Abstract
In a hypothesis that has remained controversial since its inception, Darwin suggested that long-tubed flowers and long-tongued pollinators evolved together in a coevolutionary race, with each selecting for increasing length in the other. Although the selective pressures that flowers impose on tongue length are relatively straightforward, in that longer tongues allow access to more nectar, selective pressures that pollinators impose on flower length are less clear. Here, we test for such selective pressures in the highly specialized mutualism between the nectar bat Anoura fistulata, which can extend its tongue twice as far as other nectar bats, and Centropogon nigricans, which has flowers of a similar length (8–9 cm). We used flight cage experiments to examine the effects of artificially manipulated flower lengths on (i) bat behaviour and (ii) pollen transfer. Increased length produced longer visits, but did not affect the force bats applied during visits. In the second experiment, flower length increased both the male and female components of flower function: long male flowers delivered more pollen grains and long female flowers received more pollen grains. However, pollen transfer was not correlated with visit duration, so the mechanism behind differences in pollen transfer remains unclear. By demonstrating that bats select for increasing flower length, these results are consistent with the hypothesis that A. fistulata evolved its remarkable tongue in a coevolutionary race with long-tubed flowers similar to that envisioned by Darwin.
Keywords: Anoura fistulata, Centropogon nigricans, chiropterophily, bat pollination, coevolution, Darwin's race
1. Introduction
Numerous animal taxa have adapted to a nectarivorous lifestyle, including clades of bees, flies, moths, birds and bats (Pijl 1961; Stebbins 1970; Fenster et al. 2004; Fleming & Muchhala 2008). Elongated mouthparts are one of the most consistent and obvious adaptations to this mode of feeding. For nearly all of these radiations, there exists an outlier species, with extremely long mouthparts, that visit plants with flowers of a corresponding length. Examples include the sword-billed hummingbird (Ensifera ensifera) with a 10 cm long bill (Snow & Snow 1980), the mega-nosed fly (Moegistorynchus longirostris) with a 5.7 cm long proboscis (Johnson & Steiner 1997) and the giant hawkmoth (Xanthopan morgani praedicta) with a 25 cm long proboscis (Nilsson et al. 1985).
Darwin (1862) and Wallace (1867) provided a possible explanation for such extreme elongation, suggesting that the long nectar spur of the Malagasy star orchid (Angraecum sesquipedale) evolved in a coevolutionary race with a giant hawkmoth. According to this model, selection on the hawkmoth favours longer tongues to better reach the orchid's nectar, while selection on the orchid favours nectar spurs that are longer than hawkmoth tongues because this ensures contact with the orchid's reproductive parts (thus maximizing pollen transfer).
In this study, we test the hypothesis that a similar coevolutionary race has occurred in a highly specialized bat–flower mutualism. The nectar bat Anoura fistulata has evolved the ability to extend its tongue to 8.5 cm (150% of its body length; Muchhala 2006a), which is nearly double the tongue extension of any other nectar bat (Winter & von Helversen 2003). Novel adaptations in tongue morphology enable this bat to store a large portion of the tongue in its thoracic cavity. An important component of this bat's diet is flowers of Centropogon nigricans (Campanulaceae), which have 8–9 cm long corollas and are pollinated exclusively by A. fistulata (Muchhala 2006a). Could coevolution with C. nigricans or similar long-tubed flowers have led to the extreme tongue length of A. fistulata?
