Abstract
Migratory silvereyes treated with a strong magnetic pulse shift their headings by approximately 90°, indicating an involvement of magnetite-based receptors in the orientation process. Structures containing superparamagnetic magnetite have been described in the inner skin at the edges of the upper beak of birds, while single-domain magnetite particles are indicated in the nasal cavity. To test which of these structures mediate the pulse effect, we subjected migratory silvereyes, Zosterops l. lateralis, to a strong pulse, and then tested their orientation, while the skin of their upper beak was anaesthetized with a local anaesthetic to temporarily deactivate the magnetite-containing structures there. After the pulse, birds without anaesthesia showed the typical shift, whereas when their beak was anaesthetized, they maintained their original headings. This indicates that the superparamagnetic magnetite-containing structures in the skin of the upper beak are most likely the magnetoreceptors that cause the change in headings observed after pulse treatment.
Keywords: migratory orientation, magnetoreception, magnetite, magnetic pulse, magnetite-containing structures, Australian Silvereyes
1. Introduction
Inspired by the compass needle, Yorke (1979, 1981) was the first to suggest magnetoreception in birds on the basis of chains of ferromagnetic crystals. A biogenic ferromagnetic substance, magnetite, a specific iron oxide Fe3O4, was already known from chitons (Lowenstam 1962) and was subsequently found in a variety of organisms ranging from bacteria to arthropods and members of all major groups of vertebrates (for summary, see Kirschvink et al. 1985). Depending on size, magnetite particles have different magnetic properties: those of more than 1 μm are multidomains with little net magnetization, whereas crystals smaller than 1 μm are mostly single domains carrying a stable magnetic moment. Even smaller ones with sizes below 0.05 μm are superparamagnetic; they lack a stable magnetic moment, but their moments can be aligned by an external magnetic field (Kirschvink 1989). Kirschvink & Gould (1981) considered theoretically a number of ways in which receptors based on magnetite crystals might work, and several competing models on the functional mode of receptors have been forwarded and discussed since, some based on single domains, others on superparamagnetic particles and even others were hybrid models based on both (e.g. Kirschvink & Walker 1985; Edmonds 1992; Shcherbakov & Winklhofer 1999; Davila et al. 2003, 2005; Fleissner et al. 2007; Solov'yov & Greiner 2007; Walker 2008).
Biogenic magnetite has also been found in birds. Based on remanence measurements, Beason & Nichols (1984) and Beason & Brennon (1986) identified magnetic particles in the ethmoid region, associated with the ophthalmic branch of the trigeminal nerve. Iron-rich particles in that area, in particular in the nasal cavity, were also indicated by histological studies (Beason & Nichols 1984; Williams & Wild 2001) and assumed to be single-domain magnetite particles. Hanzlik et al. (2000) and Winklhofer et al. (2001), on the other hand, reported structures containing very small iron-rich particles at a specific location in the mucous skin at the inside edges of the upper beak, which were identified as superparamagnetic magnetite by crystallographic and magnetometric methods (see also Tian et al. 2007). Subsequent histological studies revealed that they are associated with a series of iron-rich platelets, all embedded within the sensory terminals of the ophthalmic nerve (Fleissner et al. 2003, 2007). These structures were first described in pigeons, but corresponding structures have also been found in domestic chickens and two passerine species, the European robin, Erithacus rubecula, and the garden warbler, Sylvia borin (Fleissner et al. 2007; Stahl et al. 2007), so that they appear to be a common feature of all birds.
