Abstract
Chagas disease is caused by the parasite Trypanosoma cruzi it is the most common cause of heart disease in endemic areas of Latin America. The year 2009 marks the 100th anniversary of the discovery of T. cruzi infection and Chagas disease by the Brazilian physician Carlos Chagas. Chagasic cardiomyopathy develops in from 10 to 30 percent of persons who are chronically infected with this parasite. Echocardiography and magnetic resonance imaging are important modalities in the evaluation and prognosis of individuals with chagasic heart disease. The etiology of chagasic heart disease likely is multifactorial. Parasite persistence, autoimmunity, and microvascular abnormalities have been studied extensively as possible pathogenic mechanisms. Experimental studies suggest that alterations in cardiac gap junctions may be etiologic in the pathogenesis of conduction abnormalities. The diagnosis of chronic Chagas disease is made by serology. The treatment of this infection has shortcomings that need to be addressed. Cardiac transplantation and bone marrow stem cell therapy for persons with Chagas disease have received increasing research attention in recent years.
Keywords: Trypanosoma cruzi, Chagas disease, cardiomyopathy
Introduction
Chagas disease, an important cause of heart disease in Latin America, is the result of infection with the protozoan parasite Trypanosoma cruzi. This infection is life-long and the serious cardiac and gastrointestinal problems that characterize chronic symptomatic Chagas disease develop in approximately 10 to 30 % of infected persons.
The year 2009 marks the 100th anniversary of the discovery of the disease that bears the name of the physician who first described the illness and its causative organism. There has been a dramatic increase in the understanding of the epidemiological, clinical, and pathophysiological aspects of Chagas disease since our review in 1992 [1]. Recently, several excellent reviews on various aspects on this disease have appeared [2–5]. The intent in this article is not to present an exhaustive review of the disease, but rather to put forth recent perspectives relating to the effects of T. cruzi on the heart, which is the organ most commonly affected in persons with chronic infection. This article is an outgrowth of our association with Dr. Edmund Sonnenblick, a coeditor of this journal who died in 2007. He had a life-long interest in cardiomyopathy and encouraged us to write this contribution.
Carlos Justiniano Ribeiro das Chagas was born in the state of Minas Gerais, Brazil, on July 9, 1879 to José Justiniano das Chagas and Mariana Cándida Chagas. After his basic schooling and a brief stint as an engineering student in the city of Ouro Preto in the state of Minas Gerais, he decided to study medicine in Rio de Janeiro and trained under Dr. Oswaldo Cruz, one of the most renowned scientists of that generation. After graduation from medical school, Chagas worked as a malaria control officer. In 1909, while working in a malaria control campaign in Lassance, Minas Gerais, Chagas observed flagellated organisms in the blood a febrile child named Berenice. After the fever abated, he no longer saw any parasites in her blood. He named the organisms “Trypanosoma cruzi” in honor of his mentor. In a period of several months, working almost entirely on his own, he described the pathogen, its vector, and the clinical features of Chagas disease, an accomplishment unique in the history of medicine, and published descriptions of the parasite, its vector, and the disease in humans [6.7]. In the 1960s, many years after the death of Carlos Chagas, Bernice was located and was found to be seropositive for Chagas disease but free of any of the stigmata of her chronic infection. She died of other causes in 1973.
Although the discovery of this “new” disease was hailed by many as an outstanding scientific achievement, Carlos Chagas never was awarded the Nobel Prize [8]. He died in 1935, but his vision lives on in the work of the many scientists and clinicians who continue to investigate this fascinating disease. Interestingly but not surprisingly, it has been shown that Chagas disease was present in South America long before it was discovered in 1909, as paleoparasitological studies showed that T. cruzi was present in tissues from mummies in costal northern Chile from the period 4000 BC to 1400 AD [9].
Chagas disease is endemic in all Latin America countries with the exception of the Caribbean nations. The eggs of the triatome vectors usually hatch over a range of 16 to 34°C with the highest fertility occurring at 21 to 32°C. Fertility is also related to humidity. Most triatome vectors are nocturnal and they are attracted to warmth, carbon dioxide and odor.
In recent decades the rate of emigration from Chagas-endemic countries to the United States, Canada, and the European Union has increased markedly. Currently an estimated 13 million immigrants from endemic regions live in the United States and presumably 100,000 or so of these persons chronically harbor T. cruzi. Seven instances of transmission of T. cruzi by blood transfusion have been reported in Canada and the United States, and five instances of transmission by organ transplantation in the latter have been described. The presence of increased numbers of T. cruzi-infected immigrants and these instances of transmission here have prompted a growing interest in Chagas disease in many quarters and led to the initiation of serological screening of donated blood in January 2007 [10,11].
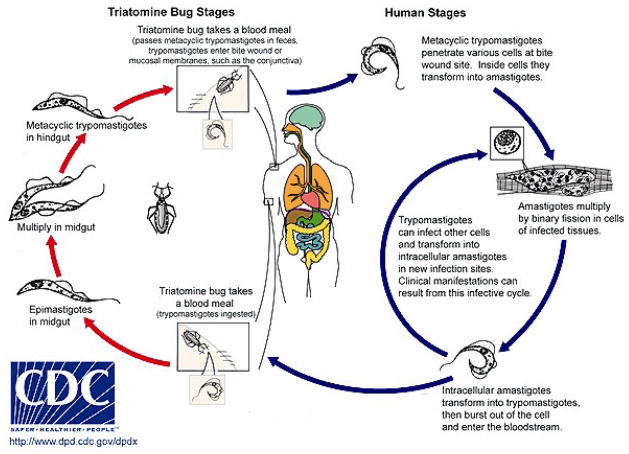
T. cruzi has a complex life cycle (Figure 1) consisting of four morphologically and biochemically distinct forms. During a blood meal from an infected mammalian host, the insect vector ingests blood form trypomastigotes (BFTs), which once in the midgut of the vector transform into epimastigotes that are capable of dividing. After 3–4 weeks, infective, non-dividing metacyclic trypomastigotes (MTs) are present in the hindgut of the vector and are deposited with the feces of the vector during subsequent blood meals. Transmission to the new host takes place when the parasite-laden feces contaminate oral or nasal mucous membranes, the conjunctivas, or other vulnerable surfaces. When the parasites enter a host cell they are first observed in a parasitophorous vacuole after transformation to amastigotes (AMAs), where they may be killed by cytocidal mechanisms or may evade this onslaught and enter the cytoplasm. Once there, the AMAs multiply by binary fission. (figure 3) As sizable numbers accumulate the AMAs somehow sense that the life of their host cell is ending and they transform to BFTs. The latter parasites are released as the host cell ruptures and they then disseminate through the lymphatics and the bloodstream to find new cells to invade. This process continues asynchronously for the life of the host. Although any nucleated mammalian cell can be parasitized by these organisms, cells of the reticuloendothelial, nervous and muscle systems, including the heart, appear to be favored. In addition, recent observations suggest that adipocytes are readily invaded by BFTs and may serve as a reservoir from which the infection may be reactivated [12]. Also, small numbers of BFTs may be ingested in blood meals taken by vectors. These organisms then transform into epimastigotes in the midgut of their new host, thus completing the cycle. Vector-borne transmission T cruzi infection usually occurs in persons who live in primitive houses in areas where the sylvatic cycle is active. The living quarters are invaded by infected vectors which become domiciliary and feed at night on the humans, dogs [13], and other mammals that live there.
Figure 1.
Life cycle of Trypanosoma cruzi (CDC website)
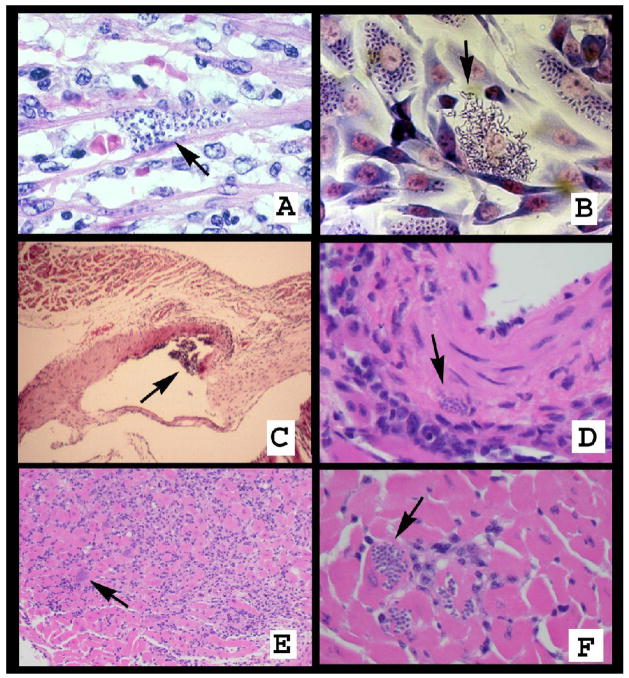
Figure 3.
