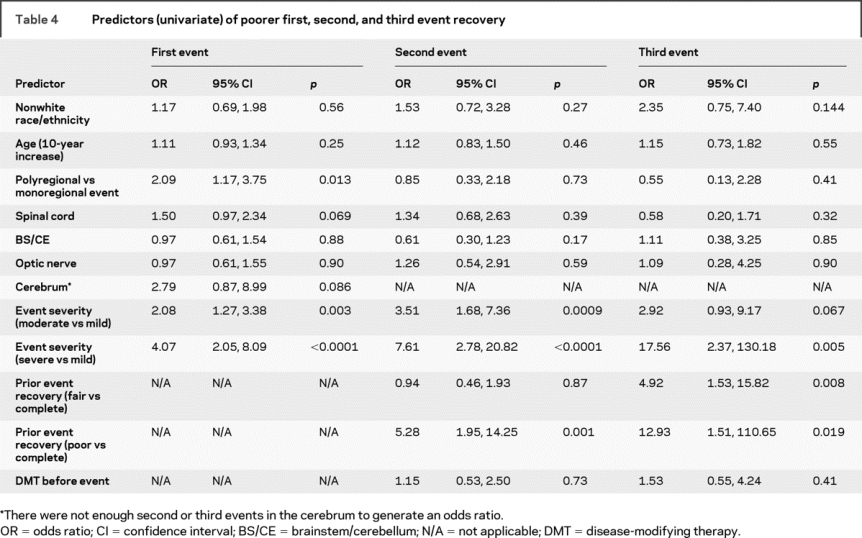
The most important predictors of relapse recovery were the concurrent event severity and the degree of recovery from the prior event (table 4). A moderate vs mild event was associated with a two- to threefold increase in the odds of poorer recovery of the first, second, and third events, while a severe vs mild event was associated with a 4- to 17-fold increase in the odds of worse recovery, although the CIs surrounding the ORs for the second and third events are wide due to the lower number of patients who had them (table 1).
Table 4 Predictors (univariate) of poorer first, second, and third event recovery
Multivariate analyses.
In the multivariate model for IDE recovery, which included age, race, event location, and IDE severity, IDE severity remained a strong predictor of worse recovery (OR for moderate vs mild severity = 2.38, 95% CI [1.38, 4.07], p = 0.002; OR for severe vs mild IDE = 5.08, 95% CI [2.42, 10.69], p < 0.0001) (table 5). Increased age was weakly associated with a worse recovery; nonwhite race remained an apparently unimportant predictor. IDE onset in the spinal cord, but not other locations, was associated with poorer recovery (OR = 2.85, 95% CI [1.30, 6.28], p = 0.009).
Table 5 Multivariate predictors of poorer first, second, and third event recovery
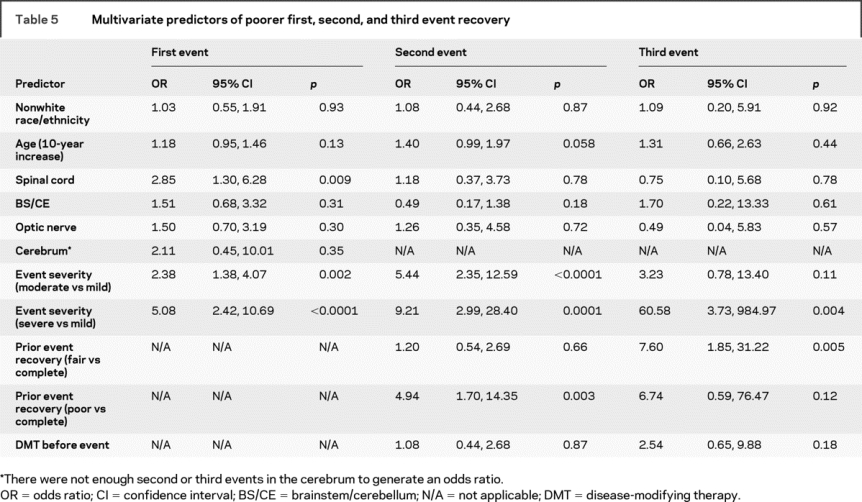
The multivariate model for second event recovery included age, race/ethnicity, location, DMT, IDE recovery, and second event severity (table 5). The severity of the second relapse was an even stronger predictor of poor recovery than in the univariate analyses (OR for moderate vs mild event = 5.44, 95% CI [2.35, 12.59], p < 0.0001; OR for severe vs mild event = 9.21, 95% CI [2.99, 28.40], p = 0.0001). The ORs associated with age, IDE recovery, location, DMT, and race/ethnicity did not substantially differ from those obtained in the univariate models.
