Abstract
Microglia are found throughout the central nervous system, respond rapidly to pathology and are involved in several components of the neuroinflammatory response. Iba1 is a marker for microglial cells and previous immunocytochemical studies have utilized this and other microglial-specific antibodies to demonstrate the morphological features of microglial cells at the light microscopic level. However, there is a paucity of studies that have used microglial-specific antibodies to describe the ultrastructural features of microglial cells and their processes. The goal of the present study is to use Iba1 immuno-electron microscopy to elucidate the fine structural features of microglial cells and their processes in the hilar region of the dentate gyrus of adult Sprague-Dawley rats. Iba1-labeled cell bodies were observed adjacent to neurons and capillaries, as well as dispersed in the neuropil. The nuclei of these cells had dense heterochromatin next to the nuclear envelope and lighter chromatin in their center. Iba1-immunolabeling was found within the thin shell of perikaryal cytoplasm that contained the usual organelles, including mitochondria, cisternae of endoplasmic reticulum and Golgi complex. Iba1-labeled cell bodies also commonly displayed an inclusion body. Iba1-labeled cell bodies gave rise to processes that often had a small side branch arise within 5 μm of the microglial cell body. These data showing “resting” Iba-1 labeled microglial cells in the normal adult rat dentate gyrus provide a basis for comparison with the morphology of microglial cells in disease and injury models where they are activated or phagocytotic.
Keywords: Hippocampus, hilus, peri-neuronal, peri-capillary, microglial processes
1. Introduction
Microglia are the smallest of the glial cell types in the central nervous system. Several studies have shown that microglial cell bodies are only 2–5 μm in diameter and that their processes are relatively short compared to those of astrocytes (Peters et al., 1991). In the central nervous system microglia respond rapidly to pathology and are involved in several components of the neuroinflammatory response. These include antigen recognition and presentation, as well as cytotoxic and phagocytotic responses (Gehrmann et al., 1995). The microglial cells are also closely associated with neurons and astrocytes in neurodegeneration and regeneration (Aarum et al., 2003, Borges et al., 2003, Ziv et al., 2006).
An interesting feature of these cells is that their diverse functions can be observed and defined using morphological criteria. For example, microglial cells in the normal brain exist in a quiescent state, where they have a round cell body and thin processes with simple ramifications that constantly monitor the physiological environment (Nimmerjahn et al., 2005). Following CNS insult such as traumatic brain injury, ischemia or seizures (Wiessner et al., 1993; Fujita et al., 1998; Shapiro et al., 2008), microglial cells rapidly proliferate to increase their numbers (Niquet et al., 1994; Huttmann et al., 2003) and undergo morphological alterations (Gehrmann, 1995; Davalos et al., 2005). In their initial state of activation, the cell bodies of the microglial cells enlarge and their processes retract and become thickened (Kreutzberg, 1996). As they progress onto their pleomorphic and phagocytic forms, they typically take on an amoeboid shape (Thomas, 1992). This shape most likely reflects the cells’ active movement while in the process of clearing necrotic areas (Nakajima and Kohsaka, 1993). If the necrosis is incomplete, or the neuroinflammation is chronic, their cell bodies will remain elongated or rod-like. However, if phagocytosis is complete, the microglial cells appear as compound granular corpuscles, also known as Gitter cells (Das, 1976). Thus, it is essential to define the complex morphological features of these cells to understand how they react to various types of neuropathology.
Ionized calcium binding adaptor molecule 1 (Iba1) is a marker for microglial cells and previous immunocytochemical studies have utilized Iba1-specific antibodies to demonstrate the morphological features of microglial cells at the light and confocal microscopic levels (Ito et al., 1998; Okere and Kaba, 2000; Hirayama et al., 2001; Shapiro et al. 2008). In contrast with other microglial markers like CR3 complement receptor that is only present in ramified microglia or ED2 that is exclusive of perivascular microglia or MUC 101 and MUC 102 that are present, respectively, in white or gray matter microglia, Iba1 is expressed by all these microglial cell subpopulations (Ito et al., 1998). However, there is a paucity of studies that have described the ultrastructural features of microglial cells and their processes using this unique microglial marker (Sasaki et al., 2008). Therefore, the goal of the present study is to elucidate the fine structural features of microglial cells and their processes. This analysis was performed in the hippocampal dentate gyrus of adult Sprague-Dawley rats to show their normal distribution and their association with capillaries, neurons and astrocytes.
