Abstract
In addition to those with savant skills, many individuals with autism spectrum conditions (ASCs) show superior perceptual and attentional skills relative to the general population. These superior skills and savant abilities raise important theoretical questions, including whether they develop as compensations for other underdeveloped cognitive mechanisms, and whether one skill is inversely related to another weakness via a common underlying neurocognitive mechanism. We discuss studies of perception and visual processing that show that this inverse hypothesis rarely holds true. Instead, they suggest that enhanced performance is not always accompanied by a complementary deficit and that there are undeniable difficulties in some aspects of perception that are not related to compensating strengths. Our discussion emphasizes the qualitative differences in perceptual processing revealed in these studies between individuals with and without ASCs. We argue that this research is important not only in furthering our understanding of the nature of the qualitative differences in perceptual processing in ASCs, but can also be used to highlight to society at large the exceptional skills and talent that individuals with ASCs are able to contribute in domains such as engineering, computing and mathematics that are highly valued in industry.
Keywords: enhanced perceptual processing, gestalt, grouping, intelligence
1. Theories of superior abilities
The phenomenal talents of some savant individuals with autism spectrum conditions (ASCs) have attracted enormous media attention, because many are in domains highly prized by Western societies, such as art and music, and others go beyond what most neurotypical individuals can achieve, such as calendrical calculation. In whatever domain the skills are displayed, society reacts with astonishment and delight towards performances and exhibitions of these talents. But for those researchers who assess perception and attention in individuals with ASCs in experimental studies, the exceptional performance demonstrated by the participants with ASCs compared with neurotypical control participants is no less thrilling. For example, some years ago, we reported a series of studies examining visual search in ASCs, in which we asked children to detect targets hidden among distractors as quickly and as accurately as possible (Plaisted et al. 1998a). Although we reported the graphical representations and statistical analyses that demonstrated the superior rapidity of visual search in children with ASCs compared with neurotypical children, we did not, in the context of those formal experimental papers, report our experience of astonishment and admiration while watching the children with ASCs complete the tasks with such remarkable skilful speed.
Other studies have shown superior abilities compared with neurotypicals in studies of block design and embedded figures (Shah & Frith 1983, 1993), memory for pitch (Heaton et al. 1998; Heaton 2003), attentional focus (Townsend & Courchesne 1994), local processing (Plaisted et al. 1999; Mottron et al. 2003) and discrimination (Plaisted et al. 1998b).
What psychological processes might underpin these exceptional skills? Could there be a single underlying process that can explain all the savant skills and the exceptional performance seen in some tests in psychological studies? Probably not. Yet, there is a surprising conceptual similarity between a class of theory that has been put forward to explain savant skills in art, music and calculation and those that have been proposed to explain the exceptional performance in perceptual and attentional tasks in experimental studies. Each of these theories, although different in specific detail, propose that these abilities result from low-level processing mechanisms that operate exceptionally well to compensate for deficits in higher level mechanisms. All broadly predict inverse relationships between performance on tasks that primarily marshal lower level processes and complementary tasks that heavily rely on higher level processes.
For example, in a prominent theory of savant skills, Snyder proposes that these exceptional skills result from privileged access to lower level processes responsible for supporting drawing, calculation and so on. This privileged access is a consequence of compromise to other brain areas responsible for conceptual holistic processing. This raises the astonishing possibility that even neurotypicals possess latent savant abilities, but that these are prevented from expression as a consequence of the masking of the lower level processes by the operation of higher order conceptual processes (Snyder et al. 2003). There are direct parallels between this theory of savant skills and those theories that have been debated in the literature concerning superior performance on tests of visual perception and attention. For example, the reduced generalization hypothesis proposes that individuals with ASC have a reduced perception of similarity, resulting in enhanced abilities to discriminate, on the one hand, and a reduced ability to categorize, on the other hand (Plaisted 2000, 2001). Similarly, Mottron and colleagues (e.g. Mottron & Burack 2001) have proposed a model of enhanced perceptual functioning, suggesting that the superior skills of individuals with autism arise as a consequence of overdeveloped perceptual functioning. According to the theory, this overdevelopment occurs as a consequence of underdevelopment of higher level cognitive processes (although in a later version of the theory, Mottron et al. (2006) emphasize a difference in the relation between lower and higher level processing in ASCs, the latter being optional for individuals with ASCs but mandatory for neurotypicals). Perhaps the best known, and certainly the seminal theory in this area of research, is the weak central coherence theory (Frith 1989). In its original form, this proposed that the exceptional part-based processing seen in performance on tasks such as block design and embedded figures results from deficits in integration processes that serve to draw information together as a meaningful whole.