Several studies have provided support for Darwin's hypothesis by demonstrating instances of pollinators imposing directional selection on flower length (Nilsson 1988; Johnson & Steiner 1997; Alexandersson & Johnson 2002; Anderson & Johnson 2008). However, the hypothesis has also met with resistance since it was first published, and various alternative models have been proposed. One idea is that matches between plant and pollinator lengths do not, in fact, entail pairwise coevolution, but rather one-sided plant evolution, with plants switching between pollinators and then adapting to their already-established tongue lengths (Wasserthal 1997; Whittall & Hodges 2007). Although such shifts between pollinators could well account for the majority of cases of floral tube elongation (e.g. Whittall & Hodges 2007; Wilson et al. 2007; Tripp & Manos 2008), they cannot explain the extreme cases because they cannot account for instances of flowers or tongues continuing to evolve beyond that of existing mutualist partners. That is, the longest flower in a habitat would not continue to evolve to extreme lengths because no pollinators would exist to ‘shift’ to. In fact, Darwin was not proposing a general mechanism for the evolution of corolla tube length, but was specifically interested in the extreme case of A. sesquipedale (Darwin 1862). Furthermore, in expounding on Darwin's idea, Wallace (1867) actually envisioned that initial stages in tube elongation would involve pollinator shifts, and suggested that a coevolutionary race would begin only when the tube length corresponded to the tongue length of the largest hawkmoth in the habitat.
Wasserthal (1997) suggested a possible alternative explanation for such extreme cases. He posited that the ‘driving force’ for elongation involves the evolution of longer hawkmoth tongues to better evade predators, rather than coevolution between plant and pollinator. This model is unlikely to apply to the mutualism between A. fistulata and C. nigricans because of the differences in the mechanics of nectar feeding in bats and moths. A moth's proboscis functions as a straw and needs to be fully extended before it is inserted in a flower. Nectar bats such as Anoura, on the other hand, lap nectar through rapid extensions and retractions of the tongue (Winter & von Helversen 2003). Thus, even while visiting short flowers, A. fistulata fully inserts its head and then extends its tongue only as needed to reach the nectar (N. Muchhala 2005, personal observation). Clearly, the long tongue does not allow it to avoid flower-based predators.
Another possible alternative hypothesis involves selective pressure for plants to specialize on pollinators (Belt 1874; Rodríguez-Gironés & Santamaría 2007; Rodríguez-Gironés & Llandres 2008). Specialization may have been important in the initial stages of this mutualism; that is, a hypothetical progenitor to C. nigricans with a short corolla tube may have faced initial selective pressure to elongate in order to prevent other bats from visiting. However, other bats can only extend their tongues to 4 cm; once the corolla tube had reached 6 cm, these would have been effectively excluded. Further elongation from 6 to 8.5 cm cannot be explained by selective pressure to specialize.
Thus, the pollinator shift, predator avoidance and specialization models are unlikely explanations for the extreme lengths observed in this bat–flower mutualism. To test the plausibility of Darwin's model, here we explore the plant side of the putative coevolutionary race. Selective pressures on bat tongue length are relatively straightforward, in that longer tongues would allow access to more nectar. However, what selective benefits would the plant gain in evolving a longer floral tube? As mentioned above, our system differs from that of Darwin's hawkmoth and orchid in the mechanics of flower feeding, in that bats fully insert their heads in all flowers and then extend the tongue only as needed. Thus, possessing a tongue longer than the corolla does not preclude contact with the flower's reproductive parts, and bats (unlike hawkmoths) can potentially effectively pollinate short flowers. However, we hypothesize that corolla length may still positively correlate with pollen transfer by increasing either the duration of bat visits or the force of contact with the flower parts, especially if long corollas place some of the nectar just out of reach. To evaluate these hypotheses, we conducted two sets of controlled flight cage experiments, designed to test the effects of flower length on (i) bat behaviour and (ii) pollen transfer.
2. Material and methods
Centropogon nigricans is endemic to the western slopes of the Andes of Ecuador (Jeppesen 1981). Flowers open at dusk and emit a strong, musky odour throughout anthesis. Corollas are bilaterally symmetrical, with nectar held at the base of 8–9 cm corolla tubes. Reproductive parts are exserted and positioned above the corolla opening to place pollen on, and receive it from, the foreheads of bats (figure 1b). Centropogon and related genera exhibit ‘secondary pollen presentation’ (Erbar & Leins 1995); the five anthers are fused together into an anther tube, which holds the pollen and dispenses it through a distal opening during pollinator visits. Flowers are protandrous; the male phase lasts for several days as the stigma slowly elongates within the tube (pushing pollen towards the distal opening). The female phase begins on the third or fourth night, when the stigma emerges from the distal end of the tube, unfolds and becomes receptive.