A. Pseudocyst in the heart containing intracellular amastigotes (arrow). B. Infected cultured fibroblasts containing intracellular amastigotes. Some cells have ruptured and trypomastigotes are observed leaving the host cell (arrow). C. Vessel of an infected mouse demonstrating a vasculitis. D. Pseudocyst in the wall of a blood vessel. E. Acute myocarditis in the heart of a T. cruzi-infected mouse. There are many inflammatory cells and pseudocysts (arrow). F. Pseudocysts in the heart of a T. cruzi–infected mouse (arrow).
In the past most transmission of T cruzi to humans has been vector-borne, with other modes such as transplacental [1], through contaminated food or drink, and laboratory accidents accounting for relatively few new cases. This situation has changed considerably in recent years due to the successful implementation of vector control programs in many of the endemic countries. Much of the progress in this regard has been achieved under the aegis of the Southern Cone Initiative (SCI), which began in 1991 in Argentina, Bolivia, Brazil, Chile, Paraguay, and Uruguay. Uruguay was declared transmission-free in 1997 and was followed by Chile in 1999 and most recently Brazil in 2006. Major progress has been made in the other SCI countries, particularly in Argentina, and similar programs are being developed in the Andean nations and Central America. Moreover, transfusion transmission of T. cruzi has been essentially eliminated throughout much of the endemic range. All the endemic countries except Mexico have mandated testing of donated blood for evidence of T. cruzi infection and efficient screening programs have been implemented. Although much remains to be done in some endemic areas, the progress achieved to date prompted the attendees at a WHO conference on Chagas disease convened in Geneva in 2007 to set 2010 as the target date for the elimination of transmission of the parasite.
Chagas heart disease
In general, persons seropositive for Chagas disease who are identified in epidemiologic studies or through blood donor screening do not recall having had acute Chagas disease and do not know that they are chronically infected with T. cruzi. This is because the persons at highest risk for acquiring T. cruzi infection, i.e., the rural poor, typically have little access to medical care, and also because acute Chagas disease generally is a mild illness. Nonetheless, after a minimum incubation period of a week or two, some newly-infected persons may develop severe signs and symptoms. These can include fever, chills, nausea, vomiting, diarrhea, rash, and meningeal irritation. Moreover, a raised inflammatory lesion at the site of parasite entry (a chagoma), unilateral periorbital edema (Romaña sign), conjunctivitis, lymphadenopathy, and hepatosplenomegaly have all been described in patients with acute Chagas disease. Laboratory abnormalities are non-specific and may include anemia, thrombocytopenia, and elevated liver and cardiac enzymes. The diagnosis of acute Chagas disease is primarily parasitologic. Motile BFTs can be observed in wet preparations of blood and cerebrospinal fluid in many patients with the acute disease. Serologic tests for parasite-specific IgG are often negative during this stage, and assays for IgM have not been standardized and are not widely available.
During the acute phase of the illness, asynchronous cycles of parasite multiplication, host cell destruction, and infection of new cells occur. Myocarditis, cardiomegaly, and congestive heart failure (CHF) develop in a small percentage of acutely-infected patients [14, 15]. Arrhythmias, heart block, or CHF in the setting of acute T. cruzi infection are indicative of a poor prognosis [16]. It is not known if the severity of acute Chagas disease affects the likelihood of development of chronic cardiac or gastrointestinal manifestations years later. A small percentage of acutely-infected patients, often children, die of acute myocarditis or meningoencephalitis. In most patients, however, as specific cellular and humoral immune responses develop, the parasitemia wanes and signs and symptoms resolve completely, usually in two to four months. These individuals then enter the indeterminate phase of T. cruzi infection, which is characterized by detectable specific antibodies and an absence of clinical manifestations attributable to the infection. The indeterminate phase may last from months to an entire lifetime, and as noted most chronically infected persons never develop clinical manifestations attributable to the persistence of the parasites. Even so, each year an estimated 20,000 people die of chronic Chagas heart disease (CCHD) in the endemic countries [17], and CCHD annually may kill 250 or so T. cruzi-infected immigrants in the United States.
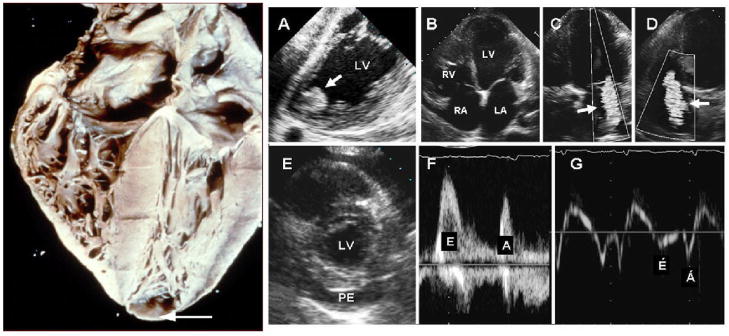
CCHD may present insidiously as CHF or abruptly with arrhythmias and/or thromboembolic events. Dilated congestive cardiomyopathy is an important manifestation of CCHD that typically occurs years or even decades after a person first becomes infected. Apical aneurysm of the left ventricle is one of the hallmarks of CCHD as observed by cardiac imaging and at autopsy (Figure 2). Histology of cardiac tissue from patients with CCHD shows contraction-band necrosis and myocytolysis. Focal and diffuse areas of myocellular hypertrophy are observed with or without inflammatory infiltrates, and fibrosis replacing previously damaged myocardial tissue is evident. The destruction of conduction tissue results in AV and intraventricular conduction abnormalities. In areas where the disease is endemic, the presence of RBBB, associated with an anterior fascicular block, is highly suggestive of chagasic cardiomyopathy and these conduction defects may necessitate the placement of a pacemaker. Increases in levels of brain natriuretic peptide (BNP) have been shown to be of value in the evaluation of patients with CCHD [18]. It is not understood why some patients in the indeterminate phase of T. cruzi infection develop CCHD while most do not. It would seem that the infecting parasite strain, host immunogenetics [19], and personal health issues would dictate the final outcome, but specific predictive parameters have not been identified. On the other hand, measurement of anatomic, electrocardiographic, radiologic, and functional parameters in patients who have developed some degree of CCHD has been shown to be useful for predicting mortality [20].
Figure 2.
The right panel is a heart obtained from an individual with chronic Chagas cardiomyopathy. There is four-chambered enlargement of the heart and an apical aneurysm. (Courtesy of Armed forces Institute of Pathology). The left panel shows the echocardiographic findings in Chagas heart disease. A. Transesophageal echocardiography demonstrating an apical aneurysm containing a large, round, and protruding thrombus (arrow); B–D. Transthoracic apical 4-chamber views of the heart showing dilated cardiac chambers (B), and functional mitral (C) and tricuspid (D) regurgitation (arrows). E. Parasternal short-axis view of the heart showing a large pericardial effusion (PE). F and G. Transmitral pulsed-Doppler (F) and lateral annulus tissue Doppler (G) demonstrating apparently normal peak early (E) and late (A) transmitral velocities, E/A ratio, and E-wave deceleration time (F) but abnormal early (E′) and late (A′) velocities (G) consistent with advanced diastolic dysfunction. LA=left atrium; LV=left ventricle; PE=pericardial effusion; RA=right atrium; RV=right ventricle.
Echocardiography
Echocardiography is an important tool for the initial assessment and long-term follow-up of persons with cardiac Chagas disease. During acute T. cruzi infection echocardiographic findings may include pericardial effusion [21] and segmental LV wall motion abnormalities. Overall LV systolic function is usually preserved at this stage, although a small minority of patients with cardiac involvement may present with dysfunction caused by severe myocarditis. In rare instances, pericardial effusion can occur and may be hemodynamically significant.
During the indeterminate phase there may be no ECG or x-ray abnormalities and echocardiography is often normal at this stage; however, stress testing and more sensitive echocardiographic techniques may disclose latent myocardial abnormalities. As an example, abnormal myocardial relaxation indexes have been demonstrated by tissue Doppler imaging in some patients with otherwise normal echocardiograms [22]. In addition, the advent of harmonic and contrast echocardiography has resulted in improved detection of subtle regional LV wall motion abnormality [23]. Using dobutamine stress echocardiography, blunted heart rate and LV contractile reserve have been demonstrated in some patients with normal resting echocardiograms [24]. Ischemic regional LV wall motion abnormality may also be detected on dobutamine stress echocardiography or by myocardial perfusion studies. These latter findings support the notion of impaired coronary flow reserve due to abnormal myocardial microvasculature [24, 25].
