Abstract
Species-specific behaviours gradually emerge, via incomplete patterns, to the final complete adult form. A classical example is birdsong, a learned behaviour ideally suited for studying the neural and molecular substrates of vocal learning. Young songbirds gradually transform primitive unstructured vocalizations (subsong, akin to human babbling) into complex, stereotyped sequences of syllables that constitute adult song. In comparison with birdsong, territorial and mating calls of vocal non-learner species are thought to exhibit little change during development. We revisited this issue using the crowing behaviour of domestic Japanese quail (Coturnix coturnix japonica). Crowing activity was continuously recorded in young males maintained in social isolation from the age of three weeks to four months. We observed developmental changes in crow structure, both the temporal and the spectral levels. Speed and trajectories of these developmental changes exhibited an unexpected high inter-individual variability. Mechanisms used by quails to transform sounds during ontogeny resemble those described in oscines during the sensorimotor phase of song learning. Studies on vocal non-learners could shed light on the specificity and evolution of vocal learning.
Keywords: birdsong, vocal learning, development, vocalization, Japanese quail
1. Introduction
Birdsong is a fruitful model in behavioural neurobiology. Several features of birdsong can explain this interest: (i) song is a reproducible and quantifiable behaviour, (ii) there are many similarities between the acquisition of birdsong and human speech, (iii) song production and song learning are controlled by a set of discrete brain nuclei, and (iv) early stages of vocal ontogeny (subsong) resemble babbling in babies and song becomes progressively stereotyped as the bird matures (Brainard & Doupe 2002; Catchpole & Slater 2008). During this developmental process, songbirds use a number of motor strategies to adjust their own vocal output to approach the temporal and acoustic features of a learned auditory model (Tchernichovski et al. 2001). In contrast to birdsong, studies mainly performed on Galliforms and Columbiforms concluded that learning had no influence on vocal development and, therefore, that their vocalizations exhibit a strong genetic determinism (Konishi 1963; Lade & Thorpe 1964; Konishi & Nottebohm 1969; Nottebohm & Nottebohm 1971; Baker & Bailey 1987; Baptista 1996; Derégnaucourt et al. 2001). Indeed, various manipulations, including cross-fostering (Lade & Thorpe 1964; Baker & Bailey 1987), hybridization (Lade & Thorpe 1964; Baptista 1996; Derégnaucourt et al. 2001), deafening (Konishi 1963; Nottebohm & Nottebohm 1971) and transplantation of embryonic brain tissue between quail and chicken (Balaban 1997), failed to influence the structure of vocalizations in juveniles. Thus, song-learning birds such as the songbird species appear to have evolved a number of specific motor strategies that help to transform the initially unstructured vocalization into a structured pattern under the guidance of a learned model by means of sensorimotor feedback. However, these motor changes might not directly be linked to vocal production learning since they have also been observed in young oscines raised in social isolation and even in deaf birds (Price 1979).
Nevertheless, changes have been observed in the vocalizations of non-songbird species. In adult birds, Walcott et al. (2006) have recently described spectral and temporal changes in the yodel of male loons (Gavia immer), following the establishment of new territories. Changes in pitch were recorded in Galliforms during the breeding season (Beani et al. 2000) and across seasons (Rotella & Ratti 1988). Vocal changes in adult birds can also be triggered artificially by testosterone administration (Beani et al. 1995, 2000). In young birds, vocal changes in pitch, amplitude and stereotypy occur spontaneously in Anseriforms, Columbiforms, Galliforms and Lariforms during ontogeny (Guyomarc'h 1974; Abs 1980; Meinert & Bergmann 1983; Ten Thoren & Bergmann 1987; Groothuis 1992a; Ballintjin & ten Cate 1997). In young Galliforms of both sexes, administration of testosterone elicits crowing behaviour, as early as on the day of hatching (Hamilton 1938; Marler et al. 1962; Cariou 1969; Schleidt & Shalter 1973). As the birds age, the crow becomes longer through fragmenting into distinct parts, and loses many of the details of the frequency pattern seen in the young birds (Marler et al. 1962; Cariou 1969). It was believed that the crowing patterns induced by testosterone at a young age do not occur in the normal development of crowing, which first appears when adult males reach sexual maturity (Marler et al. 1962).
The evidence for variability in the vocalizations of adult non-vocal learners prompted us to re-examine the degree of stereotypy and plasticity in the calls of a vocal non-learner, the domestic Japanese quail (Coturnix coturnix japonica). The ease with which quail breed in captivity and their relatively rapid ontogenetic development have made them attractive for research (Mills et al. 1997). The Japanese quail constitutes a model of choice for studies of behaviour and its genetic, neurophysiological and neuroendocrine bases (Mills et al. 1997). In particular, its vocal behaviour is well documented and has been the subject of several studies (Cariou 1969; Guyomarc'h 1974; Guyomarc'h & Guyomarc'h 1996; Balaban 1997). We employed a recently developed method for continuously recording and analysing all vocalizations produced during ontogeny by a bird. Given that this method revealed new insights into developmental learning in songbirds (Derégnaucourt et al. 2005), application of this method to the Japanese quail might reveal unsuspected vocal changes during development in this vocal non-learner.
2. Material and methods
(a) Subjects and housing
We used 10 male domestic Japanese quails (Coturnix c. japonica) for this study. The birds were three weeks old when they were purchased from a local supplier. Immediately after their arrival in the laboratory, they were placed in a cage (50×40×30 cm), in an individual anechoic chamber. Food (vitamin-supplemented pellets) and water were supplied ad libitum.
Spontaneous crowing can be observed in both male and female day-old chicks (Cariou 1969; Balaban 1997). Thereafter, crowing is not observed until the quails reach sexual maturity, except in steroid-implanted birds (Cariou 1969; Balaban 1997). Although we could have placed quails in isolation earlier, we would have faced technical problems to maintain high temperature and appropriate ventilation in the sound box. Indeed, quails require an external heat source during the first three weeks of life (Arad & Itsakiglucklich 1991).
(b) Physiological development
Body mass of the quails was measured on a weekly basis with an electronic balance (precision of 0.1 g). In addition, sexual development was estimated by measuring the length of the cloacal vent (Sachs 1969) with calipers to the nearest 0.1 mm. This increase is generally associated with the production of foam from the proctodeal gland. Measurements were performed at approximately the same time of day on each day of sampling.