2. Experimental studies of perceptual grouping
Experiments designed to ‘drill down’ to identify the mechanisms suggested by these inverse theories have provided little evidence to support them. Taking the weak central coherence hypothesis as an example, a range of experimental approaches to identifying deficits in integration processes leading to deficits in global-level processing have revealed intact integration instead. For example, several studies have used hierarchical stimuli (e.g. a large global letter constructed from small letters) to tap grouping processes, finding that individuals with ASCs process the global level with the same efficiency as neurotypical individuals (Mottron & Belleville 1993; Ozonoff et al. 1994). In the light of such findings, Happé and colleagues (Happé & Frith 2006; Happé & Booth 2008) have argued that the local superiority bias is independent of global processing operations in ASCs.
This is not to say, however, that grouping processes in individuals with ASCs are the same as those in neurotypicals. Instead, individuals with ASCs demonstrate a much more complex and subtle pattern of perceptual and cognitive processing. For example, when given a choice between processing the global or the local level, individuals with autism choose to prioritize the local level (Plaisted et al. 1999). Furthermore, in recent studies of gestalt grouping, it has been found that individuals with ASCs show selective grouping abilities and biases in comparison with neurotypicals. For example, Brosnan et al. (2004) found that children with ASCs tended not to process gestalt stimuli based on nature relationships (such as grouping white dots and black dots displayed in the same array in two separate groups). They concluded that individuals with ASCs may show selective deficits in grouping by principles such as similarity rather than grouping based on place relationships, such as is required when processing hierarchical stimuli.
However, a recent study using different methodologies complicates the picture of grouping processes in ASCs still further. We have recently suggested that the methodology employed by previous studies does not always allow for performance to be based on the initial perceptual representation and does not therefore adequately tap the nature of the gestalt experienced by individuals with ASCs (Falter 2007). For example, many studies employ tasks that require participants to identify or detect the presence of a stimulus at the global level, or draw the stimulus, all of which introduce a substantial delay between percept and action to exacerbate the distorting influence of cognitive, attentional and motoric factors on perception. The studies do not necessarily therefore provide the most sensitive and accurate reflection of the nature of the initial gestalt representation in individuals with ASCs.
Accordingly, we employed a procedure that has recently been successfully employed in neurotypical adults to measure grouping processes without explicitly asking observers to introspect on these processes (Feldman 2007). This procedure relies on the well-documented tendency of observers to pay attention to shapes that are grouped together, rather than shapes that are not grouped. Typically, when observers are asked to make a judgement concerning two ‘features’ (in our study, to say whether two oriented lines had the same or different orientations), they do so more rapidly and/or accurately when these features appear on two grouped objects than when they appear on two ungrouped objects (e.g. Duncan 1984). This effect of grouping can therefore be used to assess an observer's grouping processes, even though the observer does not need to report whether they saw grouping in the display, or not.
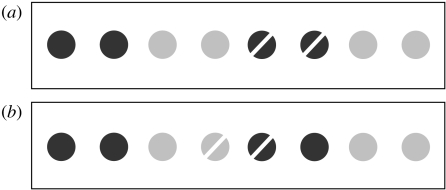
In our task (Falter et al. in preparation), we presented a row of circles, some coloured blue and others red. Adjacent circles could be of the same colour (such that we expected them to be perceptually grouped together owing to the established perceptual principle that neurotypicals ‘group by similarity’) or different colours (such that they should be less well grouped). We also varied how near dots were to each other (nearer dots should, we expected, be perceptually grouped together as neurotypicals have been shown to ‘group by proximity’). Pairs of oriented lines were then presented on adjacent circles in each trial and the observer's task was simply to determine whether these lines had the same orientation or different orientations. These could either appear on circles we expected to be grouped (figure 1a) or circles we expected not to be grouped (figure 1b and accompanying legend). Each array of circles could be horizontally oriented (as illustrated in figure 1) or vertically oriented (as was the case for the results provided in figure 2).
Figure 1.