Early echocardiographic studies were focused on patients with symptomatic Chagas cardiac disease, nearly half of whom were shown to have typical apical LV aneurysms with extension towards the apical and mid-portions of the inferolateral wall. Moreover, approximately 25% of the patients in this group had a dilated and diffusely hypokinetic LV. Segmental wall motion abnormalities occurred in the absence of epicardial coronary artery disease. Additional common findings were apical thrombus, basal infero-posterior hypokinesis or akinesis, LA enlargement, and RV dilatation, and systolic dysfunction. Some patients were found to have four-chamber dilatation and biventricular systolic dysfunction. Thus, advanced chagasic heart disease phenotypically mimics either chronic ischemic or idiopathic dilated cardiomyopathy. Altered anatomy is a significant predictor of progressive adverse LV remodeling in this disease. Mitral and tricuspid regurgitation are often present in patients with regional or global ventricular dysfunction. The presence of echocardiographic abnormalities is highly predictive of poor outcomes in CCHD [23]. The spectrum of cardiac abnormalities in Chagas disease is shown in Figure 2. The mouse model of T. cruzi infection faithfully recapitulates the human disease. Our laboratory pioneered the use of echocardiography in the investigations of T. cruzi infections in mice. We found that in chronically infected mice there is a significant reduction in the percent fractional shortening accompanied by an increase in the left ventricular end diastolic diameter and thinning of the ventricular wall [26]. These observations are similar to those found in human chronic chagasic cardiomyopathy.
Cardiac magnetic resonance imaging
Cardiac gated magnetic resonance imaging (MRI) is a non-invasive method with excellent spatial resolution and tissue contrast that permits the evaluation of cardiac anatomy and function. It has been applied to study both clinical and experimental chagasic cardiomyopathy. Previously it was suggested that MRI would be useful in the diagnosis and management of CCHD. Ueno et al [27] reported an MRI study of a 50-year-old Brazilian woman who underwent MRI in Japan to evaluate the cause of dyspnea on exertion. This was one of the first cases reported in which cardiac MRI was used to evaluate a patient with Chagas disease. The MRI revealed localized thinning and a small apical LV aneurysm. Subsequently several articles have been published that describe the use of MRI for the evaluation of myocarditis in Chagas disease [28,29,30]. MRI with myocardial tagging can visualize damaged areas of the heart with wall motion abnormalities.
Our group showed the utility of MRI and centerline analysis to evaluate heart wall motion abnormalities in Chagas disease in mice [34]. This latter study demonstrated the utility of MRI and centerline analysis as a straightforward method for monitoring regional LV wall motion in T. cruzi-infected mice. The first MRI study of experimental Chagas disease in mice infected with T. cruzi was reported by Huang et al.[31,]. This was followed by several reports demonstrating that in mice MRI was ideally suited for detection of RV dilatation, a hallmark of CCHD, and that this approach could be used to evaluate the effects of drug therapy and the roles of various genes in the etiology of chagasic cardiomyopathy [32–34].
Pathology and pathogenesis
During acute infection there is an intense inflammatory reaction consisting primarily of leukocytes, including eosinophils and macrophages, accompanied by increased expression of inflammatory mediators such as cytokines, chemokines, and nitric oxide synthase [31, 35]. There are parasitic pseudocysts (parasitized host cells filled with amastigotes) with myonecrosis, myocytolysis and an intense vasculitis (figure 3). Mast cells have also been observed in inflamed tissues.
Trypomastigotes gain access to the cardiac myocytes (CMs) by invading endothelial cells, vascular smooth muscle cells, and the interstitial areas of the vasculature and the myocardium. Subsequently, CMs are invaded and destroyed. Portions of the vasculature may also be destroyed but this is not universal. The parasite passes through two basal laminae areas and two layers of the extracellular matrix (ECM) of the myocardium as well as the interstitial matrix between the two basal laminae. Parasites-derived enzymes play an important role in the degradation of the ECM and subsequent parasite invasion. In an experimental model it was recently shown that the expression and activity of myocardial zinc-dependent metalloproteinases are upregulated (MMP-2, MMP-9), and that inhibition of these enzymes reduces the inflammation in the myocardium [36].
In the heart there are three layers of CMs that are obliquely oriented to each other and meet at the apex. As a result of ischemia or inflammation and necrosis there is degradation of the ECM as a result of ischemia and inflammation-induced damage which leads to slippage of the ventricular layers leading to mural thinning and apical aneurysm formation. As noted, damage to this area of the myocardium is common in CCHD. Remodeling in the context of CCHD refers to the structural changes associated with inflammation, necrosis, hypertrophy and ventricular dilation. Myocytolysis, myonecrosis and contraction band necrosis are frequently observed. Myocytolysis follows the differentiation of amastigotes into BFTs. Contraction band necrosis is a result of hypoperfusion followed by reperfusion such as that seen after local vasospasm of the branches of the coronary microvasculature. There are bands of fibrous tissue replacing CMs. An important feature of CCHD is the accumulation of extracellular collagen that encloses fibers or groups of fibers. All areas of the heart, including the conduction pathways, may be involved. Microvascular involvement manifested by basement membrane thickening has been demonstrated. The irreversible pathological changes lead to structural and functional alterations. The remodeling process results in damage to the ECM and the replacement of CMs and vascular cells by fibrous tissue [37]. Thus, all these events in concert lead to thinning of the myocardium and cardiac hypertrophy. CD4+ and CD8+ T-cell are present in the inflammatory infiltrate of the myocardium, but in the in chronic chagasic cardiomyopathy CD8+ T-cells predominate [38, 39].
In the myocardium of infected mice there is upregulation of the mitogen-activated protein kinase pathway as well as cell cycle regulatory proteins. Infection of the myocardium results in cell proliferation of cells other than CMs [40,41], however, it is unclear whether infection can also cause CMs, which are terminally differentiated, to re-enter the cell cycle. Interestingly, while cyclins A and E are abundant in fetal/neonatal CMs and are presumably involved in driving the proliferative capacity of CM’s in the developing heart [42, 43], cyclins A and E are not normally found in postmitotic adult hearts. In contrast, following T. cruzi infection, CMs expressing both of these fetal/neonatal cell cycle markers are frequently observed. The reappearance of cyclin E-positive and cyclin A-positive cells in the adult myocardium raises the possibility that either infection is able to partially dedifferentiate adult CMs, thereby enabling their re-entry of into the cell cycle, or infection of the myocardium results in the recruitment and expansion of CM precursor cells, thereby contributing to infection-induced cardiomyopathy.
The question of whether apoptosis occurs in CCHD has not been fully investigated. Apoptosis was observed in T. cruzi-infected cultured CMs [44]. In human heart tissue Rossi and Souza [45] did not observe apoptosis of CMs, but in a dog model apoptosis was observed in CMs as well as in endothelial and inflammatory cells [46].
The paucity of parasite pseudocysts as the infection becomes chronic led to the erroneous conclusion that that there was no direct relationship between the parasite and the evolution of CCHD. In recent years, however, it has become evident using more sensitive methods that there is indeed parasite persistence in many tissues, including the heart, and that the presence of parasites correlates with areas of tissue inflammation [47, 48].
The parasite-endothelial cell interactions are among the first to occur during acute T. cruzi infection and in recent years the nature of these interactions as well as their consequences have received increased scrutiny. Since the vasculature comprises approximately 35% of the volume of the myocardium it would appear reasonable that the interaction of the parasite and the endothelium would be an important element in the pathogenic process. The early descriptions of a T. cruzi–induced vasculitis were described by Jorg [49, 50]] and Rossi and Ramos [51]. In the 1980s it was demonstrated that microvascular compromise was an important contributing factor in the pathogenesis of experimental and human cardiomyopathies of various etiologies and that the calcium-blocking agent verapamil could mitigate this process [52]. Although at that time CCHD had been well described, the association between vascular compromise and CCHD had not. In a mouse model of acute T. cruzi infection, Factor et al [53] demonstrated vasospasm and saccular aneurysms in the subendocardial microvasculature, similar to that described in other cardiomyopathies. Furthermore, it was suggested by these authors that these observations, made during acute infection, might contribute to the development of the typical dilated cardiomyopathy observed in chronic chagasic cardiomyopathy [43, 53]. Subsequently, it was demonstrated that T. cruzi infection resulted in reduced blood flow in the microvacular bed which was reversed by treatment with verapamil [54].