(c) Photoperiod
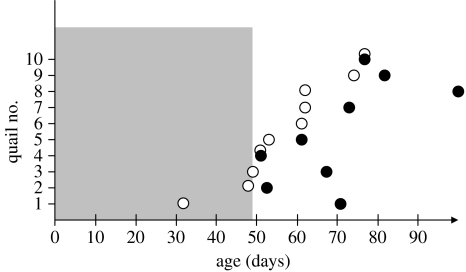
The birds were first raised in 12 L : 12 D. In contrast to what would be expected according to previous studies (Follett & Maung 1978; Mills et al. 1997), this photoperiodic regimen did not enhance the sexual development of the quails. Only three quails out of 10 started to produce crows in 12 L : 12 D (quail 1: day post-hatch (dph) 32; quail 2: dph 48; quail 3: dph 49; figure 1). Therefore, after four weeks in 12 L : 12 D (dph 49), quails were transferred to 14 L : 10 D for eight weeks. A significant increase in the length of the cloacal vent (accompanied by foam production from the proctodeal gland; Wilcoxon, n=10, p<0.002) and in the weight (Wilcoxon, n=10, p<0.004) was observed in the week following the transfer to 14 L : 10 D. No significant changes were observed before or afterwards. The seven remaining quails started to crow from 2 to 28 days following the transfer from 12 L : 12 D to 14 L : 10 D (median=13 days). Temperature was maintained at 20°C (±2°C) during the whole experiment.
Figure 2.
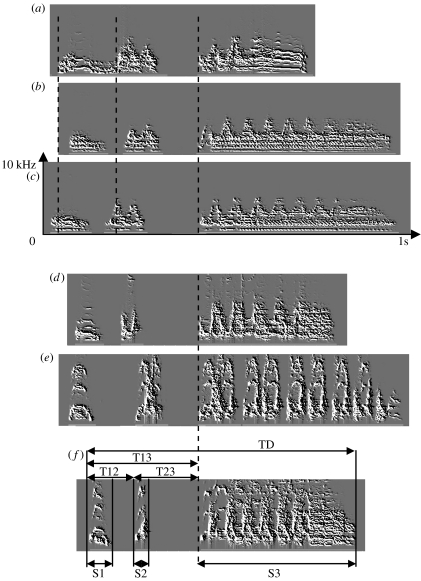
Spectrograms of crows produced by adult male Japanese quails. (a–c) Three stages of crow development recorded in the same bird. (a) First crow produced by the quail in its life. (b) First segmented crow. (c) Complete form recorded at the end of the experiment. (d–f) Complete forms of crows recorded from three different individuals at the end of the experiment. S1–S3, measures of the duration of syllables 1–3; T12–T13–T23, measures of the intersyllabic gaps, TD, measure of the total duration of the crow. The dashed line aligns the different crows at the beginning of the final trill, thereby illustrating changes (within individual) and/or differences (between individuals) in the temporal structure of the Japanese quail crow.
(d) Recording and analysis of the Japanese quail crow
Online detection of the production of crows: in Japanese quails, males but not females crow (Guyomarc'h & Guyomarc'h 1996). Males crow only during the breeding season (Guyomarc'h & Guyomarc'h 1996) and the vocalization is dependent on circulating androgens (Beach & Inman 1965; Schumacher & Balthazart 1983). The Japanese quail crow is composed of three syllables: two short ones followed by a long trill (figure 2; Guyomarc'h & Guyomarc'h 1996). This crow is accompanied by a synchronized postural display with the neck extended, followed by several patterned ‘head bobs’ (Shaw 2000). Playback of crows of male Japanese quail enhances sexual development of females (Guyomarc'h & Guyomarc'h 1984). It also elicits phonotaxis (Goodson & Adkins-Regan 1997) and rally call production in females (Derégnaucourt & Guyomarc'h 2003).
During the whole experiment (12 weeks), vocal activity of each individual bird was continuously recorded using the Sound Analysis Pro (SAP) software (Tchernichovski et al. 2004). The program was run on a PC equipped with an Edirol UA1000 sound card (16 bits, sampling frequency: 44.1 kHz), connected to multidirectional Earthworks TC20 microphones (one per sound box) placed above the cage. Each bird's vocalizations were monitored continuously. Because both adult and steroid-implanted juveniles produce very low amplitude crows at times (Cariou 1969; Potash 1972), we adjusted the settings of the software (amplitude threshold: 40 dB; duration threshold: 100 ms) to make sure that all crows would be automatically identified and recorded. This results in recordings of occasional cage noises and other vocalizations (contact calls, ‘growling’; Guyomarc'h & Guyomarc'h 1996), which were subsequently filtered out.
Crows were subsequently analysed offline using the batch module of SAP. The software then performed multitaper spectral analysis to compute spectral derivatives and acoustic features as documented in the SAP user manual. Subsequent analysis was based on the six acoustic features that were computed in each spectral frame: amplitude; pitch; Wiener entropy; frequency modulation; continuity; and goodness of pitch (Tchernichovski et al. 2000). We adjusted amplitude and Wiener entropy thresholds so that each crow would be segmented in syllables. For each syllable, various acoustic features, based on the main ones mentioned above (see table 1 for a complete list), were automatically calculated by the software, and the results were stored in MySQL v. 4.0 tables (http://mySQL.com; one daily table per quail). Final stages of analysis were performed with Microsoft Excel and Matlab (The Mathworks, Natick, MA).
Table 1.
Vocal changes during crowing development. (Comparison between the first crows produced by the quails (column ‘beginning’) and the last crows produced at the end of the experiment (column ‘end’). Component PCA: component matrix of the principal component analysis calculated using spectral features only. Last column: percentage of syllables that do exhibit an increase for each spectral feature.)
| component PCA | ||||||
|---|---|---|---|---|---|---|
| acoustic feature | beginning (mean±s.e.) | end (mean±s.e.) | 1 | 2 | 3 | % |
| T13 (ms) | 289.87±16.13 | 342.68±12.75 | ||||
| S3 (ms) | 364.18±27.07 | 444.3±20.44 | ||||
| TD (ms) | 655.05±30.59 | 786.98±26.42 | ||||
| mean FM | 39.85±1.68 | 46.70±1.81 | 0.88 | 0.29 | −0.24 | 95 |
| mean AM | 0.03±0.001 | 0.04±0.001 | 0.75 | 0.63 | −0.09 | 80 |
| mean entropy | −4.17±0.32 | −3.79±0.38 | 0.7 | −0.61 | 0.01 | 85 |
| mean frequency (Hz) | 2592.21±116.91 | 3141.76±331.35 | 0.83 | −0.39 | 0.28 | 85 |
| variance FM | 573.11±24.05 | 569.44±18.43 | −0.16 | 0.46 | 0.81 | 40 |
| variance AM | 0.03±0.001 | 0.04±0.001 | 0.75 | 0.61 | −0.11 | 75 |
| variance entropy | 0.66±0.10 | 0.70±0.09 | 0.02 | 0.75 | 0.25 | 65 |
| variance mean frequency (kHz) | 339.76±23.16 | 573.48±120.68 | 0.62 | −0.61 | 0.41 | 80 |
For each bird, daily means for each acoustic parameter were then computed. To estimate vocal changes, we compared crows produced at the beginning of the development with crows produced during the last day of the experiment.
To characterize developmental changes in the temporal structure of the crow, we designed a raster plot analysis. We used an amplitude threshold that segmented all the sounds produced by the birds into syllables. Syllables longer than 300 ms (characteristics of the last syllable of the Japanese quail crow) were then detected and aligned at their beginning.
(e) Circadian changes in crow production
To investigate circadian changes in crow production, we calculated for each day the number of crows emitted per hour.