2. Results
2.1. Light and confocal microscopy
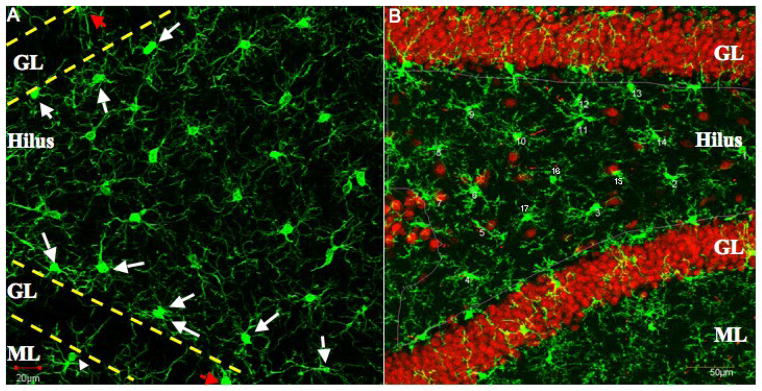
Iba1-immunolabeled cells were examined at the light and confocal microscopic level to determine their distribution in the rat dentate gyrus. Consistent with previous reports of the distribution of microglial cells using other markers (Dalmau et al., 1998; Moga et al., 2005), Iba1-labeled microglial cells were found throughout the hilus, at the border of the hilus and granule cell layer and in the molecular layer (Fig. 1A–B). Iba1-labeled cells in the granule cell layer were relatively infrequent. The spacing between the Iba1-labeled cell bodies in the hilus was relatively constant, and the quantitative data obtained for numbers of Iba1-labeled cell bodies per 10,000 μm2 of hilar area were consistent with that observation (2.05 per unit area; N=250 cells). The processes arising from these Iba1-labeled cell bodies appeared to be uniformly distributed in the hilus, such that each microglial cell with its processes had its own domain that rarely overlapped with adjacent microglial cells (Fig. 1). A concentration of Iba1-labeled cells was found at the border between the hilus and the granule cell layer, i.e., the subgranular zone (Fig. 1). The cells at this location had a more flattened cell body as compared to those in the deep hilus which had round cell bodies. The molecular layer had a relatively even distribution of Iba1 labeled microglial cells (Fig. 1). However, some of them were concentrated at the granule cell layer border with the molecular layer and these cells had processes extending into the granule cell layer (Fig. 1). The percentage of Iba1-labeled cells with cell bodies apposed to a NeuN-labeled neuronal soma was 31% (N = 82 cells; range, 25–40%), which is approximately 1 of 3 Iba1-labeled cells in the hilus.
Fig. 1.
Confocal Z-stack merged images depicting Iba1-immunolabeled cells and Neu-N labeled neurons found in the dentate gyrus. A depicts Iba1-immunolabeled cells located at the border of the hilus and granule cell layer (GL), in the molecular layer (ML) and in the hilus. Note the relatively even spacing and uniform distribution between the Iba1-immunolabeled cell bodies in the hilus. Many Iba1-labeled cells are concentrated at the border between the hilus and GL, i.e., the subgranular zone (arrows). Other cells with processes extending into the granule cell layer have their cell bodies (arrowhead) in the ML. Iba1-labeled cells in the GL (red arrows) are relatively infrequent. B shows both Iba1-immunolabeled cells and Neu-N labeled neuronal somata located within the dentate gyrus. The hilar region was traced (red line) in order to determine the frequency of Iba1-immunolabeled cell bodies apposed to Neu-N neuronal somata, and examples of this apposition are indicated (white arrows). Scale bar = 20 μm for A and B.