To demonstrate an involvement of magnetite-based receptors in avian navigation, migrating birds and homing pigeons have been treated with a magnetic pulse, a treatment designed to selectively affect magnetite. With an intensity of 0.5 T, the pulse was strong enough to alter the magnetization of single domains, and with a duration of approximately 4 ms, it was brief enough to prevent the particles from mechanically rotating with the pulse and thus escaping remagnetization. The pulse would also have a marked effect on clusters of superparamagnetic particles, temporarily disrupting them and/or changing their shape (Davila et al. 2005). Applying this pulse to migratory Australian silvereyes, Zosterops l. lateralis, had indeed a marked effect on their orientation behaviour: instead of preferring their seasonally appropriate migratory direction, the birds showed an approximate 90° shift in heading, turning towards east, with a certain tendency to prefer the east–west axis (Wiltschko, W. et al. 1994, 1998, 2006). Obviously, receptors based on magnetizable material, probably magnetite, were involved in the birds' orientation.
Thus, magnetite particles have been described in birds, including the garden warbler which is now considered to belong to the same family as the Australian silvereye, and pulse experiments indicated an involvement of magnetite-based receptors in avian orientation, but a direct link between these two groups of findings has not yet been established. In particular, it has not been established which type of magnetite receptors were involved in the response to the pulse—single domains in the nasal cavity or superparamagnetic particles in the beak. Since the superparamagnetic particles described by Fleissner et al. (2003, 2007) in the upper beak are concentrated in six distinct structures in the skin along edges inside the upper mandible, we decided to temporarily deactivate them with a local anaesthetic to observe whether birds would respond to a pulse under these conditions. Here, we report the results of such experiments with Australian silvereyes.
2. Material and methods
The experiments took place in Armidale, NSW, Australia (30°30′ S, 151°40′ E), during southern spring from 28 September to 13 October 2006.
(a) Test birds
The test birds were Australian silvereyes of the partially migratory Tasmanian population. Most birds of this subspecies spend their winter on the Australian continent, moving north as far as northern New South Wales and southern Queensland, to return to Tasmania in southern spring. They migrate in flocks predominantly during the twilight hours at dawn and dusk (Lane & Battam 1971).
On 14 September 2006, 15 individuals—10 adults and 5 juveniles—were mist-netted on the Campus of the University of New England in Armidale, not far from the later test site. They were kept as a flock in an outside aviary until 26 September 2006, when they were moved into housing cages (80×40×40 cm) in groups of four in an indoor room under an artificial light regime that was synchronized with the local photoperiod. When the tests were completed, the birds were kept for 10 more days to ensure that any effect of the pulse had worn off (see Wiltschko, W. et al. 1998), and then they were released near the place of capture.
(b) Test procedure and data collection
The tests took place indoors in a wooden building where the local geomagnetic field (56 μT, −62° inclination) was unchanged. The testing room was lit by dim ‘white’ light from an incandescent light bulb, with light levels in the test cages between 24 and 29 mW m−2. The light passed through a diffuser before it reached the bird in the cage. We tested the birds every second day for 75 min beginning an hour before sunset.
The test protocol replicated that of previous studies (Wiltschko, W. et al. 1994, 1998; Munro et al. 1997). Testing began with six control tests for each bird in the local geomagnetic field (mN=360°, 56 μT, −62° inclination) to determine the directional preference of each individual in order to assure that the birds showed appropriate migratory orientation.1 Then the birds were subjected to a pulse with an intensity of 0.5 T and a duration of approximately 4–5 ms, which was administered in the same way as before: a solenoid was aligned in an east–west direction; the birds were placed into the solenoid facing east with the head pointing straightforward to the end where the magnetic south pole of the pulse field was induced (‘south anterior’ as defined by Beason et al. 1995, 1997). The first critical tests followed immediately after pulsing, with half of the birds having their upper beak locally anaesthetized, in order to temporarily deactivate the iron-containing structures described by Fleissner et al. (2003, 2007). Anaesthesia was achieved by gently rubbing a cotton bud soaked in Xylocaine 2 per cent (Astra Zeneca, Wedel, Germany: active substance lidocaine hydrochloride) along the mucous skin at the inner edges of the upper mandible. The other half of the birds were tested without anaesthesia. The next tests followed 2 days later, and this time the groups were reversed: the birds that had their beak anaesthetized before were now tested without anaesthesia, and vice versa.