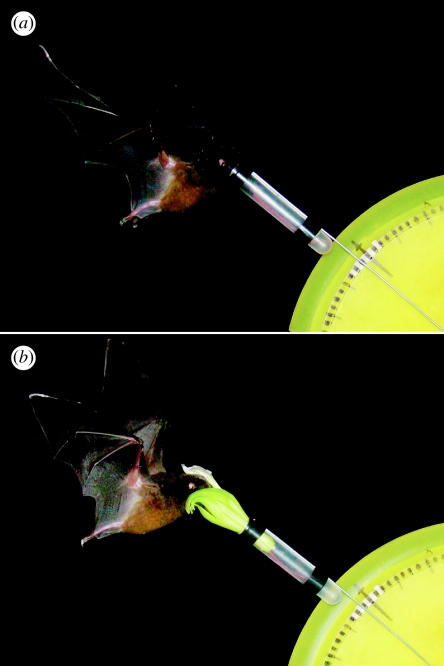
Figure 1.
Photos of the flight cage experiments. (a) A bat visiting a 73 mm tube in experiment 1, which was designed to test the effects of flower length on bat behaviour. Note the plate positioned behind the flower to measure visit force in degrees. (b) A bat visiting an artificially lengthened female flower in experiment 2, which was designed to test the effects of flower length on pollen transfer.
We conducted research from September 2007 to March 2008 in the Bellavista Cloud Forest Reserve (elev. 2000–2400 m.a.s.l.), which is located in the Pichincha Province of Ecuador (00°01′ S, 78°41′ W). We captured four individuals of A. fistulata with mist nets and placed them in separate flight cages (3×3×2 m screen tents). All were released after the study. On the night a bat was captured, we allowed it to acclimatize to the cage and drink freely from a test tube filled with a sugar-water solution (approx. 20% sucrose). On the following four nights, we conducted two sets of experiments, the first involving test tubes of varying lengths and the second involving real flowers with artificially manipulated corolla lengths. We videotaped all experiments with Sony digital camcorders using nightshot mode.
(a) Experiment 1: effects of flower length on bat behaviour
We videotaped bats visiting six different lengths of artificial ‘flowers’ to explore how a broad range of lengths affects pollinator behaviour in terms of visit duration and force applied during visits. We cut polypropylene test tubes (12×75 mm, 5 ml; BD Falcon, Franklin Lakes, NJ) as needed to make five lengths that differed from each other by 10 mm. The actual internal diameter of the tubes was 10.6 mm and internal length was 73.0 mm, thus the functional lengths of the five tubes were 73, 63, 53, 43 and 33 mm. Tubes were affixed to a wire ‘stem’ and, for the latter four lengths, leftover portions were also affixed behind the tube in order to minimize potential effects of weight differences on the force measurements. We made an additional length (83 mm) by adding a 10 mm portion to a sixth tube. In the flight cage, we positioned tubes on a bench 1 m above the ground. As visiting bats strove to reach the nectar, their push into the flower was transduced, at least partially, into angular displacement of the wire. To measure this displacement, we made a protractor-like scale from a plastic plate mounted behind the tube, with marks along the edge at every 2.81° (32 marks per 90°; figure 1a). Before each trial, we aligned the wire stem with the 45° mark. This allowed us to estimate the maximum instantaneous force exerted during the visit in terms of the maximum displacement from 45°. We presented bats with individual tubes (each containing 0.1 ml of sugar-water) in blocks of six, with the order of lengths randomized. We ran a total of 20 trials for each tube length and each of the four bat subjects, for a grand total of 480 trials. During slow-motion replay of the videos, we recorded the maximum displacement in degrees and the total visit duration in seconds. Given that the repetitions for each bat are not independent, data analyses were conducted using the means for each bat (i.e. n=4 observations per tube length). We conducted an ANOVA to test for effects of length on the two response variables, with a linear post hoc analysis to test the prediction that increasing length corresponds with increasing visit force and visit duration.