2.2. Electron microscopy
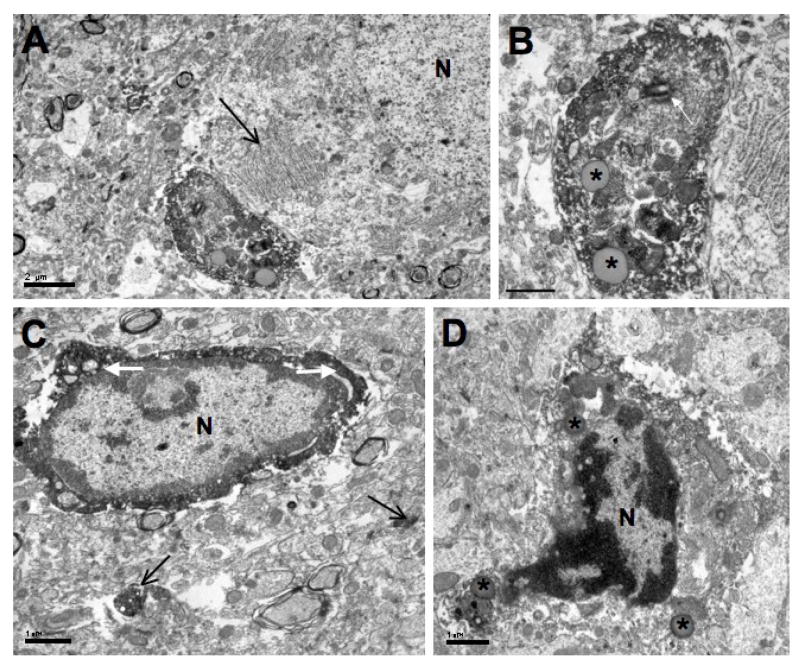
Ultrastructurally, Iba1-immunolabeled cell bodies in the dentate gyrus were identified by having electron-dense, immunoreaction product within their perikaryal cytoplasm (Figs. 2 – 6). This distribution of immunoreaction product was consistent with that observed in confocal image stacks used to form a movie (see Supplemental Material ). The Iba1-labeled microglial cell bodies generally had elongated nuclei but some nuclei had either round or triangular shaped nuclei (Fig. 2). Labeled cell bodies had nuclei with electron dense heterochromatin adjacent to their nuclear envelopes while the center of these nuclei had lighter chromatin. The extent of electron dense heterochromatin varied because patches could also be found in the central portion of the nuclei. Despite the dense immunoreaction product in the perikaryal cytoplasm of these cells, organelles were identified, and they included mitochondria, cisternae of endoplasmic reticulum, Golgi complexes, and inclusion bodies which appeared to be either lysosomes or lipid globules. The Iba1-labeled cell bodies that were fusiform generally had a length of about 6 μm and a width of about 3–4 μm.
Fig. 2.
Electron micrographs of microglial cell bodies found in the hilus of the dentate gyrus. A shows a peri-neuronal Iba1-labeled cell body, with the Nissl body (large arrow) of a nearby neuron (N) located close to this microglial cell’s plasma membrane. B is an enlargement of the microglial cell body. Note the inclusion bodies (asterisks) and a prominent centrosome (white arrow) within its perikaryon. In C, a dark band of Iba1 immunoreaction product (white arrows) is observed surrounding the cell nucleus (N) of another microglial cell. Most of the dense heterochromatin within the nucleus is found near the nuclear envelope. Two Iba1-labeled profiles (black arrows) appear in the neuropil. D is an Iba1-labeled cell body with a boot-shaped nucleus (N), a triangular-shaped cell body, and multiple inclusion bodies (asterisks). Scale bars = 2 μm for A and 1 μm for B–D.
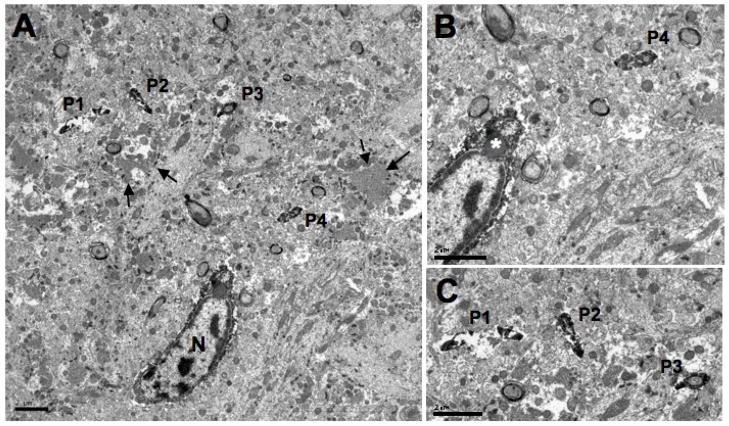
Fig. 6.
Electron micrographs of an Iba1-labeled microglial cell with Iba1-labeled processes in the hilus of the dentate gyrus. A shows an Iba1-labeled cell body with four cross-sectioned Iba1-labeled processes found approximately 4.6 μm (P4) and 11 μm (P1-P3) away from the cell’s nucleus (N). Mossy fibers (arrows) can also be seen scattered throughout the neuropil. B is an enlargement of the Iba1-labeled cell found in A and shows an inclusion body (asterisk) located its perikaryon. One of the Iba1-labeled processes (P4) is also shown. C is an enlargement of the three cross-sectioned Iba1-labeled processes (P1–P3). Note the arrangement of the cross-sectioned processes, demonstrating what appears to be a central process (P2) with two side branches (P1, P3). Scale bars = 2 μm for A–C.