For recording the birds' directional tendencies, we used funnel-shaped cages whose inclined walls were lined with coated paper (BIC, Germany, formerly Tipp-Ex; for details, see Wiltschko, W. et al. 1994, 1998). When moving, the birds left scratches on the coating, which documented the distribution of their activity.
(c) Data analysis
For evaluation, the coated paper was removed from the test cage, divided into 24 sectors, and the number of scratches in each sector was counted. One recording with fewer than 35 scratches was excluded due to insufficient migratory activity.
From the distribution of activity, we calculated the heading of each recording. Based on the headings of the 15 birds, we calculated by vector addition a mean vector of each testing day. To characterize the behaviour during the control phase, we also determined the individual birds' mean vectors from the five to six control headings per bird and comprised these mean headings in a grand mean vector for the control period before pulsing. After pulsing, the headings of the birds when tested without and with anaesthesia of their upper beak were summarized in mean vectors. All mean vectors were tested with the Rayleigh test for directional preference (Batschelet 1981).
The behaviour of the birds after pulse treatment with and without local anaesthesia of the beak was compared with the behaviour during the control phase before treatment, based on (i) headings of all 15 birds on each of the various testing days and (ii) the 15 mean headings of the individual birds. The behaviour with and without anaesthesia of the upper beak was also compared. For these comparisons, we used the non-parametric Mardia–Watson–Wheeler test indicating differences in distribution (Batschelet 1981).
3. Results
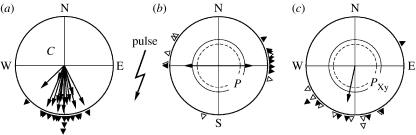
During the control phase before pulsing, the birds were well oriented in their southerly migratory directions: on each testing day, the vector based on the 15 headings is significant (p<0.001, Rayleigh test), with mean directions between 164° and 194° (table 1). There is no statistical difference between any two of these days (p>0.05, Mardia–Watson–Wheeler test). The six headings of the individual birds add up to long vectors with a median length of 0.81 and individual mean directions between 163° and 226° (figure 1a).
Table 1.
Orientation of the 15 silvereyes on the test days before and after pulsing. (C, control data obtained before pulsing; P, data obtained after pulsing without anaesthesia. Asterisks at rm indicate a significant preference by the Rayleigh test; asterisks at the differences indicate significance by the Mardia–Watson–Wheeler test. ΔC, difference from the control sample; ΔP difference from the sample after pulsing without anaesthesia. Significance levels: ***p<0.001; n.s., not significant.)
| mean vector | comparison | ||||
|---|---|---|---|---|---|
| testing day | n | αm | rm | ΔC | ΔP |
| before pulsing | |||||
| day 1 | 15 | 182° | 0.70*** | ||
| day 2 | 15 | 181° | 0.84*** | ||
| day 3 | 15 | 194° | 0.76*** | ||
| day 4 | 15 | 193° | 0.68*** | ||
| day 5 | 15 | 184° | 0.70*** | ||
| day 6 | 14 | 164° | 0.77*** | ||
| second-order mean based on 15 means of individual birds (days 1–6) | 15 | 184° | 0.95*** | C | |
| after pulsing | |||||
| without anaesthesia | 15 | 90°–270° | 0.71*** | −94°,+86° *** | P |
| beak anaesthetized | 15 | 194° | 0.74*** | +10° n.s. | −104°,+76° *** |
Figure 1.
Orientation of Australian silvereyes before and after being treated with a brief, strong magnetic pulse. (a) Orientation during the control phase before pulse treatment (C): the vectors based on the five or six recordings from each bird are shown as arrows, with the mean directions of individuals marked by triangles at the periphery of the circle. (b,c) Orientation after pulse treatment: (b) without anaesthesia (P); (c) upper beak anaesthetized with the local anaesthetic Xylocaine (PXy). The headings of the individual birds are given as triangles at the periphery of the circle, with filled triangles indicating headings obtained immediately after pulsing and open triangles indicating those obtained 2 days later. The arrows indicate the mean axis and the vector, respectively, and the two inner circles represent the 5% (dashed) and the 1% significance border of the Rayleigh test. For numerical data, see table 1, last two lines.