(b) Experiment 2: effects of flower length on pollen transfer
For the second set of experiments, we used the same experimental set-up described above, except that we placed fresh flowers in the tubes (figure 1b). We collected male- and female-phase flowers of C. nigricans at dusk on the day of, or the day before, experiments. We cut flowers approximately 55 mm below the corolla opening (30 mm above the corolla base) and placed them in test tubes of two different lengths: 73 or 33 mm. Flowers could be readily swapped between test tubes since they fitted the tubes snugly, without the need for adhesives. Within blocks of experimental trials (see below), we used the same flower in the different tubes, thus allowing only corolla length to vary between the ‘short’ and ‘long’ flowers. Corolla lengths in nature are 80–90 mm, so the 33 mm test tubes artificially shortened corollas to approximately 70 mm, and the 73 mm test tubes artificially lengthened corollas to approximately 110 mm. We chose this length because bats typically push their heads approximately 25 mm into C. nigricans corollas during visits; thus, given a mean maximum tongue extension of 85 mm (Muchhala 2006a), bats should just be able to reach nectar in the artificially lengthened flowers.
An experimental block comprised four trials. For each trial, a bat was presented with a set of three flowers: first a male (pollen donor); then a female of one length (first pollen recipient); then a female of the other length (second pollen recipient). We covered the reproductive parts (stigma and anther tube) of the female flowers with a layer of parafilm and placed a small piece of double-sided tape (12.5×5 mm) on the end to collect pollen transferred. Reusing the same three flowers, we ran randomized blocks of four trials for the four possible combinations of short (S) and long (L) flowers (i.e. S–L–S, S–S–L, L–L–S and L–S–L). Prior to each visit, we stocked the plastic corollas with 0.1 ml of sugar-water each, and gently squeezed the anther tube of the male flower to push pollen to the distal end of the tube. The same male flower could often be used for multiple experimental blocks, given the large amount of pollen contained in the anther tube. Following each trial, the tape samples were collected from female flowers, placed on a microscope slide and covered with single-sided tape. We placed new tape ‘stigmas’ on the two female flowers, and swapped the flowers among the artificial corollas so as to complete a block of four trials, in random order.
Pollen transfer was estimated by using a light microscope to count pollen grains along a vertical and horizontal transect through the centre of the tape samples. For data analysis, we calculated the following four response variables for each block of four trials: total pollen delivered by the two L males (each delivered pollen to two stigmas, a long and a short); similarly, total pollen delivered by the two S males; total pollen received by the four L females; and total pollen received by the four S females. We conducted 10 replicates of the blocks for each of the four bats, for a grand total of 160 experimental trials (480 single visits). To improve normality, the raw pollen counts were transformed by adding 0.5 and taking the square root. Again, data analyses were conducted using the means for each bat, thus n=4. Paired t-tests were used to determine whether corolla length (L versus S) of a male flower affected the amount of pollen it delivered and whether the corolla length of a female flower affected the amount of pollen it received.
These experimental trials were also videotaped in order to document visit duration and visit force, as described above for experiment 1. We pooled data from all flower visits (i.e. males, first females and second females) to test the effects of flower length (long versus short) on duration (n=462) and force (n=347). Although the experimental trials included 480 visits, these sample sizes are lower because occasional videotape errors precluded obtaining information on duration and/or force. We also tested for correlations between the amount of pollen transferred to female flowers and visit duration or force, using all data as well as subsets based on bat individual and flower type.