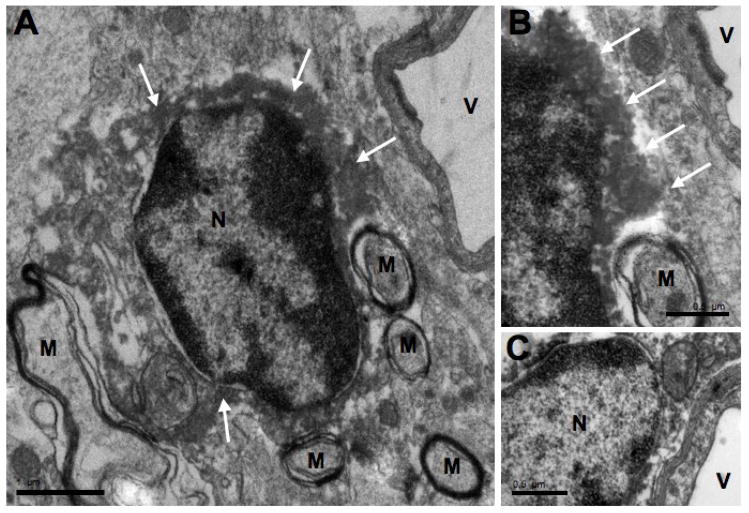
Iba1-labeled cell bodies were observed adjacent to neurons (consistent with the light microscopic data) and capillaries as well as dispersed in the neuropil. Peri-neuronal Iba1-labeled cell bodies were located adjacent to large somata in the hilus (Fig. 2A and B) and to granule cells in the granule cell layer (not shown). The Iba1-labeled cell bodies either apposed these neuronal somata and/or were separated from these somata by a thin astrocytic process. No axon terminals were found between the cell bodies of these peri-neuronal Iba1-labeled microglial cells and the cell bodies of neurons they apposed (Fig. 3). However, the neuronal cell bodies displayed remnants of postsynaptic densities at these sites of apposition (Fig. 3). The peri-capillary Iba1-labeled cell bodies were located within 1.0 μm from the basal lamina of capillary walls (Fig. 4). The perikaryal cytoplasmic rim on the side apposed to the capillary was thinner than the rim on the other side of the Iba1-labeled cell body (Fig. 4A). Peri-capillary Iba1-labeled cell bodies were sometimes separated from the basal lamina of capillary walls by only a thin process of an astrocyte (Fig. 4C). The Iba1-labeled cell bodies in the neuropil were often apposed by thin processes of astrocytes. They had shapes that were round, fusiform, cuboidal or triangular (Figs. 2C, 2D, 3A, 5A and 6A). Both myelinated axons (Fig. 5) and mossy fibers (Fig. 6) were found in the neuropil adjacent to Iba-1 labeled microglial cell bodies.
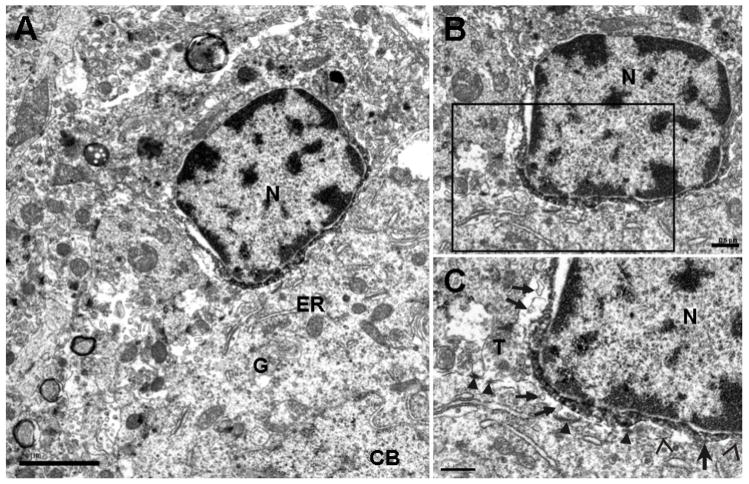
Fig. 3.