Examples of stimuli used in grouping experiments. Note that circles in actual displays were red and blue (indicated by light and dark greys, respectively). The observer's task was to determine as quickly and accurately as possible whether the two bars were of the same or different orientations. Stimuli in (a), but not (b), are considered grouped by similarity for neurotypical observers.
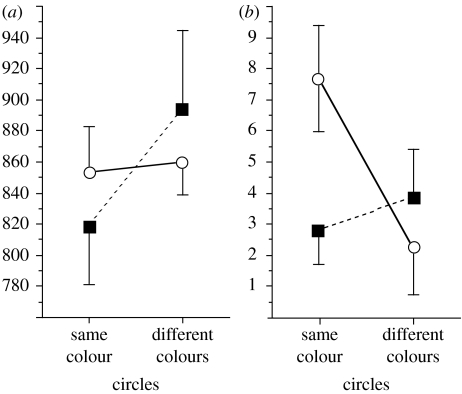
Figure 2.
(a) Reaction time and (b) accuracy data for the neurotypical children (filled squares) and children with ASCs (open circles).
We compared patterns of performance on these tasks in 46 children with ASCs and 46 neurotypical children. The children were matched for chronological age (mean 12.9 years, range 8–16) and general mental functioning as assessed by the Standard Progressive Raven's Matrices (Raven et al. 1998; mean raw score 43). We found that children with ASCs showed robust grouping by proximity (i.e. grouping together circles that were near to each other) at least to the same extent as neurotypicals.
However, the most interesting results arose in displays where circles of the same colour versus different colours were equidistant, such that only similarity, not proximity, was available as a cue for grouping. Here, as expected, neurotypical children exhibited more efficient responses in terms of faster reaction times (RTs; figure 2a) and the same trend in errors (figure 2b) when the two oriented lines appeared on circles of the same colour than when the lines appeared on circles of different colours, showing that these had been perceptually grouped together. However, the ASC children showed no such pattern in RTs (figure 2a) and robustly the opposite pattern in terms of their error rates (figure 2b), in that they more efficiently (accurately) compared the line segments' orientations when they appeared on circles of different colours than when they appeared on circles of the same colour.
This finding could not be explained by appealing to a possible tendency for ASD individuals to ignore colour information when grouping elements in a scene; such a tendency would have predicted no effect of the circles' colours on their performance. Nor, indeed, was there any bias among the errors made by ASC children that might suggest a role for response competition (‘Stroop’) effects in this result—they did not, for example, find it particularly difficult to say that the two lines' orientations were different when they appeared on circles of the same colour. Rather, it was clear that the ASC children had processed the circles' colours, and these had affected their grouping of the scene, but in a manner that was qualitatively different from grouping in neurotypicals. Our ASC children appeared to have grouped together circles of different colours rather than of the same colour.
This short review of studies of global-level processing in ASCs thus provides little evidence for the proposal that the enhanced local and part-based processing often observed in individuals with ASCs results from deficits in higher level global processing. Instead, a far more complex profile of perceptual processing emerges that cannot be captured by theoretical models that propose straightforward inverse relationships.
3. Difficulties in perception
A general assumption made by the inverse theories is that lower level processing is enhanced in ASCs, and thus responsible for their superior performance on many tasks compared with individuals without ASCs. However, a recent line of research has revealed that at least some perceptual processes are adversely affected in ASCs. Several studies have now shown that individuals with ASCs show higher thresholds for the perception of motion coherence (e.g. Spencer et al. 2000; Milne et al. 2002; Pellicano et al. 2005). Two proposals have been advanced for this relative insensitivity to motion coherence. One is that higher levels of the dorsal visual stream, typically responsible for the integration of motion signals, are adversely affected in ASCs. The other is that motion integration difficulties result from deficits in early perceptual processes that drive the dorsal visual stream, in particular the magnocellular pathway. Insofar as inverse theories predict deficits in higher level processes and enhanced processing in lower level systems, then they propose that the difficulties in motion coherence observed in ASCs result from abnormalities in areas higher in the dorsal visual stream, such as area MT/V5.
We are currently assessing this assumption in a series of studies examining visual dorsal stream processing in ASCs. The emerging evidence suggests that, far from there being deficits at higher levels of processing in the dorsal visual stream, the causal deficit originates even before vision information reaches the visual cortex, in low levels of perceptual processing by magnocells in the thalamus.