3. Results
(a) Experiment 1: effects of flower length on bat behaviour
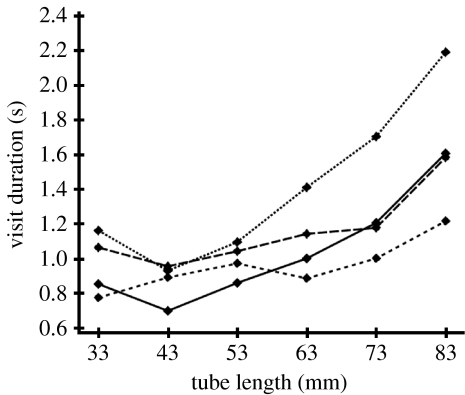
Although there was a trend towards increased visit force at longer tubes, this was only marginally significant (F5,18=2.36, p=0.08). Visit duration, on the other hand, varied with tube length (F5,18=5.37, p=0.03; figure 2). A post hoc linear contrast test showed a significant linear component (F=21.48, p<0.001). The shortest tubes (33 mm) received a mean visit duration of 0.93 (±0.18 s.d.; n=4). Over the following lengths, visit duration increased steadily from a mean of 0.87 (±0.12 s.d.) for 43 mm tubes to 1.55 (±0.40 s.d.) for 83 mm tubes.
Figure 2.
Mean visit duration (s) of four A. fistulata individuals to test tubes of six different lengths (dotted line, bat A; small-dashed line, bat B; dashed line, bat C; solid line, bat D).
(b) Experiment 2: effects of flower length on pollen transfer
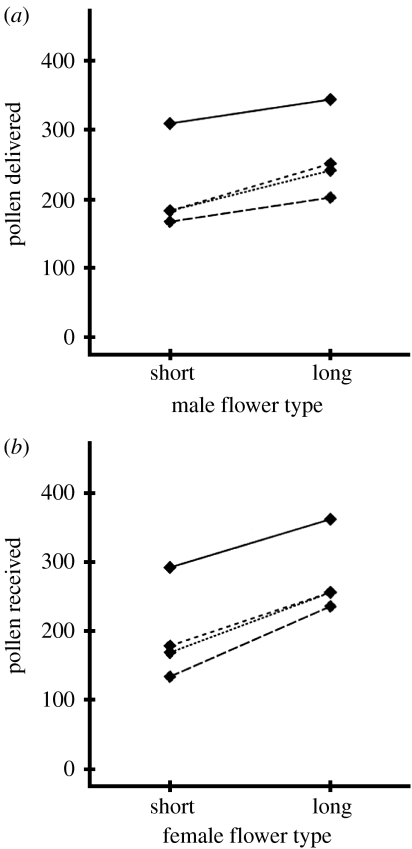
Long flowers both delivered and received more pollen than short flowers. As a component of male function, long male flowers delivered more pollen than short male flowers by a factor of 1.23 (figure 3a; paired t-test: t=5.74, d.f.=3, p=0.0105). As a component of female function, long female flowers received more pollen than short female flowers by a factor of 1.44 (figure 3b; paired t-test: t=9.167, d.f.=3, p=0.0027). The fitness advantage of long flowers is not context dependent; that is, the increase in pollen delivered by long male flowers is similar when long and short recipients are analysed separately, and the increase in pollen received by long female flowers is similar when long and short donors are analysed separately (see fig. S1 in the electronic supplementary material). Of the four bat individuals, bat D transferred much more pollen than the other three (figure 3a,b). Although we cannot rule out the possibility that this difference is due to unmeasured behavioural differences, it is probably attributable to bat D having a shorter tongue. We measured tongue extension by noting the maximum depth bats could access nectar in a modified straw (sensu Muchhala 2006a): bat D reached only 75.4 mm while the other bats reached 87.8, 88.0 and 88.1 mm (A, B and C, respectively). Thus, for bat D, short flowers may have been functionally equivalent to long flowers for the other bats.
Figure 3.