Electron micrographs of a peri-neuronal Iba1-labeled microglial cell found in the hilus of the dentate gyrus. A shows the nucleus (N) of this Iba1-labeled microglial cell that apposes a neuronal cell body (CB). Note that the immuno-reaction product is limited to the perikaryal cytoplasm of the microglial cell and its electron density is increased at the site of apposition to the neuronal cell body. Some of the cellular organelles observed within the neuronal cell body include the Golgi apparatus (G) and granular endoplasmic reticulum (ER). B is an enlargement of the same Iba1-labeled microglial cell and neuronal cell body found in A. Note the typical chromatin pattern for the nucleus (N) of this microglial cell with heterochromatin mainly concentrated adjacent to the nuclear envelope. C is an enlargement of the box in B. Thin astrocytic processes (open arrows) separate small portions of the apposition between the microglial cell and the neuronal cell body while a direct apposition is found at just one site (large arrow). Several postsynaptic densities (arrowheads) are found in the neuronal cell body at this apposition while others are involved in a synapse with an axon terminal (T). Several exosomes (small arrows) are located in the extracellular space surrounding the Iba1-labeled microglial cell body. Scale bars = 2 μm for A and 0.5 μm for B and C.
Fig. 4.
Electron micrographs of peri-capillary Iba1-labeled microglial cell bodies found in the hilus. A shows an Iba1-labeled microglial cell body near a blood vessel (V) with its characteristic nucleus (N) and immunoreaction product (white arrows) in its perikaryal cytoplasm. Note the myelinated axons (M) in the adjacent neuropil. B is an enlargement of an adjacent serial section showing the peri-capillary cell found in A. One of the myelinated axons (M) is shown as well as the immunoreaction product (white arrows). The microglial cell’s plasma membrane and the basal lamina of the endothelial cell lining this capillary (V) are only separated by about 0.5 μm. C demonstrates another example of a peri-capillary, Iba1-labeled cell body with the typical morphology of its nucleus (N). Also, this cell’s plasma membrane and the basal lamina of the endothelial cell are separated by only 0.16 μm. Scale bars = 1 μm for A and 0.5 μm for B and C.
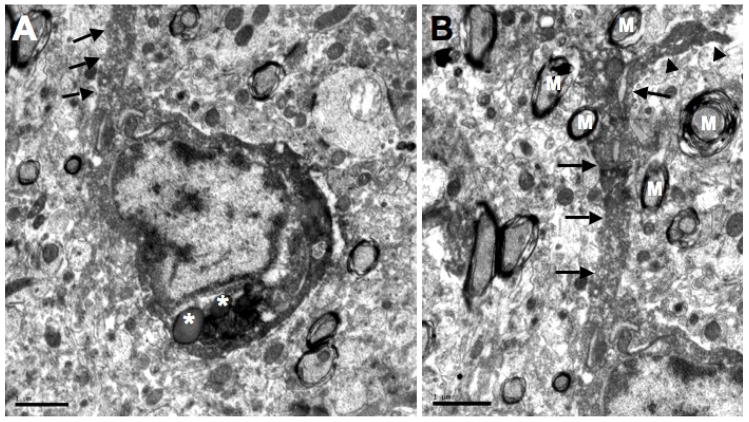
Fig. 5.
Electron micrographs of an Iba1-labeled microglial cell body with a labeled process found in the adjacent neuropil. A shows the labeled cell body and a few inclusion bodies (asterisks) found within its cytoplasm. The proximal part of its process (arrows) is shown in A while panel B shows a more distal portion of this process. Note that this Iba1-labeled process (arrows) extends into the neuropil where several myelinated axons (M) are found. A pedunculated side branch (arrowheads) arises from this labeled process. Scale bars = 1 μm for A and B.
Processes were observed to arise from Iba1-labeled cell bodies and these often had a small side branch arise within 5 μm of their cell body (Fig. 5). Often, these processes were separated from their cell body of origin by neuropil in any given thin section (Figs. 2C and 6A). However, they could be traced back to the parent cell body in adjacent serial sections. Iba1-labeled processes in the hilus were apposed to mossy fibers that had atypical morphology with clear zones in their centers (Fig. 6C).
Another interesting observation for these Iba1-labeled cells was the unusual space observed around the cell bodies. Thus, a gap in the adjacent neuropil appeared around the Iba1-labeled cell bodies (Fig. 2D) and processes (Fig. 6C). This clear area commonly displayed vesicles that were larger than synaptic vesicles and are referred to as exosomes. The common frequency of this clear zone suggested that it was not an artifact of fixation but could have been caused by the removal of reaction product from the tissue block during thin sectioning.