For example, in one of our first experiments, we presented participants with a task designed to target selectively the information processed by magnocells or parvocells (Greenaway 2005). Effective targeting of one or other of these two types of cell using behavioural psychophysical measures is notoriously difficult, owing to the often rather opaque relationship between individual cell responses and the visual system's overall response, the presence of a third broad class of cell in the LGN (‘koniocells’) and heterogeneity of response within each class of cell. Indeed, many previous studies of human observers have employed stimuli that should be preferred by individual magnocells, yet as Skottun (2000) has demonstrated, such studies have not effectively measured either magnocellular or parvocellular function.
An elegant procedure that comes closest to targeting magnocellular functions is that developed by Pokorny & Smith (1997). It relies on the presence of robust differences in response to ‘luminance contrast’ between magnocells and parvocells. Luminance contrast is a measure of the magnitude of differences in light coming from different parts of a stimulus. At low contrast levels (faint stimuli), magnocells respond much more robustly than parvocells, but this response soon reaches a maximum. Parvocells' responses to low contrast stimuli are poor, but continue to increase as the contrast of the stimulus is increased. These two response properties give rise to two patterns of findings. Magnocells are more sensitive than parvocells to single low contrast stimuli, whereas parvocells are more sensitive than parvocells to differences between higher contrast stimuli.
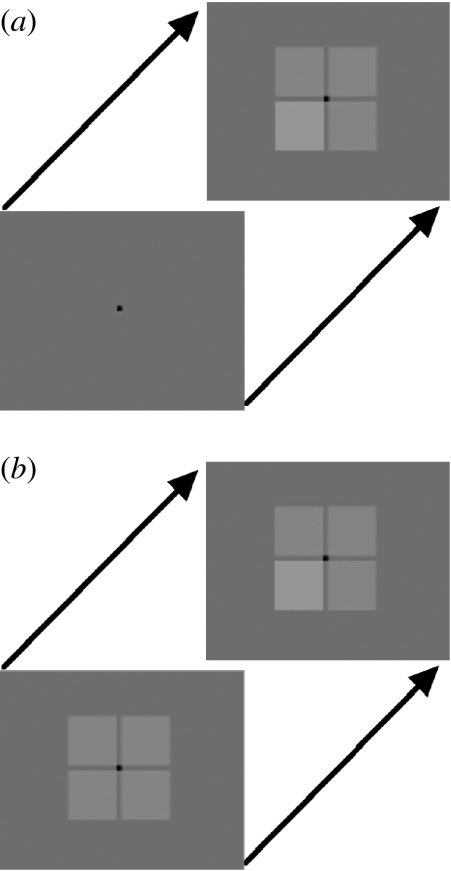
Pokorny & Smith's (1997) procedure exploits this pattern of responses. On each trial, a ‘pedestal’ of four squares is presented. After looking at the pedestal for a while, one of the squares becomes momentarily slightly darker or lighter (figure 3b). Because only one aspect of the display has changed, magnocells are very good at detecting even very faint changes in these stimuli (better than parvocells or indeed koniocells), so performance on this condition should be governed by how efficiently a person's magnocells are functioning. Accordingly, for our current purposes, we refer to this type of trial as the ‘magnocell condition’.
Figure 3.
Typical display sequences for the study of (a) magnocellular and (b) parvocellular function.
In a second type of trial, the pedestal of four squares, one of which is slightly lighter or darker than the others, is presented simultaneously on a grey background (figure 3a). The observer must detect which of the squares is slightly darker or brighter than the others. Now, because all stimuli are presented at once, the task is effectively to distinguish between different levels of light in the four squares (rather than simply to detect a single light change). Accordingly, parvocells should govern performance under these conditions rather than magnocells.
We compared 17 children with ASCs and 17 neurotypical children, matched for chronological age (mean 12 years, range 9–14) and general mental functioning (mean raw Raven's matrices score 40). Each child's threshold was measured using a two-down, one-up staircase procedure (i.e. two correct responses led to a decrease in luminance increment and one incorrect response led to an increase in luminance increment). The task continued until 10 reversals had been reached, and the threshold was calculated by taking the mean of the last eight reversals.