Infection-associated vasculitis, vasospasm, vasoconstriction, platelet aggregation, and a reduction in blood flow have been described in acute Chagas disease. Infected cultured endothelial cells display increased expression of leukocyte adhesion molecules [55]. When verapamil, which increases coronary blood flow, was administered to mice soon after infection there was a reduction of the subsequent cardiomyopathy in comparison to untreated controls [56, 57]. In recent years the contributions of thromboxane A2 [58] and endothelin-1 [26] to the pathogenesis of Chagas disease have been detailed. Both are pro-inflammatory and cause vascular spasm and platelet aggregation.
The paucity of parasites in the myocardium has also led to severaltheories as to the etiology of CCHD including microvascular compromise (see above) autoimmunity [59] andneurogenic [3, 60]. More recently, the group headed by Garg has presented evidence that T. cruzi infection results in oxidative stress in the myocardium that can be monitored by measurements of malonylaldehyde, glutathione disulfide (oxidative stress markers) and declining antioxidant defenses (superoxide dismutase, MnSOD, catalase) in the peripheral blood [61–63]. Furthermore, targeted therapy can reverse these alterations (64, and Garg, personal communication). The implications for clinical management of patients with chagasic heart disease have yet to be determined.
As noted, a common feature of chagasic heart disease is conduction/rhythm disturbances [65]. Because cardiac conduction requires the presence and appropriate distribution of gap junction channels between the myocytes and because aberrant gap junction expression and distribution is a common factor in various cardiomyopathies [66], an area of interest has been whether T. cruzi infection alters expression, function or distribution of gap junction proteins in cardiac myocytes (CMs). In studies performed on cultured rat CMs, expression of the major cardiac gap junction protein connexin43 (Cx43) was not significantly altered at either protein or mRNA level; however, there was a marked disturbance in subcellular localization, with a prominent loss of appositional plaque formation [67, 68]. This loss of correct localization between CMs was associated with a loss of electrical coupling and intercellular diffusion of the fluorescent dye Lucifer Yellow. More recent studies in which microarray methodology has been used to examine gene expression changes in hearts of T. cruzi-infected mice have also failed to detect differences in expression of the gap junction protein Cx43 [69, 70 and unpublished results]. However, slight but significant differences in overall Cx43 abundance is observed at some timepoints following acute infection of mice with the Y strain of T. cruzi. These data indicate that perturbations of Cx43 biotrafficking, abundance and functional coupling may contribute to the high incidence of arrhythmias in Chagas disease [71].
Some experts in the field adhere to only one theory to explain the development of chronic chagasic heart disease to the exclusion of others. Recently, microarray analysis has been employed to understand the pathogenesis of CCHD [69]. We believe that the etiology likely is multifactorial.
The laboratory diagnosis of Chagas Disease
The diagnosis of acute T. cruzi infection is usually made by the detection of parasites. Blood form trypomastigotes (BFTs)can be observed by microscopic examination of fresh blood or buffy coat. BFTs also can be seen in Giemsa-stained thin and thick blood smears. If the parasites cannot be detected by these methods, inoculation of blood into specialized liquid medium or into mice may be appropriate. However, these methods lack sensitivity because parasites may not be observed for several weeks. Assays based on the polymerase chain reaction (PCR), first developed 20 years ago, may be the most sensitive method for detecting acute and congenital T. cruzi infections. If acute Chagas disease is suspected in an immunocompromised patient and these parasitologic methods fail to demonstrate the presence of parasites, tissue specimens should be examined. Such patients pose difficult diagnostic problems because they may present with fulminant clinical disease but with low parasitemias that cannot be detected. Parasites may at times be observed in other sites, such as pericardial fluid, bone marrow, brain, skin, and lymph nodes, and these tissues should also be investigated if feasible.
The diagnosis of chronic Chagas disease is generally based on detecting specific antibodies that bind to T. cruzi antigens. Several serological assays are employed in Latin America for detecting antibodies, such as the indirect immunofluorescence test (IFA) and the enzyme-linked immunosorbent assay (ELISA). These and other serologic assays are used widely for clinical diagnosis and screening donated blood, as well as in epidemiological studies. A persistent problem has been the presence of false negative and false positive reactions. There are two FDA-approved tests available in the United States for clinical testing. One is a lysate-based ELISA (Hemagen Chagas Kit; Hemagen Diagnostics, Inc., Columbia, MD), and the other is an ELISA based on recombinant antigens (Chagatest Elisa Recombinante; Laboratorios Wiener, Rosario, Argentina). Screening of the United States blood supply currently is being done with a lysate-based ELISA (Ortho T. cruzi ELISA Test System; Ortho-Clinical Diagnostics, Raritan, NJ). An automated blood screening assay based on four chimeric recombinant antigens is being developed (PRISM Chagas Assay; Abbott Laboratories, Abbott Park, IL) [72] and a test based on a blot format is being developed with the same antigens for confirmatory testing (Abbott Chagas Immunoblot Assay) [73].
An immunoprecipitation assay based on iodinated T. cruzi proteins (RIPA), developed by Louis V. Kirchhoff of the University of Iowa has been demonstrated to be highly specific as well as sensitive when used in clinically and geographically diverse groups of infected and uninfected people. The RIPA currently is being used as the confirmatory assay to test all donor samples that are positive in the Ortho screening assay, and it is also available for clinical testing.
The detection of chronic infection by testing for parasite antigens in blood and urine has been studied. This approach has not achieved results comparable to those obtained by serologic methods. PCR-based assays for detecting chronic T. cruzi infections have been studied extensively. However, their usefulness in this context has not been established definitively. For many years it was hoped that this method would be well-suited for detecting the low number of parasites circulating in the blood of chronically infected persons. However, there are sampling issues because parasitemias are extremely low and may in fact be intermittent, thus limiting the sensitivity of the assays. Moreover, false positive results may be an issue. A likely niche for PCR-based assays is in the diagnosis congenital T. cruzi infections immediately after birth.
Anti-parasitic treatment
The treatment of T. cruzi is not satisfactory. There are two drugs available Nifurtimox (Lampit, Bayer 2502) and benznidazole (Rochagan, Roche 7–1051). They lack efficacy and must be taken for extended periods. In addition, they may cause severe side effects. These drugs reduce the severity of acute Chagas disease. It is generally thought that approximately 70% of persons with acute infection are cured parasitologically with a full course of either drug, but there are no sizable studies that support this success rate. This cure rate is thought to decrease as a function of the time patients have been infected and perhaps less than 10% in individuals with individuals with long-standing chronic infection can be cured. There are no convincing data from properly controlled trials that treatment with either nifurtimox or benznidazole is beneficial in persons with long-standing infections. Experts in Brazil and Argentina currently recommend specific treatment only for patients with acute and congenital T. cruzi infections, and for chronically infected children. Therapy for adults assumed to have long-standing infections is not recommended, regardless of clinical status, although the reality is that many such persons do get treatment. A large trial designed to address the efficacy of benznidazole is under way (the BENEFIT Multicentric Trial). Allopurinol and several antifungal azoles have been shown to have some anti-T. cruzi activity in in vitro experiments and in animal studies. But there are no data that would warrant their use in place of nifurtimox or benznidazole. The question of anti-parasitic drug treatment in persons who are found be seropositive while being screened as blood donors has been addressed in a recent publication [74].
Persons with severe CCHD with dilated cardiomyopathy and congestive heart failure (Class III and Class IV) may benefit from heart transplantation. More than 100 such heart transplants have been done in Brazil, and roughly a couple of dozen have been done in the United States as well. A major concern in the recipients of transplantation is the consequences of immunosuppression including the reactivation of T. cruzi infection [75]. It is interesting, but not unexpected, that the overall survival of heart transplant patients with CCHD is longer than that of persons transplanted for heart disease resulting from other etiologies. Stem cell transplantation currently is being evaluated in patients with severe heart failure associated with CCHD (see section on Stem cell treatment below).