Effects of flower length on pollen transfer by A. fistulata in flight cage experiments. (a) Mean number of pollen grains delivered by short and long male flowers and (b) mean number of pollen grains received by short and long female flowers (dotted line, bat A; small-dashed line, bat B; dashed line, bat C; solid line, bat D).
Visits to long flowers lasted 2.37 s (±0.09 s.e.), while visits to short flowers lasted 1.3 s (±0.05). This represents a significant difference (t=10.1, d.f.=461, p<0.001) and suggests that the observed differences in pollen transfer may be due to longer visits to long flowers. However, no correlation was found between visit duration and amount of pollen received by a female flower, either when all results were pooled (r=−0.062, n=314, p=0.27) or when analysed separately by bat individual and flower type (results not shown; of 16 regressions of pollen receipt on visit duration, slopes of regression lines were positive for 7, negative for 9 and none showed a significant Pearson correlation). Visits to long flowers exerted more force (5.35°±1.33 s.d.) than those to short flowers (4.38°±0.98; t=7.92, d.f.=347, p<0.001). However, as with visit duration, visit force did not correlate with pollen transfer when all results were pooled (r=−0.05, n=236, p=0.44) or broken down into bat and treatment combinations (results not shown). Thus, the observed differences in pollen transfer are not explained by either visit duration or visit force.
4. Discussion
This study found a selective advantage to increased corolla length for C. nigricans flowers pollinated by A. fistulata. This advantage was detected for both the male and the female components of fitness. Long male flowers exported more pollen than short male flowers by a factor of 1.23 (figure 3a), and long female flowers received more pollen than short female flowers by a factor of 1.44 (figure 3b). These results are consistent with the hypothesis that the extreme tongue length of A. fistulata evolved with long-tubed flowers in a coevolutionary race as envisioned by Darwin (1862) for hawkmoths and star orchids. As floral tube lengths increased over evolutionary time to maximize pollen transfer, tongue lengths of A. fistulata would be expected to show a concurrent evolutionary increase because exploitative competition would favour long-tongued individuals. Exploitative competition has been shown to have a strong effect on nectar bats in an experimental setting (von Helversen & Winter 2003). When two species of different sizes shared the same limited nectar resource, individuals of the species with larger body size (which require larger amounts of nectar to meet their daily energetic needs) declined in body weight while those of the smaller species were unaffected. In a similar fashion, A. fistulata individuals with longer tongues would be favoured since they can reach nectar not accessible to other bats.
The controlled experimental design of this study implies that differences in bat behaviour must be responsible for the observed differences in pollen transfer. However, the exact mechanism remains unclear. The two behavioural components tested here, visit duration and visit force, did not explain variation in pollen transfer. Although visit duration to the six artificial flowers significantly increased with tube length (figure 2), visit duration to the actual flowers in the second experiment did not correlate with pollen transfer. The force that bats applied during visits neither depended on tube length for artificial flowers nor did it correlate with pollen deposition for actual flowers. However, observations suggest that another component of force, not explicitly measured in this study, may be the actual behavioural difference affecting pollen transfer. We quantified force as the displacement in degrees of the stem; this may measure only the component of force perpendicular to the main axis of the flower, rather than the force along that axis with which the bat pushes into the flower. With longer flowers, bats should push into the flower to better reach the nectar, thereby picking up and depositing more pollen. In fact, the videos showed that the bats frequently pushed their heads further into long tubes (past their eyes), while a larger portion of the head and snout typically remained outside of the tube during visits to shorter flowers. Further studies would be useful to isolate this component of force and test its effect on pollen transfer.