After the birds had been treated with the pulse, their behaviour depended on whether or not their upper beak had been locally anaesthetized: without anaesthesia, they showed a significant preference for an east–west axis, with the birds tested immediately after pulsing significantly preferring easterly directions (n=8, 78°, r=0.73, p<0.01), while the headings of the birds tested 2 days later were axially distributed (figure 1b). These directions were significantly different from that on any day before pulsing (at least p<0.01). With their upper beak anaesthetized, by contrast, the birds continued in their normal southerly migratory direction (figure 1c), and their behaviour was not different from that on any of the days before pulsing (all comparisons: p>0.05), but was significantly different from that when their beak was anaesthetized (p<0.001). A difference between the adult and the juvenile birds was not observed.
4. Discussion
Our findings clearly show that local anaesthesia of the mucous membrane inside the upper beak suppresses the effect of the magnetic pulse. The way in which we applied the anaesthetizing substance—it was not injected, but applied externally with a cotton bud to the skin without breaking it—meant that the anaesthetic could easily reach the receptors in the skin, but it is highly unlikely that it also affected any other iron-rich structures described further within the tissue of the nasal cavity (see Beason & Nichols 1984; Williams & Wild 2001). Our results thus indicate that the superparamagnetic magnetite-containing structures in the skin at the inside edge of the upper mandible described by Fleissner et al. (2003) are the magnetoreceptors that mediate the pulse effect. This is in agreement with the observation that the effect of the pulse wears off rather fast (Wiltschko, W. et al. 1994, 1998, 2007)—a finding that is hard to explain on the basis of single domains (see Wiltschko, W. et al. 2007 for discussion).
Previous experiments (e.g. Beason et al. 1995, 1997) suggest that the magnetic pulse used in the present study does not silence the receptors altogether, but rather causes them to produce altered information that induces the birds to alter their compass courses. By local anaesthesia, we prevented this false information from being provided, and the birds headed south as did the controls. Here, our findings represent a parallel to those of Beason & Semm (1996) who anaesthetized the ophthalmic nerve and found that after this treatment, their test birds, bobolinks, Dolichonyx oryzivorus, no longer showed an effect of the pulse. But while these authors disrupted the transmission through the nervous system, our approach was to stop the receptors themselves—in both cases, false information from the magnetite-based receptors did not reach the brain, and hence the pulse had no effect.
At the same time, the normal input from these receptors was also missing, but this does not seem to have caused any obvious deficits: the birds preferred their innate migratory direction and had no problem locating it with their compass.
This leads to the question of what type of magnetic information the magnetite-based receptors in the upper beak provide. The observation that the birds could continue in their normal migratory direction after pulse treatment if the receptors were deactivated (present study) or the transmission of their input by the ophthalmic nerve was disrupted (Beason & Semm 1996) indicates that they are not involved in the avian inclination compass. Munro et al. (1997) obtained results that pointed out the same: young, inexperienced migrants are not affected by the pulse, but continue to prefer their migratory direction. Even adult birds that alter their headings after pulse treatment have been shown to locate these altered headings using their inclination compass (Wiltschko, W. et al. 2006). All these studies clearly show that the inclination compass itself is not affected by the pulse. Experiments with silvereyes and European robins (not involving a pulse) also showed that the avian inclination compass works normally when the receptors in the upper beak are anaesthetized (e.g. Wiltschko, R. et al. 2007, 2008b). The inclination compass thus works independently of the magnetite-based receptors; it appears to be entirely based on radical-pair processes in the eye (Wiltschko, W. et al. 2002; Ritz et al. 2004; Thalau et al. 2005), with the respective information mediated by the optical nerve.