Most quails crowed exclusively at night, but crows were also recorded during the day. To investigate overnight changes in the temporal pattern of the crows, we compared crows emitted at night (‘night crows’) with those emitted during the day (‘day crows’), taken into account in the last 15 days of the experiment. For quails that did crow exclusively during the night, we compared crows emitted at the beginning of the night (‘dusk crows’) with those produced before the lights were switched on in the cage (‘dawn crows’). For statistical reasons, we pooled the two groups (day crows with ‘dusk’; night crows with ‘dawn’).
(f) Potential of individual coding
As in the European quail (Guyomarc'h et al. 1998), the temporal pattern of this call is highly stereotyped in the Japanese quail and might play a role in inter-individual recognition. The last 30 crows produced by each individual at the end of the experiment were used to measure the potential of individual coding (PIC) of call parameters (Aubin et al. 2007). Spectral features were computed over the complete crow (i.e. one single value for each acoustic feature per crow). We also measured different temporal parameters such as syllable duration and intersyllabic intervals (figure 2). For each acoustic feature, we computed the between-individual and within-individual coefficients of variations (respectively CVbi and CVwi). To assess the PIC for each acoustic parameter, we calculated the ratio CVbi/mean CVwi (Aubin et al. 2007). For a given parameter, a PIC value greater than 1 means that this parameter may be used for individual recognition since its intra-individual variability is smaller than its inter-individual variability. Individual variation in call parameters was also quantified by calculating the repeatability. We then performed a principal component analysis with all acoustic parameters, followed by a discriminant analysis.
(g) Statistical analysis
To avoid multiple testing, we reduced the dimensionality of acoustic parameters obtained by SAP with principal components analyses.
Changes within individuals were estimated using the Wilcoxon matched-pair signed-rank test (Siegel & Castellan 1988).
The statistical packages SPSS v. 15.0 and Statistica v. 2.0 were used for analysis.
3. Results
(a) Vocal changes during crow development
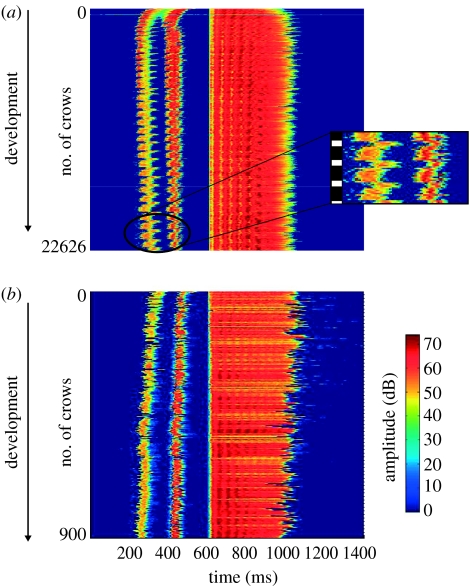
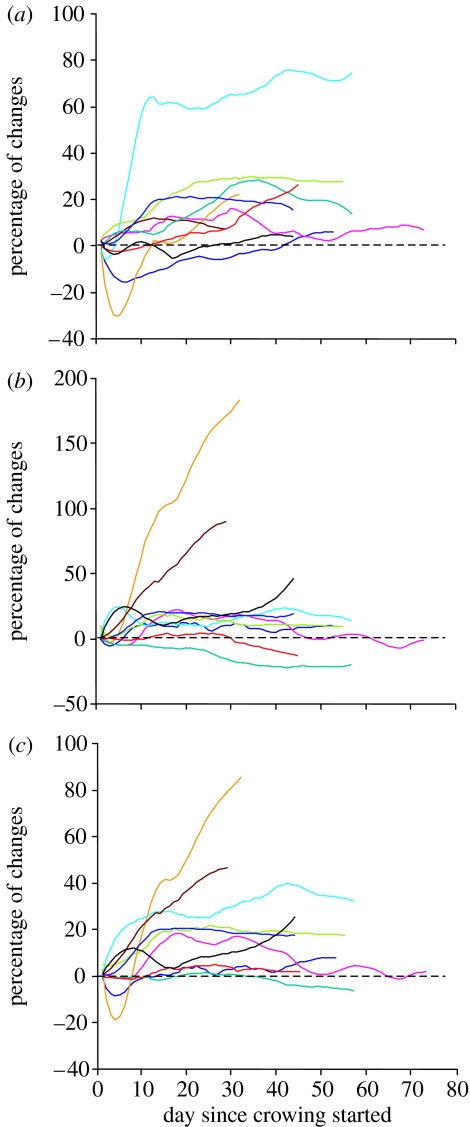
In all the birds, we observed some changes in the temporal structure of the crow (figures 1–3). In 8 birds out of 10, the first crow was composed of two segments (figures 2a and 3a). For the two remaining birds, the three syllables of the first crow were already separated by silences (figure 3b). For those that produced a first crow composed of two segments, silence insertion was observed in the first segment for seven quails, giving rise to two short syllables (figure 2b). Silence insertion was observed in the second segment (final trill) for one quail. Silence insertion occurred from 4 to 56 days following the production of the first crow (median=11 days, n=7; figure 1). The remaining quail continued to produce crows segmented in two parts until the end of the experiment. Within the first few days following segmentation into three parts, the birds would still occasionally produce earlier non-segmented versions of the crow. Intersyllabic temporal structure changed during development for all quails. The time interval between the beginning of the crow and the beginning of the final trill (T13, figure 2f) significantly increased in all birds during development (Wilcoxon, p<0.002; table 1; figure 4). The length of the final trill (S3) increased for 7 birds out of 10. Overall, the total duration increased in 8 birds out of 10 (table 1; figure 4).
Figure 1.
Occurrence of the first crow (open circles) and the first segmented crow (filled circles) during the experiment. Transfer from 12 L : 12 D to 14 L : 10 D was done on day 49. Note that quail no. 6 did not produce segmented crows during the experiment.
Figure 3.
Raster plots of the entire development for two quails. Colour indicates amplitude values. Each horizontal line in a plot represents one crow. Crows were aligned at 600 ms by the occurrence of the third syllable. (a) Quail that produced first crows composed of two segments. The first segment then splits into two syllables. Note that the intersyllabic intervals increase during development, together with the third syllable. Circadian changes in the duration of crows were also observed: crows emitted during the dark phase were longer than those emitted during the light phase (the dark phase and the light phase are indicated by the black square and white square, respectively, to the left of the zoom window). (b) Quail that produced first crows that were already segmented into three distinct syllables. Note that in this case, the third syllable duration decreases during development.
Figure 4.
Developmental trajectories of the duration of crows. Each colour indicates a different quail. (a) T13 (interval between the beginning of the crow and the beginning of the third syllable (final trill)). (b) S3: duration of the last syllable (trill) of the crow. (c) TD: total duration of the crow. Daily data were smoothed using a Savitzky–Golay (polynomial) smoothing filter (polynomial order: 3, frame size: 15). A dashed line indicates baseline value (zero): for each day, we subtracted the daily mean value of the first day of recording from the daily mean value of the day.