The multivariate model for third event recovery included age, race/ethnicity, location, DMT, second event recovery, and third event severity. Only the latter two predictors appeared to be meaningful, but the CIs surrounding the ORs were large (table 5). Age, location, and nonwhite race did not appear to substantially impact recovery. There was a trend for DMT to be associated with a worse recovery (OR = 2.54, 95% CI [0.65, 9.88], p = 0.18).
DISCUSSION
Our finding that individuals with MS inherently experience relatively similar severity of and recovery from relapses over time early in the disease course substantiates the concept that at the individual level, patients with MS may have predetermined disease features. This notion is supported by our previous reports that a given patient with RRMS is likely to have consecutive relapses in the same location within the nervous system and that there may be pathologic homogeneity within, but not between, individuals with MS.12,16,18 Whether stereotyped severity, recovery, and location of exacerbations is explained by genetic polymorphisms or other underlying biologic processes remains to be determined.
Since poor recovery from early events predicts a worse long-term MS prognosis, interventions that modify this tendency toward more severe relapses with poor recovery are needed.1,3–5,7,9 Incomplete recovery may result from a more severe initial injury, such as axonal damage,19 or from limited repair processes. Studying relapse severity and recovery as secondary outcome measures in trials of neuroprotective agents may give insight into the responsible mechanisms. Whether current DMTs modify relapse severity or recovery is uncertain. DMT reduced the annual rate of moderate and severe exacerbations in one study.20 Here, DMT did not seem to attenuate relapse severity or recovery, although many patients did not receive treatment prior to the second or third attack. Also, this was not a randomized study, so patients who received DMT may have been different from those who did not.
In previous studies, African Americans had more rapid disease progression or were more likely to be disabled than were Caucasians, suggesting that the long-term course of MS may be more aggressive in the former group, although predictors of such outcomes have not been fully characterized.21–24 Here, we demonstrate that nonwhite race/ethnicity was associated with a higher odds of more severe demyelinating events. We report elsewhere that nonwhites have a twofold increase of the risk of an early second demyelinating event.25 It is less clear if nonwhite race is predictive of poor recovery from events since the CIs are so wide. Extended follow-up of the cohort will help further define the long-term outcomes of this heterogeneous group of nonwhite patients.
This study confirms previous reports that poor recovery of a first MS event is associated with severe presentation and polyregional onset.7,10,11 We expand these findings by showing that the severity of the second and third events also predicts their recovery. Younger patients appeared more likely to have a more severe IDE, and there was a trend for younger patients to experience better recovery of the first and second events. These results should be confirmed in a larger cohort. If the differential effect of age exists, perhaps younger patients have attacks associated with more edema such that symptoms are worse at their peak but also resolve more completely as the edema subsides. Younger patients may also have more plasticity and therefore better repair. The effect of location, as evidenced in the multivariate models, was complex and requires further study in a larger cohort. Onset in the spinal cord did not appear to meaningfully influence relapse severity but was predictive of poor recovery of the IDE. Conversely, onset in the other three locations was associated with increased severity of the IDE (with a trend for the same when the second event involved the brainstem/cerebellum) but did not seem to meaningfully predict recovery. These data suggest that inflammation and repair processes are different in some CNS locations.