3. Discussion
Microglial cells are the macrophages in the brain and play a diverse role in the growth, maintenance and degeneration of the central nervous system. Del Rio Hortega (1932) was the first to perform detailed analyses of these cells using a modified silver staining technique. Since then, numerous studies have used light, confocal, and electron microscopic techniques to elucidate the morphological features of microglial cells. However, detailed ultrastructural analysis of their processes is lacking. Here, Iba1 immunocytochemistry was combined with electron microscopy to elucidate the fine structural morphology of the cell bodies and processes of microglial cells in the hilus of the dentate gyrus of normal adult rats. The data show that these cells have an affinity for lying adjacent to cell bodies or capillaries. It is unknown if the microglial cells at these two sites have functional differences from those found in the neuropil, where they presumably provide surveillance of the neuropil for changes that may require activation (Kreutzberg, 1996; Nimmerjahn et al., 2005). It is thought that the microglial cell processes are involved with this surveillance function, although the precise manner in which they do this has not yet been fully elaborated (Nimmerjahn, 2005).
The functional relationship between the processes of the microglial cells and neurons is not well-understood. It has been suggested that microglial cells secrete growth factors (Batchelor, 1999). It is possible that peri-neuronal processes might be monitoring the “health” of the neuron, such that it can release specific quantities of needed growth factors on demand. It is also possible that in the event that the neuron becomes denervated, or in other ways sick beyond repair, that the microglial cell is rapidly signaled to eliminate the ailing neuron before it can infect neighboring cells. In situations such as these, it has also been hypothesized that the microglial cells, true to their macrophage origin, become antigen presenting cells (Ford et al., 1995). In this case, other macrophages in the brain can be primed to detect and respond rapidly to dysfunctional or dystrophic neurons (Biber et al., 2007).
The peri-neuronal Iba1-labeled cell bodies in the present study were apposed to the surface of a neuronal cell body and there were no axon terminals forming axo-somatic synapses. This observation suggests one of two possible scenarios. The first is that the microglial cells displace or remove some of the axo-somatic synapses to gain direct juxtapositioning to the neuronal cell bodies. This possibility is supported by the fact that most neuronal cell bodies in the hilus of the dentate gyrus have a random distribution of axo-somatic synapses along their surface (Ribak and Shapiro, 2007) and these synapses are missing or denuded at the site of microglial/neuronal juxtaposition. The presence of remnants of postsynaptic densities in the neuronal cell body at these sites of juxtaposition is another indication of this suggested removal or displacement of axo-somatic terminals by microglial cells (Kelley et al., 2007). A second possibility is that the denervation precedes the direct microglial/neuronal apposition and this relationship occurs only after the axo-somatic terminals have been lost. Future studies will be needed to determine if the microglial cells are actively involved in the removal of the axo-somatic synapses, or, if denervation of these synapses precedes the direct neuronal/microglial cell apposition.
Similar to the peri-neuronal microglial cells, the peri-capillary microglial cells might also be monitoring the environment proximal to the blood brain barrier. It is well known that following many types of brain insult, the blood brain barrier is breached and rapid microglial activation occurs (Gehrmann, 1995; Davalos et al., 2005). It has also been demonstrated that peripheral macrophages infiltrate the central nervous system and this infiltration has been postulated to be increased following brain insult (Persson, 1976; Giulian et al., 1989; Chen et al., 2003). It is unclear how these macrophages may or may not differ morphologically or functionally from the endogenous population of microglial cells and if they respond differently than the resident microglial cells. Future studies are needed to further elucidate the functional significance of this ubiquitous cell type in the brain.
4. Experimental procedures
4.1. Animals
Adult (150–250g) male Sprague-Dawley rats (N = 24) were used for this study. All protocols and experiments were carried out in accordance with the Institutional Animal Care and Use Committee at the University of California, Irvine. The rats were deeply anesthetized with euthasol (1ml/kg) and transcardial perfusions were carried out using 250 ml of 0.9% sterile saline, followed by 4.0% paraformaldehyde in 0.1M phosphate buffer. The brains were allowed to post-fix in the skull for 24 hr, after which they were dissected out and placed in 4.0% paraformaldehyde for 24 hr. The brains were then transferred to phosphate-buffered saline (PBS) and 50 μm sections were cut using a vibratome.