We also observed vocal changes in the spectral components of the crow over development (figure 2; table 1). After a principal component analysis performed on the spectral features, we extracted three out of the eight principal components, which explain about 88 per cent of the total variance. Using the first component, we observed significant changes during development (Wilcoxon, p<0.01). Most acoustic features values tend to increase during development (table 1).
(b) Potential of individual coding
At the end of the experiment, we took the last 30 crows produced by each individual to perform a discriminant analysis in order to check whether the crow constitutes an individual signature. The analysis revealed that several temporal and spectral features could be used as potential cues to discriminate between individuals (table 2). On the basis of a discriminant analysis using the principal components calculated from the temporal parameters alone, we were able to correctly classify 95.7 per cent of the crows. When using only the spectral features, 91 per cent of the crows were correctly classified. Finally, when all acoustic parameters were taken into account, only 3 crows out of 300 were not correctly classified (99% of correct classification).
Table 2.
Comparison between individuals of acoustic parameters measured on crows. (Abbreviations for the statistical parameters: CVwi and CVbi, within-and between-individual coefficients of variation; PIC, potential for individual coding. Last column: repeatability score. All values above 30% (italics) are significant.)
| acoustic feature | mean±s.e. | min–max | CVbi | CVwi | mean CVwi | PIC | r (%) |
|---|---|---|---|---|---|---|---|
| S1 (ms) | 75.32±7.19 | 38.73–103.77 | 30.94 | 13.25 | 10.58 | 2.93 | 89.1 |
| S2 (ms) | 68.42±9.04 | 16.23–110.43 | 42.85 | 15.74 | 20.22 | 2.12 | 88.8 |
| S3 (ms) | 437.63±18.96 | 345.5–546.3 | 14.04 | 4.19 | 8.87 | 1.58 | 66.2 |
| T12 (ms) | 153.08±8.41 | 114.6–189.73 | 17.82 | 12.71 | 6.96 | 2.56 | 81.1 |
| T23 (ms) | 184.9±12.70 | 105.63–250.07 | 22.27 | 5.94 | 7.14 | 3.12 | 89.3 |
| TD (ms) | 775.61±24.27 | 664–903.43 | 10.14 | 2.88 | 4.92 | 2.06 | 76.9 |
| mean FM | 44.46±1.78 | 35.81–53.14 | 12.95 | 4.45 | 5.36 | 2.42 | 85 |
| mean AM | 0.004±0.001 | 0.004–0.01 | 15.18 | 4.25 | 7.38 | 2.06 | 79.3 |
| mean entropy | −3.99±0.13 | −4.68– –3.39 | −10.44 | −3.66 | −4.42 | 2.36 | 82.3 |
| mean frequency (Hz) | 2002.43±88.28 | 1558.40–2581.4 | 14.29 | 5.41 | 6.04 | 2.37 | 82.3 |
| variance FM | 600.17±14.97 | 475.37–642.33 | 8.08 | 5.32 | 6.64 | 1.22 | 57.7 |
| variance AM | 0.004±0.001 | 0.0006–0.01 | 49.79 | 15.24 | 38.66 | 1.29 | 62.4 |
| variance entropy | 1.09±0.10 | 0.72–1.76 | 31.00 | 33.67 | 15.21 | 2.04 | 78.1 |
| variance mean frequency (kHz) | 558.16±29.2 | 438.37–743.130 | 16.96 | 38.61 | 33.63 | 0.50 | 17.9 |
(c) Circadian changes in crow production
Crowing activity of quails raised in acoustic isolation exhibits an inter-individual variability (daily crowing activity at the end of the experiment: mean±s.e. (min–max), 187±77 (4–755), n=10). All the quails crowed more during the dark phase than during the light phase (Wilcoxon, p<0.002; 78.2% of crowing activity recorded during the dark phase). As previously described (Guyomarc'h & Thibout 1969; Ottinger et al. 1982), vocal activity exhibits a strong circadian pattern with two daily peaks; one major peak anticipating the beginning of the light phase and a minor peak in the hour following the beginning of the dark phase.
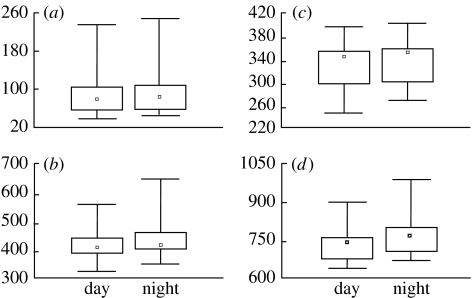
Surprisingly, we observed circadian changes in the temporal pattern of the crows (figures 3a and 5). Crows emitted at night were significantly longer than crows produced during the day (figure 5). In the same way, crows emitted at dawn (the end of the night) were longer than crows recorded at the time when lights were switched off in the cage in the previous evening. These temporal changes affected both syllables' durations and intersyllabic gaps (figure 5).
Figure 5.
Overnight changes in the temporal pattern of the crow. y-axis: duration (ms). (a) S1: duration of the first syllable, p=0.08; (b) S3: duration of the third syllable (final trill), p=0.08; (c) T13: interval between the beginning of the crow and the beginning of the final trill, p=0.036; (d) TD: total duration, p=0.005. p values: results of the Wilcoxon matched-pair test.
4. Discussion
We observed some plasticity during the development of the Japanese quail crow, and circadian variations of the temporal pattern of this acoustic signal. Ontogenetic changes resemble the developmental changes observed in vocal learners, during the transition from the amorphous subsong into mature song (Tchernichovski et al. 2001; Brainard & Doupe 2002; Aronov et al. 2008). In the same way that juvenile songbirds can use multiple strategies to learn the same song (Tchernichovski et al. 2001; Liu et al. 2004), we observed an inter-individual variability in the developmental trajectories of the crow structure. Temporal structure changed dramatically during the crow development. It involved several motor gestures that could act synergetically during development. First, silence insertion in the first or second segment gave rise to the characteristic trisyllabic structure of the Japanese quail crow. Second, time warping in syllables duration was observed. Third, time warping in intersyllabic temporal intervals occurred during development. These three vocal changes, which affect the temporal structure of the acoustic signal, have already been described in a songbird, the zebra finch (Taeniopygia guttata; Tchernichovski & Mitra 2002). Such constraints on vocal changes in the temporal pattern of the crow are defined as developmentally synchronic constraints, as opposed to developmentally diachronic constraints that act in developmental time (Tchernichovski & Mitra 2002) and that affect in our case the spectral envelope of the crow. Within a few days after the crow segmented into three parts, the birds still occasionally produced earlier non-segmented versions of the crow. Such a regression to earlier ontogenetic stages has also been observed during vocal ontogeny of songbirds (Marler & Peters 1982; Derégnaucourt et al. 2005) and suggests that vocal exploration of motor space might also exist in vocal non-learners. As songbirds, the occurrence of an incomplete display might represent a stage in which the bird is shaping its motor output through practice (Groothuis 1992b; Groothuis & Meeuwissen 1992). Young birds may use proprioceptive feedback to match the form of their motor output to some kind of template containing information about the species-specific form of the motor patterns (Brainard & Doupe 2002). Alternatively, such a parallel production of advanced and regressed vocal pattern might reflect some (hormone-dependent) instability in the development of the vocal neural circuit or vocal musculature.