We suggest that the critical first step in the coevolution of this remarkable bat–flower mutualism involved the evolution of the unique thoracic glossal tube of A. fistulata. Other nectar bats store their tongues in their jaws, so that maximum tongue extension is constrained by jaw length (Winter & von Helversen 2003; Muchhala 2006a). By allowing the bat to store a large portion of its tongue in its rib cage, the glossal tube freed A. fistulata from this functional constraint and allowed further evolution of tongue length. Because other species do not possess glossal tubes, this allowed a relatively exclusive interaction to evolve between A. fistulata and C. nigricans. Although A. fistulata also feeds from other flowers, C. nigricans is pollinated exclusively by A. fistulata (Muchhala 2006a). It has long been recognized that coevolution should occur most readily in tight, specialized interactions (Thompson 1994). In fact, in discussing the evolution of the Malagasy star orchid, Wallace (1867) pointed out that coevolution would not be expected to occur until the orchid had evolved a spur long enough to prevent all but the hawkmoth with the longest tongue from visiting it.
Once the glossal tube evolved, initial pressure selecting for corolla tube elongation in C. nigricans may have involved competition with other plants. Centropogon nigricans places its pollen on the forehead of bats, the same site that is used extensively by the many and abundant species of Burmeistera (Campanulaceae) in the Andean cloud forests (Muchhala 2006b). Although Burmeistera flowers are an important part of the diet of other species of nectar bats (Muchhala 2008), A. fistulata do not visit them. Thus, by evolving a longer corolla and specializing on A. fistulata, C. nigricans would reduce pollen loss to Burmeistera stigmas and possible stigma blockage by Burmeistera pollen (see Muchhala & Potts 2007). Since other nectar bats lack glossal tubes, they could not respond by evolving longer tongues. Thus, the key innovation of a glossal tube was critical not only in allowing A. fistulata to increase tongue length, but in preventing other nectar bats from following suit. Once the mutualism between A. fistulata and C. nigricans was sufficiently exclusive, a coevolutionary race could begin, leading to the remarkable lengths observed today.
In conclusion, this study provides support for a coevolutionary race in this extremely specialized bat–flower mutualism. Empirical evidence consistent with the hypothesis has also been found for moth–flower (Nilsson 1988; Alexandersson & Johnson 2002) and fly–flower systems (Johnson & Steiner 1997; Anderson & Johnson 2008). However, the tightly controlled experimental setting used in the present study provides especially strong support by avoiding potential confounding variables. For example, by allowing only one visit per flower, this study avoids the possibility (e.g. Nilsson 1988; Johnson & Steiner 1997) that responses of pollen deposition to corolla-length manipulation may actually be due to differences in visitation rate (because shortening tubes by tying them off makes flowers less attractive or renders nectar inaccessible). Similarly, a positive correlation between natural variation in tube length and resulting seed set (e.g. Alexandersson & Johnson 2002; Anderson & Johnson 2008) could be due to unmeasured covariates (those plants with larger flowers also produce more nectar, larger display size, more ovules, etc.; see Harder et al. 1985). The experimental design used here also allowed exploration of the effects of flower length on pollinator behaviour. Future studies would be useful to clarify the mechanism behind differences in pollen transfer and to explore broader patterns of covariation in lengths across the geographical distributions of these coevolving mutualists (sensu Anderson & Johnson 2008).
The present results, together with previous work (Nilsson 1988; Johnson & Steiner 1997; Alexandersson & Johnson 2002; Anderson & Johnson 2008), suggest that coevolutionary races may have played a central role in the evolution of extreme plant–pollinator lengths. While such extreme cases are probably quite rare, given the pervasiveness of multi-species rather than pairwise interactions in plant–pollinator mutualisms (Waser et al. 1996; Fenster et al. 2004), we argue that they have been a critical feature of the overall evolution of flower and pollinator niches. Nectar-feeding taxa consistently possess longer mouthparts than sister taxa with different feeding habits. Thus, for each nectar-feeding group, there has been an evolutionary increase in the range of mouthpart sizes, with the upper bound increasing over time. Although pollinator shifts among plants (or flower shifts among pollinators) may explain length evolution for the majority of less extreme cases of tube or tongue length evolution (Wasserthal 1997; Whittall & Hodges 2007), they cannot explain the evolution of the extreme lengths of this upper bound (because no pollinators exist beyond the bound to shift to). Coevolutionary races in the extreme outliers, such as A. fistulata and C. nigricans, may have been critical in extending this range of lengths.