4.2. Iba1 and NeuN immunocytochemistry for confocal microscopy
Sections used for immunocytochemistry were incubated in 0.5% H2O2 for 30 min, followed by 60 min in 1% H2O2, and then again for 30 min in 0.5% H2O2. Sections were then rinsed with PBS and incubated free-floating in Iba1 or Iba1 and NeuN antibody (Iba11:1000, Wako, Osaka, Japan; NeuN 1:500, Chemicon), with 3% normal goat serum, 0.05% Triton-X in PBS, for 24–48 hrs rotating at 4°C. The tissue was then rinsed 3 times for 5 min in PBS and incubated for 1 hr in fluorescent-labeled anti-rabbit IgG (Iba1, 1:200, Alexa Fluor 488, Milipore Inc., CA, USA,) and fluorescent-labeled anti-mouse IgG (NeuN 1:200, Alexa Fluor 567, Milipore Inc., CA, USA), rotating free-floating at RT.
4.3. Iba1-immuno-electron microscopy
Sections used for immunocytochemistry were incubated in 0.5% H2O2 for 30 min, followed by 60 min in 1% H2O2, and then again for 30 min in 0.5% H2O2. Sections were then rinsed with PBS and incubated free-floating in Iba1 antibody (1:1000, Wako, Osaka, Japan), with 3% normal goat serum, 0.05% Triton-X in PBS, for 24 hrs rotating at room temperature (RT). The tissue was then rinsed 3 times for 5 min in PBS and incubated for 1 hr in biotinylated anti-rabbit IgG (1:200, Vector Labs, Burlingame, CA, USA), rotating at RT. The tissue was then rinsed in PBS 3 times for 5 min each rinse, and incubated for 1 hr in ABC (Vector Labs) solution, rotating at RT. Following incubation, sections were rinsed with PBS for 20 min and were developed by incubating in 0.025% diamino-benzidine (DAB) and 0.002% hydrogen peroxide, in PBS. The DAB reaction was halted using PBS, followed by three 10 min PBS rinses.
Sections from the hippocampus were processed for electron microscopy using a previously described method (Shapiro & Ribak, 2006). Briefly, hippocampal sections were post-fixed in 1% glutaraldehyde for 1 hr, then rinsed in PBS and placed in 1% osmium tetroxide for 20–60 min, and dehydrated by ethanol and propylene oxide immersion. A flat-embedding procedure was used after which each tissue block was trimmed using a single-edged razor blade under a dissecting microscope. A short series of ultrathin (60–80 nm) sections containing the dentate gyrus from each block was cut with an ultramicrotome (UltraCut E, Reichert-Jung, Wetzlar, Germany), and sequential sections were collected on mesh and formvar-coated slot grids. The sections were stained with uranyl acetate and lead citrate to enhance contrast. Sections containing the granule cell layer and hilus were examined with a Philips CM-10 transmission electron microscope. Images of Iba1-immunolabeled microglial cells were captured with a Gatan Ultrascan digital camera.
4.4. Quantification of Iba1-labeled cells apposed to hilar neurons
To determine the percentage of Iba1-labeled cells that apposed hilar neurons, the hilus was traced (Fig. 1B) to exclude the area directly adjacent to the granule cell layer (the subgranular zone). The area of the region traced was calculated and the total number of Iba1-labeled cells that fell within this area was counted in four animals. In addition, the number of Iba1-labeled cells that had their cell body within 4 μm of the soma of a NeuN-labeled neuron was determined. These data are reported as a percentage of the total number of Iba1-labeled cells in the hilus.