Independent t-tests revealed that while the thresholds of the groups did not differ on the parvocell condition (t(32)=1.1, p=0.281), they did differ on the magnocell condition (t(32)=3.7, p=0.001). Thus, in comparison with the typically developing children, the children with ASCs exhibited clear deficits in the magnocell condition but no deficit or benefit in the parvocell condition. This finding, on the face of it, seems similar to findings of magnocellular dysfunction in other developmental disorders, such as dyslexia (e.g. Cornelissen et al. 1995). However, a debate exists as to whether there are such deficits in other developmental disorders, because it is possible that the stimulus parameters chosen in previous studies may not be sensitive enough to adequately target magnocellular processing separately from parvocellular processing. We are currently extending this study examining magnocellular processing in ASCs using flicker stimuli that target magnocellular processing far more precisely than flicker stimuli used in previous studies (e.g. Pellicano & Gibson 2008; see Skottun (2000) and Plaisted & Davis (2005) for discussions of the importance of appropriate stimulus selection in assessments of magnocellular dysfunction in discriminating developmental disorders). Further comparative research, using the kinds of procedure used here, is now urgently required to establish the degree of similarity of perceptual abnormalities between developmental disorders (Braddick et al. 2003).
For our current purposes, however, this study demonstrates a perceptual difficulty that has no obvious benefit, and clearly does not compensate for lack of development of any higher order process in a straightforward inverse manner. Thus, although there are clear demonstrations of some superior processes that lead to highly skilled performance, there are other damaged processes that are deleterious to the individual and which require amelioration.
4. The need for encouragement, education and training
This short review of studies of perception in individuals with ASCs demonstrates quite clearly that the superior performance seen in ASCs cannot be explained by a model proposing enhanced perceptual processing and defective higher level processing. Instead, the pattern of results is complex: sometimes the perceptual processes are quite different, and cannot be classified as either superior or inferior compared with those used by neurotypicals. In the case of grouping studies, individuals with ASCs parse the visual scene in different ways, resulting in a bias towards grouping by proximity and away from grouping by similarity. In other cases, perception is atypical in ways that do not enhance perceptual performance, and individuals with ASCs perform poorly compared with neurotypicals, as studies in motion processing and magnocellular processing have revealed.
This review has important implications not only for research on the psychological, neurological and genetic bases of ASCs, but also for an understanding of the contribution that individuals with ASCs can make to business and industry. At the present time, savant skills in drawing, painting and music appear to be among those most highly prized by society. Yet, an understanding of the differences in perception in ASCs should lead to an appreciation that these differences result in high-level skills and expertise in areas such as computing, engineering and mathematics. Research such as that described here makes this important point: savant abilities are relatively rare, but the skills observed in individuals with ASCs in many studies are common among the population with ASCs. These skills need as much training and encouragement as is given to any individual with talent in detailed processing, mathematics, engineering, design and so on. With such dedicated training, society, business and industry will reap the great benefits of the unusual minds of individuals with ASCs.
Footnotes
One contribution of 18 to a Discussion Meeting Issue ‘Autism and talent’.
References
- Braddick O., Atkinson J., Wattam-Bell J. Normal and anomalous development of visual motion processing: motion coherence and ‘dorsal-stream vulnerability’. Neuropsychologia. 2003;41:1769–1784. doi: 10.1016/s0028-3932(03)00178-7. doi:10.1016/S0028-3932(03)00178-7 [DOI] [PubMed] [Google Scholar]
- Brosnan M.J., Scott F.J., Fox S., Pye J. Gestalt processing in autism: failure to process perceptual relationships and the implications for contextual understanding. J. Child Psychol. Psychiatry. 2004;45:459–469. doi: 10.1111/j.1469-7610.2004.00237.x. doi:10.1111/j.1469-7610.2004.00237.x [DOI] [PubMed] [Google Scholar]
- Cornelissen P., Richardson A., Mason A., Fowler S., Stein J. Contrast sensitivity and coherent motion detection measured at photopic luminance levels in dyslexics and controls. Vision Res. 1995;35:1483–1494. doi: 10.1016/0042-6989(95)98728-r. doi:10.1016/0042-6989(95)98728-R [DOI] [PubMed] [Google Scholar]
- Duncan J. Selective attention and the organization of visual information. J. Exp. Psychol. Gen. 1984;113:501–517. doi: 10.1037//0096-3445.113.4.501. doi:10.1037/0096-3445.113.4.501 [DOI] [PubMed] [Google Scholar]
- Falter, C. M. 2007 The influence of testosterone on cognition in typical development and autism spectrum disorders. PhD thesis, University of Cambridge, Cambridge, UK.