There are some limitations to our study. Those who were determining severity and recovery of attacks were not blinded to the assignations for previous attacks. While this could raise questions of whether misclassification bias was introduced, the fact that rigid definitions based on objective examination findings were used makes it unlikely. We used 2 to 12 months as the postexacerbation time interval in which we measured recovery, which may generate concern that some patients whose recovery was documented early in this period may have experienced continued recovery later in the interval that was not captured in this analysis. However, one study demonstrated that the proportion of individuals who had incomplete recovery after a relapse did not change when recovery was measured 30 to 59, 60 to 89, or 90 or more days after the exacerbation.26 These data suggest that there is little meaningful change in recovery over time when it is measured beyond 30 days postrelapse. Our definitions of severity imply that milder events are less likely to be followed by poor recovery. While the EDSS scales are nonlinear, potentially limiting the scoring of severity and recovery, this cannot explain the within-patient tendency to experience similar severity or recovery for their first three MS exacerbations. The sample sizes for the second and particularly the third event are small, resulting in widened CIs for predictors of these events' severity and recovery. However, for the main predictors of interest, the point estimates were generally in the same direction as for the IDE model, and most of the wider CIs were similar to or encompassed those of the IDE models, indicating biologic consistency that bolsters the credibility of the findings. Finally, long-term outcomes and CNS imaging with quantitative measures of injury and repair are not available for this cohort but could add to our understanding of the pathophysiologic processes at play.
Patients with a more severe presentation and poor recovery at MS onset have an inherent tendency to continue on a similar trajectory for subsequent events. It is unclear if earlier or more aggressive treatment in patients with such poor short-term prognosis limits the long-term accrual of disability. We are currently investigating if specific genetic polymorphisms are associated with severity of and recovery from demyelinating events.
ACKNOWLEDGMENT
The authors thank the neurologists of the UCSF MS center for their participation in data collection.
Address correspondence and reprint requests to Dr. Ellen M. Mowry, Department of Neurology, Multiple Sclerosis Center, University of California, San Francisco, 350 Parnassus Avenue, Suite 908, San Francisco, CA 94117 ellen.mowry@ucsf.edu
Supported by the NMSS (RG-3692A), the Nancy Davis Foundation, a National Multiple Sclerosis Society Sylvia Lawry Fellowship Award, and the Partners MS Center Clinical Fellowship Program. Statistical analysis for this publication was made possible by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131.
Disclosure: Dr. Waubant has received research support from Biogen Idec, Genentech Inc, Pfizer, and Sanofi-Aventis, and honorarium for one educational presentation from Teva and Biogen.
Received August 25, 2008. Accepted in final form November 10, 2008.
REFERENCES
- 1.Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain 2003;126:770–782. [DOI] [PubMed] [Google Scholar]
- 2.Pittock SJ, Mayr WT, McClelland RL, et al. Change in MS-related disability in a population based cohort: a 10-year follow-up study. Neurology 2004;62:51–59. [DOI] [PubMed] [Google Scholar]
- 3.Phadke JG. Clinical aspects of multiple sclerosis in north-east Scotland with particular reference to its course and prognosis. Brain 1990;113:1597–1628. [DOI] [PubMed] [Google Scholar]
- 4.Weinshenker BG, Rice GP, Noseworthy JH, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study: 3: multivariate analysis of predictive factors and models of outcome. Brain 1991;114:1045–1056. [DOI] [PubMed] [Google Scholar]