In both vocal and vocal non-learners, vocal changes can be directly related to the maturation of the syrinx, the vocal organ (Ballintijn et al. 1995; Beani et al. 1995; Burke et al. 2007) and/or triggered by changes in the central nervous system (Beani et al. 1995; Aronov et al. 2008). Peripheral constraints may arise from having bronchi and a trachea of the necessary diameter to allow appropriate airflow, and from having syringeal muscle masses that are sufficient to open or close the syrinx (Suthers et al. 2002). For example, the silence insertion observed in the song development of zebra finches (Tchernichovski & Mitra 2002) and in quails could be achieved by reducing air pressure or by briefly closing the syrinx (Beckers et al. 2003).
Central constraints of vocal development of non-songbirds may arise from developmental changes in brainstem respiratory circuits and in the motor neurons' pool of the nucleus hypoglossus pars tracheosyringealis on one hand, and in modulatory regions directly or indirectly connected to these brainstem circuits on the other (Wild 1997). Among the latter might be the ICo (nucleus intercollicularis) regions of the midbrain or of preoptic regions of which vocalizations can be induced electrically or by hormone implants (Yazaki et al. 1997, 1999). So far, the developmental plasticity of neither the brainstem vocal control regions nor these modulatory regions in relation to vocal development of non-songbirds has been studied. In Galliforms, previous work using the quail–chick chimera technique demonstrated that the species-specific characteristics of the crow components are determined by distinct brain structures: the midbrain confers the acoustic pattern, and the caudal hindbrain confers the postural pattern (Balaban 1997; Shaw 2000). Such coordination between vocal output and postural aspects was also described in many songbirds, which often combine their songs with elaborate visual displays (Cooper & Goller 2004).
Given that both the syrinx and neural sound controlling regions are sensitive to sex hormones, testosterone and its androgenic and estrogenic metabolites might induce vocal changes (Schumacher & Balthazart 1983; Groothuis & Meeuwissen 1992; Gahr 2003, 2007; Burke et al. 2007), both during development and adulthood. For instance, incomplete forms of vocalization and subsequent developmental changes occur at an earlier than normal age in testosterone-treated young Galliforms (Marler et al. 1962; Schleidt & Shalter 1973). Moreover, testosterone can also change the vocal pattern of adults (Beani et al. 1995, 2000). Androgen levels rise as young quail age (Ottinger & Brinkley 1978). This probably contributes to the development of normal adult displays. Consequently, intermediate stages of crowing development could be associated with reduced testosterone production stemming from social stimulation (Groothuis 1992b). To rule out this hypothesis, one needs to study whether similar vocal changes occur in socially housed quail. We observed that a photoperiodic schedule of 12 L : 12 D was not sufficient to enhance significant sexual development in male quails raised in isolation, even though this photoperiod is known to induce testosterone production (Follett & Maung 1978; Mills et al. 1997). The effect of social interactions was probably underestimated in previous experiments dealing with the photoperiodic control of reproduction in quails, even if such an effect has nevertheless been already reported (Guyomarc'h & Guyomarc'h 1984). The observation that some quails produced a first crow that was already segmented into three parts might reflect individual differences in the ontogeny of testosterone levels (Ottinger & Brinkley 1978) and related differences in the developmental state of the vocal control system. The potential for performing a complete crow may thus be already present in the young quail, waiting for the right internal state, e.g. a high level of testosterone, to come about. In songbirds, chronic testosterone treatment during the sensitive period for song learning leads to an early crystallization of the song motif (Korsia & Bottjer 1991). Experimental work including hormonal measurements and treatment is required for a better understanding of the influence of testosterone in the developmental process of crowing patterns in quail. It would also be interesting to determine whether these vocal changes occur only during the first breeding season, or whether they reappear after sexual regression. Such a vocal regression is observed in the so-called ‘open-ended’ vocal learners, such as the canary, which exhibit seasonal changes in song production across years (Brainard & Doupe 2002; Catchpole & Slater 2008).
We observed circadian changes in the temporal pattern of the crow. Such day/night variations have already been described in oscines (Hultsch 1980), and could be driven by the hormone melatonin, since both songbirds and non-songbirds species exhibit melatonin receptors in brain areas that control vocal production (Cozzi et al. 1993; Gahr & Kosar 1996; Jansen et al. 2005). Alternatively, given the recent discovery that zebra finch songs slow when specific brain nuclei are cooled (Long & Fee 2008), melatonin could also influence crows indirectly through its role in the regulation of the circadian rhythm in brain and body temperature (Aschoff et al. 1973; Underwood 1994; Doi et al. 2002) and associated brain cooling at night (Aschoff et al. 1973; Doi et al. 2002). Additional experiments are required to evaluate this hypothesis.
Konishi (1985) has considered certain calls of non-vocal learners such as the crowing of roosters and the cooing of doves as functionally equivalent to the song of songbirds. At the proximate level, our study of Japanese quail demonstrates that ontogenetic changes of vocal non-learners' vocalizations exhibit some similarity to that of song in songbirds (table 3). The similarities between the mechanisms involved in song learning in songbirds and crow development in the Japanese quail suggest that the motor programmes for these vocal transformations might have appeared early in avian evolution. Moreover, this suggests that the three bird taxa that independently evolved vocal learning (i.e. songbirds, parrots and hummingbirds) needed ‘just’ to invent a mechanism to modify the produced sounds in the direction of the memorized model (either by error correction or selection; Marler 1997), but not the motor programmes to modify the sounds. Furthermore, the capacity to remember the sounds of individual conspecifics—a requisite for forming the template of the tutor song (Brainard & Doupe 2002)—is present in vocal non-learners (Guyomarc'h 1974; Lengagne et al. 1999). Although the plasticity in the ontogeny of crow production observed in quail may reflect the substrate from which vocal learning evolved, it is nonetheless also possible that quail and other vocal non-learners evolved from vocal learners that subsequently lost components of this capacity during evolution (Feenders et al. 2008). In this case, some of the similarities between quail and vocal learners may reflect the retention of components of vocal learning, rather than the precursor state that gave rise to vocal learning. More studies are required to revisit sensorimotor aspects of vocal production in non-songbirds, to clarify the specificity of vocal changes triggered by imitation, and to identify the cellular and molecular events involved in these processes.
Table 3.
Vocal changes observed in the Japanese quail and oscines.
| Japanese quail | oscines | |
|---|---|---|
| vocal development (juveniles) | ||
| stop insertion | this study | Tchernichovski & Mitra (2002) |
| increase in spectral complexity of the signal | this study | Tchernichovski et al. (2001) and Derégnaucourt et al. (2005) |
| inter-individual variability in developmental trajectories | this study | Tchernichovski et al. (2001) and Liu et al. (2004) |
| regression to earlier ontogenetic stages | this study | Marler & Peters (1982) and Derégnaucourt et al. (2005) |
| vocal production (adults) | ||
| effect of testosterone on pitch | Beani et al. (2000) | Cynx et al. (2005) |
| overnight changes in the temporal pattern | this study | Hultsch (1980) |
| Lombard effect | Potash (1972) | Cynx et al. (1998) |
Acknowledgements
The experiments were approved by the government of Upper Bavaria.