Cell-based therapy for Chagas cardiomyopathy
The interest in using cell based therapy for chagasic cardiomyopathy followed the initiation of research on the use of this modality in patients with myocardial infarction (MI). The pioneering work of Soonpa et al [76], in which labeled fetal syngeneic cardiac myocytes (CMs) were transplanted into adult mouse hearts, showed definitively that exogenous cells could be integrated into the host myocardium. Initially, most of the studies in this area of research focused on transplantation of fetal CMs, embryonic stem cells, or skeletal myoblasts into hearts that were damaged cryogenically or by MI. An important development in the use of cell therapies to improve cardiac function was based on the observations that stromal bone marrow (BM) cells could be induced to differentiate into CMs in vitro [77]. Tomita et al [78] demonstrated that autologous BM cells transplanted into cryoinjured rat hearts improved myocardial function and promoted angiogenesis. Subsequent studies, however, failed to demonstrate that the injected BM cells were in fact able to differentiate into CMs or blood vessel cells. Orlic et al [79] reported that hematopoietic stem cells from transgenic mice expressing enhanced green fluorescent protein (EGFP) transplanted into MI-damaged hearts of syngeneic mice differentiated into cardiac muscle and vascular cells. These authors believed that cells would regenerate the damaged myocardium, promoting angiogenesis and improving myocardial function. Importantly, they demonstrated complete integration of the transplanted c-kit+ BM cells, including the formation of connexin43 gap junctions between the newly formed myocardium and the surviving tissue. Others demonstrated that hematopoietic and mesenchymal stem cells derived from BM improve myocardial function in models of both cryo-injured and ischemic heart lesions [80, 81].
Cardiac regeneration by BM-derived cells has been questioned [82–84]. However, in a study where functional measurements were performed (83), improvement in heart function was detected after cell transplantation. Since then the beneficial effects of cell therapies using BM-derived cells in heart disease have been increasingly attributed to paracrine effects [85–87]. In all these cases, however, the damage to the heart was circumscribed to a specific area since the lesions were ischemic in nature.
Due to the more global nature of CCHD, an approach was developed to deliver cells systemically in a mouse model, since direct myocardial injections would have to be performed in various areas of the LV and RV, creating the possibility of myocardial damage due to the multiple injections. Therefore the first step in validating the therapy was to demonstrate that cells injected intravenously established themselves in the chagasic hearts. BM mononuclear cells were pre-incubated with Hoechst 33258 stain prior to injection into tail veins of normal and chagasic mice, and BM mononuclear cell-treated mice were sacrificed at various time points thereafter. In chagasic mice Hoechst+ cells were observed in the heart 1–7 days after BM cell injection, but fluorescent cells were not found in heart sections of normal mice injected with Hoechst 33258-stained cells. Hoechst+ cells were also found in the spleen and liver of chagasic and control BM cell-treated mice 1–2 days after transplant. In hearts of chagasic mice, Hoechst+ cells proliferated and formed clusters of cells bearing a dotted nuclear fluorescent pattern that could be observed up to 30 days after BM mononuclear cell transplant. Heart sections of BM mononuclear cell-treated mice were also stained for BM stem cell markers by immunofluorescence and Sca-1+ and cKit+ cell clusters were found in hearts of BM mononuclear cells-treated mice after cell injection [88]. Recent observations suggest that BM stem cells home to the chagasic heart, validating systemic injection as a viable approach for cell therapy in this context.
Soares et al [88] demonstrated that BM mononuclear cells from normal syngeneic donors significantly reduced cardiac inflammation and fibrosis in mice with chronic T. cruzi infections. Importantly, the improvement was observed up to six months after cell therapy. The decrease in inflammation appears to result from increased apoptosis of the infiltrating inflammatory cells as determined by TUNEL staining. The decrease in fibrosis may result from activation of metalloproteases. Although there is evidence for both trans-differentiation and fusion of the injected BM cells in the myocardium, the mechanisms of action in chagasic mouse hearts have not as yet been fully elucidated. Trans-differentiation/fusion appears to occur at an extremely low frequency and paracrine effects may be the major cause of improvement in myocardial function. In another set of experiments, it was determined that BM cells from chronically infected mice also ameliorated the pathology of infected mice [88]. This observation is important since in clinical human trials autologous BM cells were employed.
Recently, Goldenberg et al [89] demonstrated that BM mononuclear cells prevented and reversed the RV dilatation induced by T. cruzi-infection. Furthermore it was determined that repeated injections of the colony stimulating factor G-CSF decreases inflammation and fibrosis in the hearts of chagasic mice, a finding consistent with observations of Harada et al [90] that showed improvement in heart function in an ischemic mouse model. The combination of BM mononuclear cells and G-CSF enhances the effect of the cell therapy in the reduction of the inflammatory infiltrate.
Although in general the rat is a relatively poor model for reproducing human chagasic cardiomyopathy, it was reported [91] that direct LV injection of co-cultured skeletal myoblasts and mesenchymal BM-derived cells improved heart function in chronically infected rats. These findings suggest that even when injected locally stem cells are able to diffuse out and reach other regions of the heart. This is an important observation, given the widespread involvement of the myocardium in chagasic cardiomyopathy.
Based on the encouraging results in mice, investigators in Brazil initiated a clinical trial to examine the feasibility and safety of autologous BM cell transplantation in patients with CHF due to CCHD. Notably, it was reported that in a patient with chagasic cardiomyopathy BM mononuclear cells delivered by the intracoronary route were preferentially retained in diseased areas of the myocardium [92]. These patients generally have a poor prognosis, with mortality rates reaching 40% within two years of onset. At the most advanced stage of CHF the only therapeutic option is heart transplantation, but this procedure is expensive and obviously can be done in a very small number of patients. Given the uncertainties regarding the mechanisms of action of the BM mononuclear cells, the trial was designed for patients with end-stage CHF whose only therapeutic option would be heart transplantation. The trial was an open label, uncontrolled, single center clinical trial. Inclusion criteria required patients to be 20–70 years old, of either gender, with CHF due to Chagas disease, in NYHA class III or IV, with an ejection fraction of less than 40% while on optimized pharmacologic therapy for at least 4 weeks before enrollment [93]. BM cell aspiration was performed on the day of the injection and the BM mononuclear fraction was obtained. The cell suspension was injected in the coronary arteries using an angioplasty catheter. The preliminary results indicate that BM mononuclear cells therapy by intracoronary delivery is feasible and safe in chronic chagasic cardiomyopathy patients. Since this trial was designed only to evaluate safety and feasibility no conclusions could be drawn regarding the effects of the treatment. A phase II/III clinical trial is now underway.
Chagas disease and Immunosuppression
Even prior to the advent of the HIV/AIDS pandemic, reactivation of T. cruzi infection had been observed in patients undergoing immunosuppressive therapy for malignancies and organ transplantation [94–97]. Del Castillo et al [98] initially described a 19-year old man with hemophilia who had a hypodense lesion in the right frontal lobe. Pathologic examination of a biopsy of the lesion showed inflammatory perivascular infiltrates and clusters of T. cruzi amastigotes. HIV-1 and Chagas serology were positive, and the CD4 cell count was low. He was treated with nifurtimox but died of acute myocarditis. Since then many dozens of patients with HIV-T. cruzi co-infection have been described and likely many more have not been reported and have gone undetected [95, 99]. The central nervous system (CNS) and the heart are the most commonly affected sites of reactivation [99–102]. Myocarditis as the only manifestation of Chagas disease in HIV-infected patients is uncommon. Typically co-infected patients present with signs and symptoms resulting from CNS involvement and myocarditis. They may also be diagnosed at autopsy [99, 103]. Autopsy findings suggest that a substantial proportion of co-infected patients have acute cardiac disease that is clinically silent, despite the inflammation with nests of parasites in the myocardium. Clinical manifestations of reactivation can include CHF and arrhythmias.
Clinically significant Chagas disease is predominantly observed in co-infected patients with low CD4 counts and advanced AIDS. The histopathology of the CNS and the heart associated with reactivated Chagas’ disease in AIDS patients has been well described [99, 103]. In the myocardium the intensity of the inflammation provoked by the parasite varies considerably. The myocarditis may be mild or widespread and intense. Another feature of cardiac involvement is the proliferation of connective tissue. Thus, when individuals with chronic and/or asymptomatic Chagas disease acquire HIV, as immunosuppression and AIDS develops over time, there may be reactivation of the T. cruzi infection presenting as necrotizing encephalitis [104] and/or acute myocarditis. In addition, the reactivation of T. cruzi infection may increase HIV viral load [100] thus causing further immunosuppression. The reactivation of infection during periods of immunosuppression has raised questions as to where the parasites reside in the chronic stage. In that regard, Combs et al [12] demonstrated that up to 300 days post infection parasites can be found in adipose tissue in infected mice. Whether adipose tissue acts as an important reservoir of infection in human Chagas disease is not known and currently is under investigation.
Vaccine development
No vaccine is available for protecting against T., cruzi infection, despite considerable research in this area in animal models. In view of the lack of major progress in developing vaccines for other protozoan agents that are more important than T. cruzi, particularly Plasmodium falciparum and Leishmania Donovan, one might conclude that the biologic barriers to an effective vaccine for T. cruzi and other protozoan agents are unlikely to be overcome. More importantly, the widespread success in blocking transmission of T. cruzi through low-technology approaches, as mentioned above, makes justifying major investments in T. cruzi vaccine development difficult and research interest in this area has diminished considerably in recent years. However, there are several groups that are pursuing this area of research [105, 106, 107].