- Falter, C., Plaisted Grant, K. & Davis, G. In preparation. Object-based attention benefits reveal selective abnormalities of visual integration in autism. [DOI] [PubMed]
- Feldman J. Formation of visual objects in the early computation of spatial relations. Percept. Psychophys. 2007;69:816–827. doi: 10.3758/bf03193781. [DOI] [PubMed] [Google Scholar]
- Frith U. 2nd edn. 2003. Basil Blackwell; Oxford, UK: 1989. Autism: explaining the enigma. [Google Scholar]
- Greenaway, R. 2005 Factors underlying attentional abnormalities in autism. PhD thesis, University of Cambridge, Cambridge, UK.
- Happé F., Booth R. The power of the positive: revisiting weak coherence in autism spectrum disorders. Q. J. Exp. Psychol. 2008;61:50–63. doi: 10.1080/17470210701508731. doi:10.1080/17470210701508731 [DOI] [PubMed] [Google Scholar]
- Happé F., Frith U. The weak central coherence account: detail focused cognitive style in autism spectrum disorders. J. Autism Dev. Disord. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. doi:10.1007/s10803-005-0039-0 [DOI] [PubMed] [Google Scholar]
- Heaton P. Pitch memory, labeling and disembedding in autism. J. Child Psychol. Psychiatry. 2003;42:543–551. doi: 10.1111/1469-7610.00143. doi:10.1111/1469-7610.00143 [DOI] [PubMed] [Google Scholar]
- Heaton P., Hermelin B., Pring L. Autism and pitch processing: a precursor for savant musical ability. Music Percept. 1998;15:291–305. [Google Scholar]
- Milne E., Swettenham J., Hansen P., Campbell R., Jeffries H., Plaisted K. High motion coherence thresholds in children with autism. J. Child Psychol. Psychiatry Allied Discip. 2002;43:255–263. doi: 10.1111/1469-7610.00018. doi:10.1111/1469-7610.00018 [DOI] [PubMed] [Google Scholar]
- Mottron L., Belleville S. A study of perceptual analysis in a high-level autistic subject with exceptional graphic abilities. Brain Cogn. 1993;23:279–309. doi: 10.1006/brcg.1993.1060. doi:10.1006/brcg.1993.1060 [DOI] [PubMed] [Google Scholar]
- Mottron L., Burack J. Enhanced perceptual functioning in the development of autism. In: Burack J.A., Charman T., Yirmiya N., Zelazo P.R., editors. The development of autism: perspectives from theory and research. Lawrence Erlbaum Associates; Mahwah, NJ: 2001. [Google Scholar]
- Mottron L., Burack J.A., Iarocci G., Belleville S., Enns J.T. Locally oriented perception with intact global processing among adolescents with high-functioning autism: evidence from multiple paradigms. J. Child Psychol. Psychiatry Allied Discip. 2003;44:904–913. doi: 10.1111/1469-7610.00174. doi:10.1111/1469-7610.00174 [DOI] [PubMed] [Google Scholar]
- Mottron L., Dawson M., Soulières I., Hubert B., Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J. Autism Dev. Disord. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. doi:10.1007/s10803-005-0040-7 [DOI] [PubMed] [Google Scholar]
- Ozonoff S., Strayer D.L., McMahon W.M., Filloux F. Executive function abilities in autism and Tourette syndrome: an information processing approach. J. Child Psychol. Psychiatry Allied Discip. 1994;35:1015–1032. doi: 10.1111/j.1469-7610.1994.tb01807.x. doi:10.1111/j.1469-7610.1994.tb01807.x [DOI] [PubMed] [Google Scholar]
- Pellicano E., Gibson L. Investigating the functional integrity of the dorsal visual pathway in autism and dyslexia. Neuropsychologia. 2008;46:2593–2596. doi: 10.1016/j.neuropsychologia.2008.04.008. doi:10.1016/j.neuropsychologia.2008.04.008 [DOI] [PubMed] [Google Scholar]