- 5.Amato MP, Ponziani G, Bartolozzi ML, Siracusa G. A prospective study on the natural history of multiple sclerosis: clues to the conduct and interpretation of clinical trials. J Neurol Sci 1999;168:96–106. [DOI] [PubMed] [Google Scholar]
- 6.Scott TF, Schramke CJ, Novero J, Chieffe C. Short-term prognosis in early relapsing remitting multiple sclerosis. Neurology 2000;55:689–693. [DOI] [PubMed] [Google Scholar]
- 7.Runmarker B, Andersen O. Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up. Brain 1993;116:117–134. [DOI] [PubMed] [Google Scholar]
- 8.Kurtzke JF, Beebe GW, Nagler B, Kurland LT, Auth TL. Studies on the natural history of multiple sclerosis: 8: early prognostic features of the later course of the illness J Chron Dis 1977;30:819–830. [DOI] [PubMed] [Google Scholar]
- 9.Amato MP, Ponziani G. A prospective study on the prognosis of multiple sclerosis. Neurol Sci 2000;21:S831–S838. [DOI] [PubMed] [Google Scholar]
- 10.West T, Wyatt M, High A, Bostrom A, Waubant E. Are initial demyelinating event recovery and time to second event under differential control? Neurology 2006;67:809–813. [DOI] [PubMed] [Google Scholar]
- 11.Beck RW, Cleary PA, Anderson MM, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med 1992;326:581–588. [DOI] [PubMed] [Google Scholar]
- 12.Deen S, Bacchetti P, High A, Waubant E. Predictors of the location of multiple sclerosis relapse. J Neurol Neurosurg Psychiatry 2008;79:1190–1193. [DOI] [PubMed] [Google Scholar]
- 13.Polman CH, Reingold SG, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria.” Ann Neurol 2005;58:840–846. [DOI] [PubMed] [Google Scholar]
- 14.Comi G, Filippi M, Wolinsky J. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging–measured disease activity and burden in patients with relapsing multiple sclerosis. Ann Neurol 2001;49:290–297. [PubMed] [Google Scholar]
- 15.Waubant E, Goodkin DE, Sloan R, Andersson PB. A pilot study of MRI activity before and during interferon beta-1a therapy. Neurology 1999;53:874–876. [DOI] [PubMed] [Google Scholar]
- 16.Mowry EM, Deen S, Malikova I, Pelletier J, Bacchetti P, Waubant E. The onset location of multiple sclerosis predicts the location of subsequent relapses. J Neurol Neurosurg Psychiatry Epub 2008 December 9. [DOI] [PubMed]
- 17.Panitch H, Goodin DS, Francis G, et al. EVIDENCE Study Group: randomized, comparative study of interferon beta-1a treatment regimens in MS: the EVIDENCE trial. Neurology 2002;59:1496–1506. [DOI] [PubMed] [Google Scholar]
- 18.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassman H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 2000;47:707–717. [DOI] [PubMed] [Google Scholar]
- 19.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338:278–285. [DOI] [PubMed] [Google Scholar]
- 20.IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis: I: clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993;43:655–661. [DOI] [PubMed] [Google Scholar]
- 21.Cree BA, Khan O, Bourdette D, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology 2004;63:2039–2045. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman MD, Johnson SK, Moyer D, Bivens J, Norton HJ. Multiple sclerosis: severity and progression rate in African Americans compared to whites. Am J Phys Med Rehab 2003;82:582–590. [DOI] [PubMed] [Google Scholar]
- 23.Marrie RA, Cutter G, Tyry T, Vollmer T, Campagnolo D. Does multiple sclerosis-associated disability differ between races? Neurology 2006;66:1235–1240. [DOI] [PubMed] [Google Scholar]
- 24.Weinstock-Guttman B, Jacobs LD, Brownscheidle CM, et al. Multiple sclerosis characteristics in African American patients in the New York State Multiple Sclerosis Consortium. Mult Scler 2003;9:293–298. [DOI] [PubMed] [Google Scholar]
- 25.Mowry EM, Pesic M, Deen SR, Grimes B, Bacchetti P, Waubant E. Age, race, and initial demyelinating events with fewer affected functional systems predict an increased hazard of early relapse in multiple sclerosis. Mult Scler 2008;14:P563. Abstract.
- 26.Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficits in multiple sclerosis. Neurology 2003;61:1528–1532. [DOI] [PubMed] [Google Scholar]