Acknowledgments
This work was supported in part by NIH grants HL-73732, AI-076248.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tanowitz HB, Kirchhoff LV, Simon D, et al. Chagas’ Disease. Clin Microbiol Rev. 1992;4:400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kierszenbaum F. Mechanisms of pathogenesis in Chagas disease. Acta Parasitologica. 2007;52:1–12. [Google Scholar]
- 3.Marin-Neto AJ, Cunha-Neto E, Maciel BC, et al. Pathogenesis of chronic Chagas disease. Circulation. 2007;115:1109–1121. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 4.Moncayo A, Ortizyanine ML. Centenial review An update on Chagas disease (human American Trypanosomiasis) Ann Trop Med Parasitology. 2006;100:663–677. doi: 10.1179/136485906X112248. [DOI] [PubMed] [Google Scholar]
- 5.Tarleton RL, Reithinger R, Urbina JA, et al. The challenges of ChagasDisease--grim outlook or glimmer of hope. PLoS. 2007;4:1852–1857. doi: 10.1371/journal.pmed.0040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chagas C. Nova tripanosomíase humana: Estudos sobre a morfologia e o ciclo evolutivo do Schizotrypanum cruzi n.g., n.sp., agente etiológico de nova entidade mórbida no homem. Mem Inst Osw Cruz. 1909c;1:159–218. [Google Scholar]
- 7.Chagas C. Nova entidade mórbida do homem: Resumo geral de estudos etiológicos e clínicos. MemInst Osw Cruz. 1911;3:219–75. [Google Scholar]
- 8.Lewinsohn R. Prophet in his own country, Carlos Chagas and the Nobel Prize. Perspectives in biology and medicine. 2003;46:532–549. doi: 10.1353/pbm.2003.0078. [DOI] [PubMed] [Google Scholar]
- 9.Aufderheide AC, Salo W, Madden M, et al. A 9,000-year record of Chagas’ disease. Proc Natl Acad Sci U S A. 2004;101:2034–2039. doi: 10.1073/pnas.0307312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anonymous MMWR Blood Donor Screening for Chagas Disease --- United States, 2006–2007. 2007;56:141–143. [PubMed] [Google Scholar]
- 11.Bern C, Montgomery SP, Katz L, et al. Chagas disease and the US blood supply. Curr Opin Infect Dis. 2008;21:476–482. doi: 10.1097/QCO.0b013e32830ef5b6. [DOI] [PubMed] [Google Scholar]
- 12.Combs TP, Nagajyothi, Mukherjee S, et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem. 2005;280:24085–2494. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- 13.Cohen JE, Gürtler RE. Modeling household transmission of American trypanosomiasis. Science. 2001;27:293:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- 14.Laranja FS, Dias E, Nobrega G, et al. Chagas’ disease: a clinical, epidemiological and pathologic study. Circulation. 1956;14:1035–1060. doi: 10.1161/01.cir.14.6.1035. [DOI] [PubMed] [Google Scholar]
- 15.Kirchhoff LV. American trypanosomiasis. Gastro Clinics of North Amer. 1996;25:517–533. doi: 10.1016/s0889-8553(05)70261-2. [DOI] [PubMed] [Google Scholar]
- 16.Parada H, Carrasco HA, Añez N, et al. Cardiac involvement is a constant finding in acute Chagas disease: a clinical, parasitological and histopathological study. Int J Cardiol. 1997;60:49–54. doi: 10.1016/s0167-5273(97)02952-5. [DOI] [PubMed] [Google Scholar]
- 17.PAHO publication 2006
- 18.Ribeiro AL, Teixeira MM, Reis AM, et al. Brain natriuretic peptide based strategy to detect left ventricular dysfunction in Chagas disease: acomparison with the conventional approach. Int J Cardiol. 2006;109:34–40. doi: 10.1016/j.ijcard.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 19.Costa GC, Rocha MO, Moreira PR, et al. Functional IL-10 Gene Polymorphism Is Associated with Chagas Disease Cardiomyopathy. J Infect Dis. 2009;199:451–454. doi: 10.1086/596061. [DOI] [PubMed] [Google Scholar]
- 20.Rassi A, Jr, Rassi A, Little WC, et al. Development and validation of a risk score for predicting death in Chagas’ heart disease. NEJM J Med. 2006;355:799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- 21.Acquatella H. Echocardiography in Chagas heart disease. Circulation. 2007;115:1124–1131. doi: 10.1161/CIRCULATIONAHA.106.627323. [DOI] [PubMed] [Google Scholar]
- 22.Barros MVL, Rocha MOC, Ribeiro ALP, et al. Doppler tissue imaging to evaluate early myocardium damage in patients with undetermined form of Chagas disease and normal echocardiogram. Echocardiography. 2001;18:131–136. doi: 10.1046/j.1540-8175.2001.00131.x. [DOI] [PubMed] [Google Scholar]
- 23.Viotti RJ, Vigliano C, Laucella S, et al. Value of echocardiography for diagnosis and prognosis of chronic Chagas disease cardiomyopathy without heart failure. Heart. 2004;90:655–660. doi: 10.1136/hrt.2003.018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aquatella H, Schiller NB, Puigbó JJ, et al. M-mode and two-dimensional echocardiography in chronic Chagas’ heart disease. Circulation. 1980;62:787–799. doi: 10.1161/01.cir.62.4.787. [DOI] [PubMed] [Google Scholar]
- 25.Marin-Neto JA, Marzullo P, Marcassa C, et al. Myocardial perfusion defects in chronic Chagas’ disease: assessment with thallium-201 scintigraphy. Am J Cardiol. 1992;69:780–784. doi: 10.1016/0002-9149(92)90505-s. [DOI] [PubMed] [Google Scholar]
- 26.Tanowitz HB, Huang H, Jelicks LA, et al. Role of endothelin 1 in the pathogenesis of chronic chagasic heart disease. Infect Immun. 2005;73:2496–503. doi: 10.1128/IAI.73.4.2496-2503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueno Y, Nakamura Y, Takahashi M, et al. A highly suspected case of chronic Chagas’ heart disease diagnosed in Japan. Jpn Circ J. 1995;59:219–223. doi: 10.1253/jcj.59.219. [DOI] [PubMed] [Google Scholar]
- 28.Marcu CB, Beek AM, van Rossum AC. Chagas’ heart disease diagnosed on MRI: the importance of patient “geographic” history. Int J Cardiol. 2007;117:e58–60. doi: 10.1016/j.ijcard.2006.11.114. [DOI] [PubMed] [Google Scholar]
- 29.Rochitte CE, Nacif MS, Júnior AC, et al. Cardiac magnetic resonance in Chagas’ disease. Artificial Organs. 2007;31:259–267. doi: 10.1111/j.1525-1594.2007.00373.x. [DOI] [PubMed] [Google Scholar]
- 30.Sechtem U, Mahrholdt H, Vogelsberg H. Cardiac magnetic resonance in myocardial disease. Heart. 2007;93:1520–1527. doi: 10.1136/hrt.2005.067355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H, Chan J, Wittner M, Jelicks LA, et al. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J Mol Cell Cardiol. 1999;31:75–88. doi: 10.1006/jmcc.1998.0848. [DOI] [PubMed] [Google Scholar]
- 32.Jelicks LA, Shirani J, Wittner M, et al. Application of cardiac gated magnetic resonance imaging in murine Chagas’ disease. Am J Trop Med Hyg. 1999;61:207–214. doi: 10.4269/ajtmh.1999.61.207. [DOI] [PubMed] [Google Scholar]
- 33.Jelicks LA, Chandra M, Shirani J, et al. Cardioprotective effects of phosphoramidon on myocardial structure and function in murine Chagas’ disease. Int J Parasitol. 2002;32:1497–1506. doi: 10.1016/s0020-7519(02)00136-4. [DOI] [PubMed] [Google Scholar]
- 34.Durand JL, Tang B, Gutstein DE, et al. Dyskinesis in Chagasic myocardium: centerline analysis of wall motion using cardiac-gated magnetic resonance images of mice. Magn Reson Imaging. 2006;24:1051–1057. doi: 10.1016/j.mri.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machado FS, Souto JT, Rossi MA, et al. Nitric oxide synthase-2 modulates chemokine production by Trypanosoma cruzi-infected cardiac myocytes. Microbes Infect. 2008;10:1558–1566. doi: 10.1016/j.micinf.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutierrez FRS, Lalu MM, Mariano FS, et al. Increased activities of cardiac matrix metalloproteinases matrix metalloproteinase (MMP)-2 and MMP-9 are associated with mortality during the acute phase of experimental Trypanosoma cruzi Infection. J Infect Dis. 2008;197:1468–1476. doi: 10.1086/587487. [DOI] [PubMed] [Google Scholar]