That leaves a role for the magnetic information from the magnetite receptors in the navigational ‘map’, the mechanism that allows birds to determine their position and hence the compass course required to reach their goal. A role of magnetite-based receptors in the map is in agreement with the finding mentioned above that young inexperienced birds are not affected by the magnetic pulse (Munro et al. 1997)—the navigational map is built from experience rather than being innate, and the young birds, having had too little opportunity to have established a map, had no baseline to interpret the input from the magnetite-based receptors, and hence had to rely on their innate compass course alone. The shift in direction observed in experienced birds after pulsing (Wiltschko, W. et al. 1994, 1998, 2006) is also compatible with an effect on the map mechanisms under the assumption that the receptors now indicate a changed location, and therefore the need for a changed compass course. The idea that these receptors provided map rather than compass information is further supported by electrophysiological studies from the ophthalmic nerve and the trigeminal ganglion, where responses to changes in magnetic intensity have been reported (Semm & Beason 1990)—the gradient in magnetic intensity running roughly from the magnetic poles to the equator would make magnetic intensity a very suitable map component at least for latitude (see Wiltschko, W. & Wiltschko, R. 2007 for discussion).
Another recently discovered oriented response of birds could also be associated with the magnetite-based receptors in the upper beak, namely the so-called ‘fixed direction’ responses of migrants. These are directional preferences that are observed under certain abnormal light regimes that appear to disrupt the normal inclination compass—they are not related to the migratory direction and do not show the normal seasonal change between spring and autumn (Wiltschko, R. et al. 2007, 2008a; Stapput et al. 2008). Although the direction of these ‘fixed’ responses depends on the ambient light—e.g. birds prefer westerly directions under dim red light and in darkness, but easterly ones under a combination of monochromatic turquoise and yellow light—these responses disappear when the upper beak is anaesthetized as in the present study (Wiltschko, R. et al. 2007, 2008b; Stapput et al. 2008). This indicates that the information that directs birds to take these ‘fixed directions’ originate in the same magnetite-based receptors in the mucous membrane inside the upper beak (see Wiltschko, R. & Wiltschko, W. 2009 for discussion of the fixed direction responses).
These findings are very surprising in view of the electrophysiological as well as the behavioural evidence mentioned above (Semm & Beason 1990; Beason & Semm 1996; Munro et al. 1997), all indicating that the magnetite-based mechanism provides information on intensity. It means that apart from their normal function, these receptors additionally may direct the birds under certain light regimes albeit in odd directions. Magnetic directions and magnetic intensity seem to be two fundamentally different magnetic parameters, which are technically measured with different instruments—directions with a compass and intensity with a magnetometer. The observation that the receptors in the upper beak in some situations also provide information that directs the birds makes the natural role of these receptors rather puzzling. As an involvement of this type of magnetic input could only be demonstrated in situations that seem to disrupt the normal magnetic inclination compass, i.e. it would not occur under natural conditions. The functional properties of the magnetite-based receptors in the beak are still controversial (Winklhofer & Kirschvink 2008), and the theoretical background is not yet developed in detail so that their specific role at present is still poorly understood. We can only hope that further experiments will reveal their normal functions in nature and how their input is integrated with that of the radical-pair-based magnetic compass in higher centres of the brain during the navigational processes of migratory birds.
Acknowledgements
The experiments were performed in accordance with the rules and regulations of animal welfare in Australia.
Our work was supported by the Human Frontier Science Program (grant to R.W.) and the Deutsche Forschungsgemeinschaft (grant to W.W.). We sincerely thank S. Debus and G. Lollback for catching the silvereyes, and F. Geiser for logistic support.
Endnotes
After the first three tests, the birds were exposed outdoors to natural sunset and sunrise. The control data here are the data published in Wiltschko, R. et al. (2008b) in relation to another question.