We thank W. Forstmeier, D. Lipkind, H. Reers and A. ter Maat for statistical assistance, A. Leitão for criticism of an earlier draft version of this manuscript, J. Minshull for bird keeping and N. C. Rattenborg for reviewing our English manuscript. We are grateful to E. Adkins-Regan, S. Healy and two anonymous referees for their valuable comments. This work was supported by the Max Planck Society.
References
- Abs M. On the bioacoustics of the breaking of the birds' voice. Zool. Jb. Physiol. 1980;84:289–382. [Google Scholar]
- Arad Z., Itsakiglucklich S. Ontogeny of brain temperature regulation in quail chicks (Coturnix coturnix japonica) Physiol. Zool. 1991;64:1356–1370. [Google Scholar]
- Aronov D., Andalman A.S., Fee M.S. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320:630–634. doi: 10.1126/science.1155140. doi:10.1126/science.1155140 [DOI] [PubMed] [Google Scholar]
- Aschoff C., Aschoff J., von Saint Paul U. Circadian rhythms of chicken brain temperatures. J. Physiol. 1973;230:103–113. doi: 10.1113/jphysiol.1973.sp010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin T., Mathevon N., Staszewski V., Boulinier T. Acoustic communication in the Kittiwake Rissa tridactyla: potential cues for sexual and individual signatures in long calls. Polar Biol. 2007;30:1027–1033. doi:10.1007/s00300-007-0262-6 [Google Scholar]
- Baker J.A., Bailey E.D. Sources of phenotypic variation in the separation call of Northern Bobwhite (Colinus virginianus) Can. J. Zool. 1987;65:1010–1015. doi:10.1139/z87-301 [Google Scholar]
- Balaban E. Changes in multiple brain regions underlie species differences in a complex, congenital behavior. Proc. Natl Acad. Sci. USA. 1997;94:2001–2006. doi: 10.1073/pnas.94.5.2001. doi:10.1073/pnas.94.5.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballintjin M.R., ten Cate C. Vocal development and its differentiation in a non songbird: the collared dove (Streptopelia decaoto) Behaviour. 1997;134:595–621. doi:10.1163/156853997X00548 [Google Scholar]
- Ballintijn M.R., ten Cate C., Nuijens F.W., Berkhoudt H. The syrinx of the collared dove (Streptopelia decaocto): structure, inter-individual variation and source. Neth. J. Zool. 1995;45:455–479. doi:10.1163/156854295X00410 [Google Scholar]
- Baptista L.F. Nature and its nurturing in avian vocal development. In: Kroodsma D.E., Miller E.H., editors. Ecology and evolution of acoustic communication in birds. Cornell University Press; Ithaca, NY: 1996. pp. 39–60. [Google Scholar]
- Beach F.A., Inman N.G. Effects of castration and androgen replacement on mating in male quail. Proc. Natl Acad. Sci. USA. 1965;54:1426–1431. doi: 10.1073/pnas.54.5.1426. doi:10.1073/pnas.54.5.1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beani L., Panzica G., Briganti F., Persichella P., Dessi-Fulgheri F. Testosterone-induced changes of call structure, midbrain and syrinx anatomy in partridges. Physiol. Behav. 1995;58:1149–1157. doi: 10.1016/0031-9384(95)02060-8. doi:10.1016/0031-9384(95)02060-8 [DOI] [PubMed] [Google Scholar]
- Beani L., Briganti F., Campanella G., Lupo C., Dessi-Fulgheri F. Effect of androgens on structure and rate of crowing in the Japanese quail (Coturnix japonica) Behaviour. 2000;137:417–435. doi:10.1163/156853900502150 [Google Scholar]
- Beckers G.J., Suthers R.A., ten Cate C. Pure-tone birdsong by resonance filtering of harmonic overtones. Proc. Natl Acad. Sci. USA. 2003;100:7372–7376. doi: 10.1073/pnas.1232227100. doi:10.1073/pnas.1232227100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard M.S., Doupe A.J. What songbirds teach us about learning. Nature. 2002;417:351–358. doi: 10.1038/417351a. doi:10.1038/417351a [DOI] [PubMed] [Google Scholar]
- Burke M.R., Adkins-Regan E., Wade J. Laterality in syrinx muscle morphology of the Japanese quail (Coturnix japonica) Physiol. Behav. 2007;90:682–686. doi: 10.1016/j.physbeh.2006.12.007. doi:10.1016/j.physbeh.2006.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou M-L. Etude du chant de la Caille Japonaise, Coturnic coturnix japonica: variance individuelle et comparaison de trois populations. Bull. Biol. Fr. Belg. 1969;103:323–338. [Google Scholar]
- Catchpole C.K., Slater P.J.B. 2nd edn. Cambridge University Press; Cambridge, UK: 2008. Bird song: biological themes and variations. [Google Scholar]
- Cooper B.G., Goller F. Multimodal signals: enhancement and constraint of song motor patterns by visual display. Science. 2004;303:544–546. doi: 10.1126/science.1091099. doi:10.1126/science.1091099 [DOI] [PubMed] [Google Scholar]
- Cozzi B., Stankov B., Viglietti-Panzica C., Capsoni S., Aste N., Lucini V., Fraschini F., Panzica G. Distribution and characterization of melatonin receptors in the brain of the Japanese quail, Coturnix japonica. Neurosci. Lett. 1993;150:149–152. doi: 10.1016/0304-3940(93)90523-n. doi:10.1016/0304-3940(93)90523-N [DOI] [PubMed] [Google Scholar]
- Cynx J., Lewis R., Tavel B., Tse H. Amplitude regulation of vocalizations in noise by a songbird, Taeniopygia guttata. Anim. Behav. 1998;56:107–113. doi: 10.1006/anbe.1998.0746. doi:10.1006/anbe.1998.0746 [DOI] [PubMed] [Google Scholar]
- Cynx J., Bean N.J., Rossman I. Testosterone implants alter the frequency range of zebra finch songs. Horm. Behav. 2005;47:446–451. doi: 10.1016/j.yhbeh.2004.11.018. doi:10.1016/j.yhbeh.2004.11.018 [DOI] [PubMed] [Google Scholar]
- Derégnaucourt S., Guyomarc'h J.-C. Mating call discrimination in female European (Coturnix c. coturnix) and Japanese quail (Coturnix c. japonica) Ethology. 2003;109:107–119. doi:10.1046/j.1439-0310.2003.00854.x [Google Scholar]
- Derégnaucourt S., Guyomarc'h J.-C., Richard V. Classification of hybrid crows in quail using artificial neural networks. Behav. Process. 2001;56:103–112. doi: 10.1016/s0376-6357(01)00188-7. doi:10.1016/S0376-6357(01)00188-7 [DOI] [PubMed] [Google Scholar]