- 37.Higuchi M, Fukasawa S, de Brito T, et al. Different microcirculatory and interstitial matrix patterns in idiopathic dilated cardiomyopathy and Chagas’ disease: a three dimensional confocal microscopy study. Heart. 1999;82:279–286. doi: 10.1136/hrt.82.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin DL, Tarleton RL. Antigen-specific T cells maintain an effector memory phenotype during persistent Trypanosoma cruzi infection. J Immunol. 2005;174:1594–1601. doi: 10.4049/jimmunol.174.3.1594. [DOI] [PubMed] [Google Scholar]
- 39.Martin DL, Weatherly DB, Laucella SA, et al. CD8+ T-cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2006;2:e77. doi: 10.1371/journal.ppat.0020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang H, Petkova SB, Cohen AW, et al. Activation of Transcription factors (AP-1 and NF-κB) in Murine Chagasic Myocarditis. Activation of transcription factors AP-1 and NF-κB in murine Chagasic myocarditis. Infect Immun. 2003;71:2859–2567. doi: 10.1128/IAI.71.5.2859-2867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagajyothi F, Desruisseaux M, Bouzahzah B, et al. Cyclin and caveolin expression in an acute model of murine Chagasic myocarditis. Cell Cycle. 2006;5:107–112. doi: 10.4161/cc.5.1.2284. [DOI] [PubMed] [Google Scholar]
- 42.Petkova SB, Ashton A, Bouzahzah B, et al. Cell cycle molecules and diseases of the cardiovascular system. Front Biosci. 2000;5:D452–60. doi: 10.2741/petkova. [DOI] [PubMed] [Google Scholar]
- 43.Petkova SB, Huang H, Factor SM, et al. The role of endothelin in the pathogenesis of Chagas’ disease. Int J Parasitol. 2001;31:499–511. doi: 10.1016/s0020-7519(01)00168-0. [DOI] [PubMed] [Google Scholar]
- 44.Petersen CA, Krumholz KA, Carmen J, et al. Trypanosoma cruzi Infection and Nuclear Factor Kappa B Activation Prevent Apoptosis in Cardiac Cells. Infect Immun. 2006;74:1580–1587. doi: 10.1128/IAI.74.3.1580-1587.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi MA, Souza AC. Is apoptosis a mechanism of cell death of cardiomyocytes in chronic chagasic myocarditis? Int J Cardiol. 1999;68:325–331. doi: 10.1016/s0167-5273(98)00375-1. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Andrade ZA, Yu Z-X, et al. Apoptosis in acanine model of acute chagasic myocarditis. J Mol Cell Cardiol. 1999;31:581–596. doi: 10.1006/jmcc.1998.0893. [DOI] [PubMed] [Google Scholar]
- 47.Tarleton RL. Parasite persistence in the aetiology of Chagas disease. Int J Parasitol. 2001;31:550–554. doi: 10.1016/s0020-7519(01)00158-8. [DOI] [PubMed] [Google Scholar]
- 48.Jones EM, Colley DG, Tostes S, Lopes ER, Vnencak-Jones CL, McCurley TL. Amplification of a Trypanosoma cruzi DNA sequence from inflammatory lesions in human chagasic cardiomyopathy. Am J Trop Med Hyg. 1993;48:348–357. doi: 10.4269/ajtmh.1993.48.348. [DOI] [PubMed] [Google Scholar]
- 49.Jorg ME. Destruccion de vaso capilares, miocitolisis yaneurisma apical en la cardiopatia chagasica. Prensa Medica Argentina. 1980;67:490–494. [Google Scholar]
- 50.Jorg ME. Tripanosomiasis cruzi: anarquia angiotopografica por descapilarizaclon mesequimorreactiva,. cofactor patogenico de la miocardiopatia cronica. Prensa Medica Argentina. 1980;61:490–494. [Google Scholar]
- 51.Rossi M, Ramos S. Coronary microvascular abnormalities in Chagas’ disease. Am Heart J. 1996;132:207–210. doi: 10.1016/s0002-8703(96)90417-2. [DOI] [PubMed] [Google Scholar]
- 52.Sonnenblick EH, Fein F, Capasso JM, et al. Microvascular spasm as a cause of cardiomyopathies and the calcium-blocking agent verapamil as potential primary therapy. Am J Cardiol. 1985;55:179B–184B. doi: 10.1016/0002-9149(85)90629-0. [DOI] [PubMed] [Google Scholar]
- 53.Factor SM, Cho S, Wittner M, et al. Abnormalities in the microcirculation in Chagas’ disease. Am JTrop Med Hyg. 1985;34:246–253. doi: 10.4269/ajtmh.1985.34.246. [DOI] [PubMed] [Google Scholar]
- 54.Tanowitz HB, Kaul DK, Chen B, et al. Compromised microcirculation in acute Trypanosoma cruzi infection. J Parasit. 1996;82:124–130. [PubMed] [Google Scholar]
- 55.Huang H, Calderon TM, Berman JW, et al. Infection of endothelial cells with Trypansoma cruzi activates NF-κB and induces vascular adhesion molecule expression. Infect Immun. 1999;67:5434–5440. doi: 10.1128/iai.67.10.5434-5440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chandra M, Shirani J, Shtutin V, Weiss LM, et al. Cardioprotective effects of Verapamil on myocardial structure and function in a murine model of chronic Trypanosoma cruzi infection (Brazil strain): an echocardiographic study. Int J Parasitol. 2002;32:207–215. doi: 10.1016/s0020-7519(01)00320-4. [DOI] [PubMed] [Google Scholar]
- 57.de Souza AP, Tanowitz HB, Chandra M, et al. Effects of early and late verapamil administration on the development of cardiomyopathy in experimental chronic Trypanosoma cruzi (Brazil strain) infection. Parasitol Res. 2004;92:496–501. doi: 10.1007/s00436-004-1080-1. [DOI] [PubMed] [Google Scholar]
- 58.Ashton AW, Mukherjee S, Nagajyothi Fnu, et al. Thromboxane A2 is a key regulator of pathogenesis during Trypanosoma cruzi infection. J Exp Med. 2007;204:929–940. doi: 10.1084/jem.20062432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonney KM, Engman DM. Chagas heart disease pathogenesis: one mechanism or many? Curr Mol Med. 2008;8:510–518. doi: 10.2174/156652408785748004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simoes MV, Pintya AO, Bromberg-Marin G, et al. Relation of regional sympathetic denervation and myocardial perfusion disturbance to wall motion impairment in Chagas’ cardiomyopathy. Am J Cardiol. 2000;86:975–981. doi: 10.1016/s0002-9149(00)01133-4. [DOI] [PubMed] [Google Scholar]
- 61.Wen JJ, Vyatkina G, Garg N. Oxidative damage during chagasic cardiomyopathy development: role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic Biol Med. 2004;37:1821–1833. doi: 10.1016/j.freeradbiomed.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 62.Wen JJ, Yachelini PC, Sembaj A, et al. Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Rad Biol Med. 2006;41:270–276. doi: 10.1016/j.freeradbiomed.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 63.Wen JJ, Dhiman M, Whorton EB, et al. Tissue-specific oxidative imbalance and mitochondrial dysfunction during Trypanosoma cruzi infection in mice. Microbes Infect. 2008;10:1201–1209. doi: 10.1016/j.micinf.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wen JJ, Bhatia V, Popov VL, et al. Phenyl-alpha-tert-butyl nitrone reverses mitochondrial decay in acute Chagas’ disease. Am J Pathol. 2006;169:1953–1964. doi: 10.2353/ajpath.2006.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elizari MV, Chiale PA. Cardiac arrhythmias in Chagas’ heart disease. J Cardiovasc Electrophysiol. 1993;4:596–608. doi: 10.1111/j.1540-8167.1993.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 66.Severs NJ, Dupont E, Thomas N, et al. Alterations in cardiac connexin expression in cardiomyopathies. Adv Cardiol. 2008;42:228–242. doi: 10.1159/000092572. [DOI] [PubMed] [Google Scholar]
- 67.Campos de Carvalho AC, Masuda MO, et al. Conduction defects and arrhythmias in Chagas’ disease: possible role of gap junctions and humoral mechanisms. J Cardiovasc Electrophysiol. 1994;5:686–698. doi: 10.1111/j.1540-8167.1994.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 68.Campos de Carvalho AC, Tanowitz HB, Wittner M, et al. Gap junction distribution is altered between cardiac myocytes infected with Trypanosoma cruzi. Circ Res. 1992;70:733–742. doi: 10.1161/01.res.70.4.733. [DOI] [PubMed] [Google Scholar]
- 69.Mukherjee S, Belbin TJ, Spray DC, et al. Microarray analysis of changes in gene expression in a murine model of chronic chagasic cardiomyopathy. Parasitol Res. 2003;91(3):187–96. doi: 10.1007/s00436-003-0937-z. [DOI] [PubMed] [Google Scholar]