References
- Batschelet E. Academic Press; London, UK: 1981. Circular statistics in biology. [Google Scholar]
- Beason R.C., Brennon W.J. Natural and induced magnetization in the bobolink (Dolichonyx orycivorus) Ethology. 1986;91:75–80. [Google Scholar]
- Beason R.C., Nichols J.E. Magnetic orientation and magnetically sensitive material in a transequatorial migratory bird. Nature. 1984;309:151–153. doi:10.1038/309151a0 [Google Scholar]
- Beason R.C., Semm P. Does the avian ophthalmic nerve carry magnetic navigational information? J. Exp. Biol. 1996;199:1241–1244. doi: 10.1242/jeb.199.5.1241. [DOI] [PubMed] [Google Scholar]
- Beason R.C., Dussourd N., Deutschlander M. Behavioural evidence for the use of magnetic material in magnetoreception by a migratory bird. J. Exp. Biol. 1995;198:141–146. doi: 10.1242/jeb.198.1.141. [DOI] [PubMed] [Google Scholar]
- Beason R.C., Wiltschko R., Wiltschko W. Pigeon homing: effects of magnetic pulse on initial orientation. Auk. 1997;114:405–415. [Google Scholar]
- Davila A.F., Fleissner G., Winklhofer M., Petersen N. A new model for a magnetoreceptor in homing pigeons based on interacting clusters of superparamagnetic magnetite. Phys. Chem. Earth. 2003;28:647–652. doi:10.1016/S1474-7065(03)00118-9 [Google Scholar]
- Davila A.F., Winklhofer M., Shcherbakov V.P., Petersen N. Magnetic pulse affects a putative magnetoreceptor mechanism. Biophys. J. 2005;89:56–63. doi: 10.1529/biophysj.104.049346. doi:10.1529/biophysj.104.049346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds D.T. A magnetite null detector as the migrating bird's compass. Proc. R. Soc. B. 1992;249:27–31. doi:10.1098/rspb.1992.0079 [Google Scholar]
- Fleissner G., Holtkamp-Rötzler E., Hanzlik M., Winklhofer M., Fleissner G., Petersen N., Wiltschko W. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J. Comp. Neurol. 2003;458:350–360. doi: 10.1002/cne.10579. doi:10.1002/cne.10579 [DOI] [PubMed] [Google Scholar]
- Fleissner G., Stahl B., Thalau P., Falkenberg G., Fleissner G. A novel concept of Fe-mineral-based magnetoreception: histological and physicochemical data from the upper beak in homing pigeons. Naturwissenschaften. 2007;94:631–642. doi: 10.1007/s00114-007-0236-0. doi:10.1007/s00114-007-0236-0 [DOI] [PubMed] [Google Scholar]
- Hanzlik M., Heunemann C., Holzkamp-Rötzler E., Winklhofer M., Petersen N., Fleissner G. Superparamagnetic magnetite in the upper beak tissue of homing pigeons. BioMetals. 2000;13:325–331. doi: 10.1023/a:1009214526685. doi:10.1023/A:1009214526685 [DOI] [PubMed] [Google Scholar]
- Kirschvink J.L. Magnetite biomineralization and geomagnetic sensitivity in higher animals: an update and recommendation for future study. Bioelectromagnetics. 1989;10:239–259. doi: 10.1002/bem.2250100304. doi:10.1002/bem.2250100304 [DOI] [PubMed] [Google Scholar]
- Kirschvink J.L., Gould J.L. Biogenetic magnetite as a basis for magnetic field detection in animals. BioSystems. 1981;13:181–201. doi: 10.1016/0303-2647(81)90060-5. doi:10.1016/0303-2647(81)90060-5 [DOI] [PubMed] [Google Scholar]
- Kirschvink J.L., Walker M.M. Particle-size considerations for magnetite-based magnetoreceptors. In: Kirschvink J.L., Jones D.S., MacFadden B.J., editors. Magnetite biomineralization and magnetoreception in organisms. Plenum Press; New York, NY: 1985. pp. 243–256. [Google Scholar]