- Derégnaucourt S., Mitra P.P., Fehér O., Pytte C., Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433:710–716. doi: 10.1038/nature03275. doi:10.1038/nature03275 [DOI] [PubMed] [Google Scholar]
- Doi M., Nakajima Y., Okano T., Fukada Y. Light-dependent changes in the chick pineal temperature and the expression of cHsp90α gene: a potential contribution of in vivo temperature change to the photic-entrainment of the chick pineal circadian clock. Zool. Sci. 2002;19:633–641. doi: 10.2108/zsj.19.633. doi:10.2108/zsj.19.633 [DOI] [PubMed] [Google Scholar]
- Feenders G., Liedvogel M., Rivas M., Zapka M., Horita H., Hara E., Wada K., Mouritsen H., Jarvis E.D. Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PLoS ONE. 2008;3:1–27. doi: 10.1371/journal.pone.0001768. doi:10.1371/journal.pone.0001768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett B.K., Maung S.L. Rate of testicular maturation, in relation to gonadotrophin and testosterone levels, in quail exposed to various artificial photoperiods and to natural daylengths. J. Endocrinol. 1978;78:267–280. doi: 10.1677/joe.0.0780267. doi:10.1677/joe.0.0780267 [DOI] [PubMed] [Google Scholar]
- Gahr M. Male Japanese quails with female brains do not show male sexual behaviors. Proc. Natl Acad. Sci. USA. 2003;100:7959–7964. doi: 10.1073/pnas.1335934100. doi:10.1073/pnas.1335934100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M. Sexual differentiation of the vocal control system of birds. Adv. Genet. 2007;59:67–105. doi: 10.1016/S0065-2660(07)59003-6. doi:10.1016/S0065-2660(07)59003-6 [DOI] [PubMed] [Google Scholar]
- Gahr M., Kosar E. Identification, distribution, and developmental changes of a melatonin binding site in the song control system of the zebra finch. J. Comp. Neurol. 1996;367:308–318. doi: 10.1002/(SICI)1096-9861(19960401)367:2<308::AID-CNE11>3.0.CO;2-M. doi:10.1002/(SICI)1096-9861(19960401)367:2<308::AID-CNE11>3.0.CO;2-M [DOI] [PubMed] [Google Scholar]
- Goodson J.L., Adkins-Regan E. Playback of crows of male Japanese quail elicits female phonotaxis. Condor. 1997;99:990–993. doi:10.2307/1370153 [Google Scholar]
- Groothuis T.G.G. Comparison between development of bird song and development of other displays. Neth. J. Zool. 1992a;43:172–192. doi:10.1163/156854293X00287 [Google Scholar]
- Groothuis T. The influence of social experience on the development and fixation of the form of displays in the black-headed gull. Anim. Behav. 1992b;43:1–14. doi:10.1016/S0003-3472(05)80067-3 [Google Scholar]
- Groothuis T., Meeuwissen G. The influence of testosterone on the development and fixation of the form of displays in two age classes of young black-headed gulls. Anim. Behav. 1992;43:189–208. doi:10.1016/S0003-3472(05)80215-5 [Google Scholar]
- Guyomarc'h, J.-C. 1974 Les Vocalizations des Gallinacés: Structure des Sons et des Répertoires, Ontogenèse Motrice, et Acquisition de Leur Sémantique. PhD thesis, University of Rennes, France.
- Guyomarc'h C., Guyomarc'h J.-C. The influence of social factors on the onset of egg production in Japanese quail (Coturnix coturnix japonica) Biol. Behav. 1984;9:333–342. [Google Scholar]
- Guyomarc'h J.-C., Guyomarc'h C. Vocal communication in European quail; comparison with Japanese quail. CR Acad. Sci. Par. Life Sci. 1996;319:827–834. [Google Scholar]
- Guyomarc'h J.-C., Thibout E. Rythmes et cycles dans l'emission du chant chez la Caille Japonaise (Coturnix c. japonica) Rev. Comp. Anim. 1969;3:37–49. [Google Scholar]
- Guyomarc'h J.-C., Aupiais A., Guyomarc'h C. Individual differences in the long-distance vocalizations used during pair bonding in European quail (Coturnix coturnix) Ethol. Ecol. Evol. 1998;10:333–346. [Google Scholar]
- Hamilton J.B. Precocious masculine behaviour following administration of synthetic male hormone substance. Endocrinology. 1938;23:53–57. [Google Scholar]
- Hultsch, H. 1980 Beziehungen zwischen Struktur, zeitlicher Variabilität und sozialem Einsatz des Gesangs der Nachtigall Luscinia megarhynchos. PhD thesis, FU Berlin, Germany.
- Jansen R., Metzdorf R., van der Roest M., Fusani L., ter Maat A., Gahr M. Melatonin affects the temporal organization of the song of the zebra finch. FASEB J. 2005;19:848–850. doi: 10.1096/fj.04-2874fje. doi:10.1096/fj.04-2874fje [DOI] [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in the vocal behaviour of the domestic fowl. Z. Tierpsychol. 1963;20:304–367. [Google Scholar]
- Konishi M. Bird song: from behavior to neuron. Annu. Rev. Neurosci. 1985;8:125–170. doi: 10.1146/annurev.ne.08.030185.001013. doi:10.1146/annurev.ne.08.030185.001013 [DOI] [PubMed] [Google Scholar]
- Konishi M., Nottebohm F. Experimental studies in the ontogeny of avian vocalizations. In: Hinde R.A., editor. Bird vocalizations. Cambridge University Press; London, UK: 1969. pp. 29–48. [Google Scholar]
- Korsia S., Bottjer S.W. Chronic testosterone treatment impairs vocal learning in male zebra finches during a restricted period of development. J. Neurosci. 1991;11:2362–2371. doi: 10.1523/JNEUROSCI.11-08-02362.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lade B.I., Thorpe W.H. Dove songs as innately coded patterns of specific behaviour. Nature. 1964;202:366–368. doi:10.1038/202366a0 [Google Scholar]
- Lengagne T., Jouventin P., Aubin T. Finding one's mate in a king penguin colony: efficiency of acoustic communication. Behaviour. 1999;136:833–846. doi: 10.1006/anbe.1999.1086. doi:10.1163/156853999501595 [DOI] [PubMed] [Google Scholar]
- Liu W.-C., Gardner T.J., Nottebohm F. Juvenile zebra finches can use multiple strategies to learn the same song. Proc. Natl Acad. Sci. USA. 2004;101:18 177–18 182. doi: 10.1073/pnas.0408065101. doi:10.1073/pnas.0408065101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M.A., Fee M.S. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. doi:10.1038/nature07448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P. Three models of song learning: evidence from behavior. J. Neurobiol. 1997;33:501–516. doi:10.1002/(SICI)1097-4695(19971105)33:5<501::AID-NEU2>3.0.CO;2-8 [PubMed] [Google Scholar]