- 70.Mukherjee S, Nagajyothi Fnu, Mukhopadhyay A, et al. Alterations in myocardial gene expression associated with experimental Trypanosoma cruzi infection. Genomics. 2008;91:423–432. doi: 10.1016/j.ygeno.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adesse D, Garzoni LR, Huang H, Tanowitz HB, et al. Trypanosoma cruzi induces changes in cardiac connexin43 expression. Microbes Infect. 2008;10:21–28. doi: 10.1016/j.micinf.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang CD, Cheng KY, Jang LX, et al. Evaluationof a prototype Trypanosoma cruzi antibody assay with recombinant antigens on a fully automated chemiluminescence analyzer for blood donor screening. Transfusion. 2006;46:1737–1744. doi: 10.1111/j.1537-2995.2006.00965.x. [DOI] [PubMed] [Google Scholar]
- 73.Cheng KY, Chang CD, Salbilla VA, et al. Immunoblot assay using recombinant antigens as a supplemental test to confirm the presence of antibodies to Trypanosoma cruzi. Clin Vaccine Immunol. 2007;14:355–361. doi: 10.1128/CVI.00401-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bern C, Montgomery SP, Herwaldt BL, et al. Evaluation and treatment of chagas disease in the United States: a systematic review. JAMA. 2007;298:2171–2181. doi: 10.1001/jama.298.18.2171. [DOI] [PubMed] [Google Scholar]
- 75.Campos SV, Strabelli TM, Amato Neto V, et al. Risk factors for Chagas’ disease reactivation after heart transplantation. J Heart Lung Transplant. 2008;27:597–602. doi: 10.1016/j.healun.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 76.Soonpaa MK, Koh GY, Klug MG, et al. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science. 1994;264:98. doi: 10.1126/science.8140423. [DOI] [PubMed] [Google Scholar]
- 77.Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:69. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tomita S, Li R-K, Weisel RD, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100 (suppl II):247–256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 79.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 80.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 81.Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 82.Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 83.Balsam LB, Wagtner AJ, Christenson Jl, et al. Haematopoitec stem cells adopt mature haematopoitec fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 84.Nygren JM, Jovinge S, Breitbach M, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 85.Mangi A, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 86.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005 doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 87.Dawn B, Guo Y, Rezazadeh A, et al. Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function. Circ Res. 2006;98:1098–1105. doi: 10.1161/01.RES.0000218454.76784.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soares MBP, Lima RS, Rocha LL, et al. Transplanted bone marrow cells repair heart tis8ue and reduce myocarditis in chronic chagasic mice. Am J Pathol. 2004;164:441–447. doi: 10.1016/s0002-9440(10)63134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goldenberg RC, Jelicks LA, Fortes FS, et al. Bone Marrow Cell Therapy Ameliorates and Reverses Chagasic Cardiomyopathy in a Mouse Model. J Infect Dis. 2008;197:544–547. doi: 10.1086/526793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harada M, Qin Y, Takano H, et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nature Medicine. 2005;11:305–311. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- 91.Guarita-Souza LC, Carvalho KAT, Woitowicz V, et al. Simultaneous autologous transplantation of cocultured mesenchymal stem cells and skeletal myoblasts improves ventricular function in a murine model of Chagas disease. Circulation. 2006;114(1 Suppl):I120–1124. doi: 10.1161/CIRCULATIONAHA.105.000646. [DOI] [PubMed] [Google Scholar]
- 92.Jacob JLB, Vilela Salis F, Ruiz MA. Labeled stem cells transplantation to the myocardium of a patient with Chagas’ disease. Arq Bras Cardiol. 2007;89:e10–1. doi: 10.1590/s0066-782x2007001400014. [DOI] [PubMed] [Google Scholar]
- 93.Vilas-Boas F, Feitosa GS, Soares MBP, et al. Early results of bone marrow cell transplantation to the myocardium of patients with heart failure due to Chagas disease. Arq Bras Cardiol. 2006;87:159–166. doi: 10.1590/s0066-782x2006001500014. [DOI] [PubMed] [Google Scholar]
- 94.Cantarovich F, Vasquez M, Duro-Garcia, et al. Special infections in organ transplantation in South America. Transplant Proc. 1992;24:1902–1908. [PubMed] [Google Scholar]
- 95.Vaidian AK, Weiss LM, Tanowitz HB. Chagas’ disease and AIDS. Kinetoplastid Biol Dis. 2004;13;3(1):2. doi: 10.1186/1475-9292-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kohl S, Pickering LK, Frankel LS, Yaeger R. Reactivation of Chagas’ disease during therapy of acute lymphocytic leukemia. Cancer. 1992;50:827–828. doi: 10.1002/1097-0142(19820901)50:5<827::aid-cncr2820500503>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 97.Rezende R, Lescano MA, Ramalho L, et al. Reactivation of Chagas’ disease in a patient with non-Hodgkins lymphoma; gastric, oesophageal and laryngeal involvement. Trans Roy Soc Trop Med Hyg. 2006;100:74–78. doi: 10.1016/j.trstmh.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 98.Del Castillo M, Mendoza M, Oviedo J, et al. AIDS and Chagas’ disease with central nervous system tumor-like lesion. Am J Med. 1990;88:693–694. doi: 10.1016/0002-9343(90)90544-n. [DOI] [PubMed] [Google Scholar]
- 99.Sartori AM, Ibrahim KY, Nunes Westphalen EV, et al. Manifestations of Chagas disease (American trypanosomiasis) in patients with HIV/AIDS. Ann Trop Med Parasitol. 2007;101:31–50. doi: 10.1179/136485907X154629. [DOI] [PubMed] [Google Scholar]
- 100.Sartori AM, Caiaffa-Filho HH, Bezerra RC, et al. Exacerbation of HIV viral load with asymptomatic reactivation of chronic Chagas disease. Am JTrop Med Hyg. 2002;67:521–523. doi: 10.4269/ajtmh.2002.67.521. [DOI] [PubMed] [Google Scholar]
- 101.Sartori AM, Lopes MH, Benvenuti LA, et al. Reactivation of Chagas’ disease in a human immunodeficiency virus-infected patient leading to severe heart disease with a late positive direct microscopic examination of the blood. Am J Trop MedHyg. 1998;59:784–786. doi: 10.4269/ajtmh.1998.59.784. [DOI] [PubMed] [Google Scholar]
- 102.Lages-Silva E, Ramirez LE, Silva-Vergara M, Chiari E. Chagasic meningoencephalitis in a patient with acquired immunodeficiency syndrome, diagnosis, follow-up, and genetic characterization of Trypanosoma cruzi. ClinInf Dis. 2002;34:118–23. doi: 10.1086/324355. [DOI] [PubMed] [Google Scholar]
- 103.Rocha A, de Meneses AC, da Silva AM. Pathology of patients with Chagas’ disease and acquired immunodeficiency syndrome. Am J Trop Med Hyg. 1994;50:261–268. doi: 10.4269/ajtmh.1994.50.261. [DOI] [PubMed] [Google Scholar]
- 104.Cordova E, Boschi A, Ambrosioni J, et al. Reactivation of Chagas disease with central nervous system involvement in HIV-infected patients in Argentina, 1992–2007. Int J Infect Dis. 2008;12:587–592. doi: 10.1016/j.ijid.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 105.Fontanella G, De Vusser K, Laroy W, et al. Immunization with an engineered mutant trans-sialidase highly protects mice from experimental Trypanosoma cruzi infection. A vaccine candidate. 2008;26:2322–2334. doi: 10.1016/j.vaccine.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 106.Zacks MA, Garg N. Recent developments in the molecular, biochemical and functional characterization of GPI8 and the GPI-anchoring mechanism. Mol Membr Biol. 2006;23:209–225. doi: 10.1080/09687860600601494. [DOI] [PubMed] [Google Scholar]
- 107.Bhatia V, Garg NJ. Previously unrecognized vaccine candidates control Trypanosoma cruzi infection and immunopathology in mice. Clin Vaccine Immunol. 2008;15:1158–1164. doi: 10.1128/CVI.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]