- Kirschvink J.L., Jones D.S., MacFadden B.J., editors. Magnetite biomineralization and magnetoreception in organisms. Plenum Press; New York, NY: 1985. pp. 647–669. [Google Scholar]
- Lane S.G., Battam H. Silvereye movements in eastern Australia. Aust. Bird Bander. 1971;9:80–82. [Google Scholar]
- Lowenstam H.A. Magnetite in denticles capping in recent chitons (Polyplacophora) Geol. Soc. Am. Bull. 1962;73:435–438. doi:10.1130/0016-7606(1962)73[435:MIDCIR]2.0.CO;2 [Google Scholar]
- Munro U., Munro J.A., Phillips J.B., Wiltschko R., Wiltschko W. Evidence for a magnetite-based navigational ‘map’ in birds. Naturwissenschaften. 1997;84:26–28. doi:10.1007/s001140050343 [Google Scholar]
- Ritz T., Thalau P., Phillips J.B., Wiltschko R., Wiltschko W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature. 2004;429:177–180. doi: 10.1038/nature02534. doi:10.1038/nature02534 [DOI] [PubMed] [Google Scholar]
- Semm P., Beason R.C. Responses to small magnetic variation by the trigeminal system of the bobolink. Brain Res. Bull. 1990;25:735–740. doi: 10.1016/0361-9230(90)90051-z. doi:10.1016/0361-9230(90)90051-Z [DOI] [PubMed] [Google Scholar]
- Shcherbakov V.P., Winklhofer M. The osmotic magnetometer: a new model for magnetite-based magnetoreceptors in animals. Eur. Biophys. J. 1999;28:380–392. doi:10.1007/s002490050222 [Google Scholar]
- Solov'yov I.A., Greiner W. Theoretical analysis of an iron mineral-based magnetoreceptor model in birds. Biophys. J. 2007;93:1493–1509. doi: 10.1529/biophysj.107.105098. doi:10.1529/biophysj.107.105098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, B., Fleissner, G., Fleissner, G. & Falkenbegr, G. 2007 Cross-species unveiling of a putative avian magnetoreceptor, pp. 1269–1270. HASYLAB annual report 2006, Desy, Hamburg, Germany.
- Stapput K., Thalau P., Wiltschko R., Wiltschko W. Orientation of birds in total darkness. Curr. Biol. 2008;18:602–606. doi: 10.1016/j.cub.2008.03.046. doi:10.1016/j.cub.2008.03.046 [DOI] [PubMed] [Google Scholar]
- Thalau P., Ritz T., Stapput K., Wiltschko R., Wiltschko W. Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwissenschaften. 2005;92:86–90. doi: 10.1007/s00114-004-0595-8. doi:10.1007/s00114-004-0595-8 [DOI] [PubMed] [Google Scholar]
- Tian L., Xiao B., Lin W., Zhang S., Zhu R., Pan Y. Testing for the presence of magnetite in the upper-beak skin of homing pigeons. BioMetals. 2007;20:197–203. doi: 10.1007/s10534-006-9027-x. doi:10.1007/s10534-006-9027-x [DOI] [PubMed] [Google Scholar]
- Walker M.M. A model for encoding magnetic field intensity by magnetite-based magnetoreceptor cells. J. Theor. Biol. 2008;250:85–91. doi: 10.1016/j.jtbi.2007.09.030. doi:10.1016/j.jtbi.2007.09.030 [DOI] [PubMed] [Google Scholar]
- Williams M.N., Wild J.M. Trigeminally innervated iron-containing structures in the beak of homing pigeons and other birds. Brain Res. 2001;889:243–246. doi: 10.1016/s0006-8993(00)03114-0. doi:10.1016/S0006-8993(00)03114-0 [DOI] [PubMed] [Google Scholar]