- Pellicano E., Gibson L., Maybery M., Durkin K., Badcock D. Abnormal global processing along the dorsal visual pathway in autism: a possible mechanism for weak visuospatial coherence? Neuropsychologia. 2005;43:1044–1053. doi: 10.1016/j.neuropsychologia.2004.10.003. doi:10.1016/j.neuropsychologia.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Plaisted K.C. Aspects of autism that theory of mind cannot easily explain. In: Baron-Cohen S., Tager-Flusberg H., editors. Understanding other minds: perspectives from autism and cognitive neuroscience. 2nd edn. Oxford University Press; Oxford, UK: 2000. [Google Scholar]
- Plaisted K.C. Reduced generalisation in autism: an alternative to weak central coherence. In: Burack J.A., Charman A., Yirmiya N., Zelazo P.R., editors. Development and Autism: perspectives from theory and research. Lawrence Erlbaum Associates; Mahwah, NJ: 2001. [Google Scholar]
- Plaisted K.C., Davis G. Examining magnocellular processing in autism. Curr. Psychol. Cogn. 2005;23:172–180. [Google Scholar]
- Plaisted K.C., O'Riordan M.A.F., Baron-Cohen S. Enhanced visual search for a conjunctive target in autism: a research note. J. Child Psychol. Psychiatry. 1998;39:777–783. doi:10.1017/S0021963098002613 [PubMed] [Google Scholar]
- Plaisted K.C., O'Riordan M.A.F., Baron-Cohen S. Enhanced discrimination of novel highly similar stimuli by adults with autism during a perceptual learning task. J. Child Psychol. Psychiatry. 1998;39:765–775. doi:10.1017/S0021963098002601 [PubMed] [Google Scholar]
- Plaisted K.C., Swettenham J., Rees L. Children with autism show local precedence in a divided attention task and global precedence in a selective attention task. J. Child Psychol. Psychiatry. 1999;40:733–742. doi:10.1111/1469-7610.00489 [PubMed] [Google Scholar]
- Pokorny J., Smith V. Psychophysical signatures associated with magnocellular and parvocellular pathway contrast gain. J. Opt. Soc. Am. A. 1997;14:2477. doi: 10.1364/josaa.14.002477. doi:10.1364/JOSAA.14.002477 [DOI] [PubMed] [Google Scholar]
- Raven J., Raven J.C., Court J.H. Oxford Psychologists Press; Oxford, UK: 1998. Raven manual: section 3. Standard progressive matrices. [Google Scholar]
- Shah A., Frith U. An islet of ability in autistic children: a research note. J. Child Psychol. Psychiatry Allied Discip. 1983;24:613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. doi:10.1111/j.1469-7610.1983.tb00137.x [DOI] [PubMed] [Google Scholar]
- Shah A., Frith U. Why do autistic individuals show superior performance on the block design task? J. Child Psychol. Psychiatry Allied Discip. 1993;34:1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. doi:10.1111/j.1469-7610.1993.tb02095.x [DOI] [PubMed] [Google Scholar]
- Skottun B. The magnocellular deficit theory of dyslexia: the evidence from contrast sensitivity. Vision Res. 2000;40:111–127. doi: 10.1016/s0042-6989(99)00170-4. doi:10.1016/S0042-6989(99)00170-4 [DOI] [PubMed] [Google Scholar]
- Snyder A.W., Mulcahy E., Taylor J.L., Mitchell D.J., Sachdev P., Gandevia S.C. Savant-like skills exposed in normal people by suppressing the left fronto-temporal lobe. J. Integr. Neurosci. 2003;2:149–158. doi: 10.1142/s0219635203000287. doi:10.1142/S0219635203000287 [DOI] [PubMed] [Google Scholar]
- Spencer J., O'Brien J., Riggs K., Braddick O., Atkinson J., Wattam-Bell J. Motion processing in autism: evidence for a dorsal stream deficiency. Neuroreport. 2000;11:2765–2767. doi: 10.1097/00001756-200008210-00031. doi:10.1097/00001756-200008210-00031 [DOI] [PubMed] [Google Scholar]
- Townsend J., Courchesne E. Parietal damage and narrow ‘spotlight’ spatial attention. J. Cogn. Neurosci. 1994;6:220–232. doi: 10.1162/jocn.1994.6.3.220. doi:10.1162/jocn.1994.6.3.220 [DOI] [PubMed] [Google Scholar]