- Marler P., Peters S. Subsong and plastic song: their role in the vocal learning process. In: Kroodsma D.E., Miller E.H., editors. Ecology and evolution of acoustic communication in birds. Academic Press; New York, NY: 1982. pp. 25–50. [Google Scholar]
- Marler P., Kreith M., Willis E. An analysis of testosterone-induced crowing in young domestic cockerels. Anim. Behav. 1962;10:48–54. doi:10.1016/0003-3472(62)90130-6 [Google Scholar]
- Meinert U., Bergmann H.-H. Zur Jugendentwicklung Der LautäußErungen Beim Birkhuhn (Tetrao tetrix) Behaviour. 1983;85:242–258. doi:10.1163/156853983X00246 [Google Scholar]
- Mills A.D., Crawford L.L., Domjan M., Faure J.-M. The behavior of the Japanese or domestic quail (Coturnix japonica) Neurosci. Biobehav. Rev. 1997;21:261–281. doi: 10.1016/s0149-7634(96)00028-0. doi:10.1016/S0149-7634(96)00028-0 [DOI] [PubMed] [Google Scholar]
- Nottebohm F., Nottebohm M.E. Vocalizations and breeding behaviour of surgically deafened ring doves (Streptopelia risoria) Anim. Behav. 1971;19:313–327. doi: 10.1016/s0003-3472(71)80012-x. doi:10.1016/S0003-3472(71)80012-X [DOI] [PubMed] [Google Scholar]
- Ottinger M.A., Brinkley H.J. Testosterone and sex-related behavior and morphology: relationship during maturation and in the adult Japanese quail. Horm. Behav. 1978;11:175–182. doi: 10.1016/0018-506x(78)90046-6. doi:10.1016/0018-506X(78)90046-6 [DOI] [PubMed] [Google Scholar]
- Ottinger M.A., Schleidt W.M., Russek E. Daily patterns of courtship and mating behavior in the male Japanese quail. Behav. Process. 1982;7:223–233. doi: 10.1016/0376-6357(82)90037-7. doi:10.1016/0376-6357(82)90037-7 [DOI] [PubMed] [Google Scholar]
- Potash L.M. Noise-induced changes in calls of the Japanese quail. Psychon. Sci. 1972;26:252–254. [Google Scholar]
- Price P.H. Developmental determinants of structure in zebra finch song. J. Comp. Physiol. Psychol. 1979;93:260–277. doi:10.1037/h0077553 [Google Scholar]
- Rotella J.Y., Ratti J.T. Seasonal variation in gray partridge vocal behavior. Condor. 1988;90:304–310. doi:10.2307/1368558 [Google Scholar]
- Sachs B.D. Photoperiodic control of reproductive behavior and physiology of reproductive behavior and physiology of the male Japanese quail. Horm. Behav. 1969;1:7–24. doi:10.1016/0018-506X(69)90002-6 [Google Scholar]
- Schleidt W.M., Shalter M.D. Stereotypy of a fixed action pattern during ontogeny in Coturnix coturnix coturnix. Z. Tierpsychol. 1973;33:35–37. doi: 10.1111/j.1439-0310.1973.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Balthazart J. The effects of testosterone and its metabolites on sexual behavior and morphology in male and female Japanese quail. Physiol. Behav. 1983;30:335–339. doi: 10.1016/0031-9384(83)90135-x. doi:10.1016/0031-9384(83)90135-X [DOI] [PubMed] [Google Scholar]
- Shaw B.K. Involvement of a midbrain vocal nucleus in the production of both the acoustic and postural components of crowing behavior in Japanese quail. J. Comp. Physiol. A. 2000;186:747–757. doi: 10.1007/s003590000128. doi:10.1007/s003590000128 [DOI] [PubMed] [Google Scholar]
- Siegel S., Castellan N.J. McGraw-Hill Book Company; New York, NY: 1988. Nonparametric statistics for the behavioral sciences. [Google Scholar]
- Suthers R.A., Goller F., Wild M.J. Somatosensory feedback modulates the respiratory motor program of crystallised birdsong. Proc. Natl Acad. Sci. USA. 2002;99:5680–5685. doi: 10.1073/pnas.042103199. doi:10.1073/pnas.042103199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O., Mitra P.P. Towards quantification of vocal imitation in the zebra finch. J. Comp. Physiol. A. 2002;188:867–878. doi: 10.1007/s00359-002-0352-4. doi:10.1007/s00359-002-0352-4 [DOI] [PubMed] [Google Scholar]
- Tchernichovski O., Nottebohm F., Ho C.E., Pesaran B., Mitra P.P. A procedure for an automated measurement of song similarity. Anim. Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. doi:10.1006/anbe.1999.1416 [DOI] [PubMed] [Google Scholar]
- Tchernichovski O., Mitra P.P., Lints T., Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science. 2001;291:2564–2569. doi: 10.1126/science.1058522. doi:10.1126/science.1058522 [DOI] [PubMed] [Google Scholar]
- Tchernichovski O., Lints T.J., Derégnaucourt S., Cimenser A., Mitra P.P. Analysis of the entire song development: methods and rationale. Ann. NY Acad. Sci. 2004;1016:348–363. doi: 10.1196/annals.1298.031. doi:10.1196/annals.1298.031 [DOI] [PubMed] [Google Scholar]
- Ten Thoren B., Bergmann H.-H. Veränderung Und Konstanz Von Merkmalen in Der Jugendlichen Stimmentwicklung Der Nonnengans (Branta leucopsis) Behaviour. 1987;100:61–90. doi:10.1163/156853987X00071 [Google Scholar]
- Underwood H. The circadian rhythm of thermoregulation in Japanese quail. I. Role of the eyes and pineal. J. Comp. Physiol. A. 1994;175:639–653. doi: 10.1007/BF00199485. doi:10.1007/BF00199485 [DOI] [PubMed] [Google Scholar]
- Walcott C., Mager J.N., Piper W. Changing territories, changing tunes: male loons, Gavia immer, change their vocalizations when they change territories. Anim. Behav. 2006;71:673–683. doi:10.1016/j.anbehav.2005.07.011 [Google Scholar]
- Wild J.M. Neural pathways for the control of birdsong production. J. Neurobiol. 1997;33:653–670. doi: 10.1002/(sici)1097-4695(19971105)33:5<653::aid-neu11>3.0.co;2-a. doi:10.1002/(SICI)1097-4695(19971105)33:5<653::AID-NEU11>3.0.CO;2-A [DOI] [PubMed] [Google Scholar]
- Yazaki Y., Matsushima T., Aoki K. Testosterone modulates calling behavior in Japanese quail chicks. Zool. Sci. 1997;14:219–225. doi: 10.1007/s003590050302. doi:10.2108/zsj.14.219 [DOI] [PubMed] [Google Scholar]
- Yazaki Y., Matsushima T., Aoki K. Testosterone modulates stimulation-induced calling behavior in Japanese quails. J. Comp. Physiol. A. 1999;184:13–19. doi: 10.1007/s003590050302. doi:10.1007/s003590050302 [DOI] [PubMed] [Google Scholar]