- Wiltschko, R. & Wiltschko, W. 2009 ‘Fixed direction’-responses of birds in the geomagnetic field. Commun. Integr. Biol.2 100–103. See http://www.landesbioscience.com/journals/cib/article/7622 [DOI] [PMC free article] [PubMed]
- Wiltschko R., Stapput K., Ritz T., Thalau P., Wiltschko W. Magnetoreception in birds: different physical processes for different types of directional responses. HFSP J. 2007;1:41–48. doi: 10.2976/1.2714294. doi:10.2976/1.2714294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko R., Munro U., Ford H., Stapput K., Wiltschko W. Light-dependent magnetoreception: orientation behaviour of migratory birds under dim red light. J. Exp. Biol. 2008a;211:3344–3350. doi: 10.1242/jeb.020313. doi:10.1242/jeb.020313 [DOI] [PubMed] [Google Scholar]
- Wiltschko R., Munro U., Ford H., Wiltschko W. Contradictory results on the role of polarized light in compass calibration in migratory songbirds. J. Ornithol. 2008b;149:607–614. doi:10.1007/s10336-008-0324-8 [Google Scholar]
- Wiltschko W., Wiltschko R. Magnetoreception in birds: two receptors for two different tasks. J. Ornithol. 2007;148(Suppl. 1):S61–S76. doi:10.1007/s10336-007-0233-2 [Google Scholar]
- Wiltschko W., Munro U., Beason R.C., Ford H., Wiltschko R. A magnetic pulse leads to a temporary deflection in the orientation of migratory birds. Experientia. 1994;50:697–700. doi:10.1007/BF01952877 [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. Effect of a magnetic pulse on the orientation of silvereyes, Zosterops l. lateralis, during spring migration. J. Exp. Biol. 1998;201:3257–3261. doi: 10.1242/jeb.201.23.3257. [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Traudt J., Güntürkün O., Prior H., Wiltschko R. Lateralisation of magnetic compass orientation in a migratory birds. Nature. 2002;419:467–470. doi: 10.1038/nature00958. doi:10.1038/nature00958 [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. Bird navigation: what type of information does the magnetite-based receptor provide? Proc. R. Soc. B. 2006;273:2815–2820. doi: 10.1098/rspb.2006.3651. doi:10.1098/rspb.2006.3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko W., Ford H., Munro U., Winklhofer M., Wiltschko R. Magnetite-based magnetoreception: the effect of repeated pulsing on the orientation of migratory birds. J. Comp. Physiol. A. 2007;193:515–522. doi: 10.1007/s00359-006-0207-5. doi:10.1007/s00359-006-0207-5 [DOI] [PubMed] [Google Scholar]
- Winklhofer, M. & Kischvink, J. L. 2008 Does avian magnetoreception rely on both magnetite and maghemite? Phys. Biol. Phys. See http://arxiv.org/abs/0805.2249
- Winklhofer M., Holtkamp-Rötzler E., Hanzlik M., Fleissner G., Petersen N. Clusters of superparamagnetic magnetite particles in the upper-beak skin of homing pigeons: evidence of a magnetoreceptor? Eur. J. Miner. 2001;13:659–669. doi:10.1127/0935-1221/2001/0013-0659 [Google Scholar]
- Yorke E.D. A possible magnetic transducer in birds. J. Theor. Biol. 1979;77:101–105. doi: 10.1016/0022-5193(79)90140-1. doi:10.1016/0022-5193(79)90140-1 [DOI] [PubMed] [Google Scholar]
- Yorke E.D. Sensitivity of pigeons to small magnetite field variations. J. Theor. Biol. 1981;89:533–537. doi: 10.1016/0022-5193(81)90367-2. doi:10.1016/0022-5193(81)90367-2 [DOI] [PubMed] [Google Scholar]