Acknowledgments
The authors would like to recognize Dr. Zhiyin Shan for technical expertise and Dr. Christian Steinhauser for valuable discussions of our images of Iba1-labeled microglial cells. We also acknowledge support from NIH grant R01-NS38331 (to CER).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JYF, Porritt MJ, Frerichs F, Donnan JA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Chen S, Pickard JD, Harris NG. Time course of cellular pathology after controlled cortical impact injury. Exp Neurol. 2003;182:87–102. doi: 10.1016/s0014-4886(03)00002-5. [DOI] [PubMed] [Google Scholar]
- Dalmau I, Finsen B, Zimmer J, Gonzalez B, Castellano B. Development of microglia in the postnatal rat hippocampus. Hippocampus. 1998;8:458–474. doi: 10.1002/(SICI)1098-1063(1998)8:5<458::AID-HIPO6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Das GD. Gitter cells and their relationship to macrophages in the developing cerebellum: an electron microscopic study. Virchows Arch B Cell Pathol. 1976;20:299–305. doi: 10.1007/BF02890348. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Del Rio Hortega P. In: Cytology and cellular pathology of the nervous system. 2. Penfield W, editor. New York: Hoeber; 1932. pp. 483–534. [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- Fujita T, Yoshimine T, Maruno M, Hayakawa T. Cellular dynamics of macrophages and microglial cells in reaction to stab wounds in rat cerebral cortex. Acta Neurochir (Wien) 1998;140:275–279. doi: 10.1007/s007010050095. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Reviews. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Giulian D, Chen J, Ingeman JE, George JK, Noponen M. The role of mononuclear phagocytes in wound healing after traumatic injury to adult mammalian brain. J Neurosci. 1989;9:4416–4429. doi: 10.1523/JNEUROSCI.09-12-04416.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama A, Okoshi Y, Hachiya Y, Ozawa Y, Ito M, Kida Y, Imai Y, Kohsaka S, Takashima S. Early immunohistochemical detection of axonal damage and glial activation in extremely immature brains with periventricular leukomalacia. Clin Neuropathol. 2001;20:87–91. [PubMed] [Google Scholar]
- Huttmann K, Sadgrove M, Wallraff A, Hinterkeuser S, Kirchhoff F, Steinhauser C, Gray WP. Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur J Neurosci. 2003;18:2769–2778. doi: 10.1111/j.1460-9568.2003.03002.x. [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Kelley BJ, Lifshitz J, Povlishock JT. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J Neuropathol Exp Neurol. 2007;66:989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Moga M, Dempah D, Zhou D. Annexin 7-immunoreactive microglia in the hippocampus of control and adrenalectomized rats. Neurosci Lett. 2005;380:42–47. doi: 10.1016/j.neulet.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Kohsaka S. Functional roles of microglia in the brain. Neurosci Res. 1993;17:187–203. doi: 10.1016/0168-0102(93)90047-t. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Niquet J, Ben-Ari Y, Repressa A. Glial reaction after seizure induced hippocampal lesion: immunohistochemical characterization of proliferating glial cells. J Neurocytol. 1994;23:641–656. doi: 10.1007/BF01191558. [DOI] [PubMed] [Google Scholar]
- Okere CO, Kaba H. Heterogenous immunohistochemical expression of microglia-specific ionized calcium binding adaptor protein (Iba1) in the mouse olfactory bulb. Brain Res. 2000;877:85–90. doi: 10.1016/s0006-8993(00)02656-1. [DOI] [PubMed] [Google Scholar]
- Persson L. Cellular reactions to small cerebral stab wounds in the rat frontal lobe: An ultrastructural study. Virchows Arch B Cell Pathol. 1976;22:21–37. doi: 10.1007/BF02889204. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H, de F. The fine structure of the nervous system: Neurons and their supporting cells. 3. Oxford University Press; New York: 1991. [Google Scholar]
- Ribak CE, Shapiro LA. Ultrastructure and synaptic connectivity of cell types in the adult rat dentate gyrus. Prog Brain Res. 2007;163:155–166. doi: 10.1016/S0079-6123(07)63009-X. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Kawarabayashi T, Murakami T, Matsubara E, Ikeda M, Hagiwara H, Westaway D, George-Hyslop PS, Shoji M, Nakazato Y. Microglial activation in brain lesions with tau deposits: Comparison of human tauopathies and tau transgenic mice TgTauP301L. Brain Res. 2008;1214:159–168. doi: 10.1016/j.brainres.2008.02.084. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Ribak CE. Newly born dentate granule neurons after pilocarpine-induced epilepsy have hilar basal dendrites with immature synapses. Epilepsy Res. 2006;69:53–66. doi: 10.1016/j.eplepsyres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Figueroa-Aragon S, Ribak CE. Newly generated granule cells show rapid neuroplastic changes in the adult rat dentate gyrus during the first five days following pilocarpine-induced seizures. Eur J Neurosci. 2007;26:583–92. doi: 10.1111/j.1460-9568.2007.05662.x. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Wang L, Ribak CE. Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia Suppl. 2008;2:33–41. doi: 10.1111/j.1528-1167.2008.01491.x. [DOI] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: evaluation of microglia and their functions. Brain Res Rev. 1992;17:61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]
- Wiessner C, Gehrmann J, Lindholm D, Topper R, Kreutzberg GW, Hossmann KA. Expression of transforming growth factor-beta 1 and interleukin-1 beta mRNA in rat brain following transient forebrain ischemia. Acta Neuropathol. 1993;86:439–446. doi: 10.1007/BF00228578. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nature Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]