Acknowledgments
We thank Scott Armbruster, Theodore H. Fleming, Mario Vallejo-Marín, Steven Johnson and an anonymous reviewer for their comments on the manuscript, Juan Carlos Vizuete, Kylie O'Neill and Daniela Proaño for their assistance in the field, Barbara Thomson for help with statistical analyses and Bat Conservation International for financial support.
This research was approved by the Ministry of the Environment of Ecuador (permit no. 007-2007-IC-FLO/FAU-DRFP/MA).
Supplementary Material
Effects of flower length on pollen transfer by Anoura fistulata in flight cage experiments, with data further subdivided by length of donor/recipient.
References
- Alexandersson R., Johnson S.D. Pollinator-mediated selection on flower-tube length in a hawkmoth-pollinated Gladiolus (Iridaceae) Proc. R. Soc. Lond. B. 2002;269:631–636. doi: 10.1098/rspb.2001.1928. doi:10.1098/rspb.2001.1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B., Johnson S.D. The geographical mosaic of coevolution in a plant–pollinator mutualism. Evolution. 2008;62:220–225. doi: 10.1111/j.1558-5646.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Belt T. University of Chicago Press; Chicago, IL: 1874. The naturalist in Nicaragua. [Google Scholar]
- Darwin C.R. Murray; London, UK: 1862. On the various contrivances by which British and foreign orchids are fertilized. [PMC free article] [PubMed] [Google Scholar]
- Erbar C., Leins P. Portioned pollen release and the syndromes of secondary pollen presentation in the Campanulales–Asterales-complex. Flora. 1995;190:323–338. [Google Scholar]
- Fenster C.B., Armbruster W.S., Wilson P., Dudash M.R., Thomson J.D. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 2004;35:375–403. doi:10.1146/annurev.ecolsys.34.011802.132347 [Google Scholar]
- Fleming T.H., Muchhala N. Nectar-feeding bird and bat niches in two worlds: pantropical comparisons of vertebrate pollination systems. J. Biogeogr. 2008;35:764–780. doi:10.1111/j.1365-2699.2007.01833.x [Google Scholar]
- Harder L.D., Thomson J.D., Cruzan M., Unnasch R. Sexual reproduction and variation in floral morphology in an ephemeral vernal lily, Erythronium americanum. Oecologia. 1985;67:286–291. doi: 10.1007/BF00384301. doi:10.1007/BF00384301 [DOI] [PubMed] [Google Scholar]
- Jeppesen, S. 1981 Lobeliaceae. In Flora of Ecuador, vol. 14, pp. 12–48. Stockholm, Sweden: Swedish Natural Science Research Council.
- Johnson S.D., Steiner K.E. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae) Evolution. 1997;51:45–53. doi: 10.1111/j.1558-5646.1997.tb02387.x. doi:10.2307/2410959 [DOI] [PubMed] [Google Scholar]
- Muchhala N. Nectar bat stows huge tongue in its rib cage. Nature. 2006a;444:701–702. doi: 10.1038/444701a. doi:10.1038/444701a [DOI] [PubMed] [Google Scholar]
- Muchhala N. The pollination biology of Burmeistera (Campanulaceae): specialization and syndromes. Am. J. Bot. 2006b;93:1081–1089. doi: 10.3732/ajb.93.8.1081. doi:10.3732/ajb.93.8.1081 [DOI] [PubMed] [Google Scholar]
- Muchhala N. Functional significance of extensive interspecific variation in Burmeistera floral morphology: evidence from nectar bat captures in Ecuador. Biotropica. 2008;40:332–337. doi:10.1111/j.1744-7429.2007.00381.x [Google Scholar]
- Muchhala N., Potts M.D. Character displacement among bat-pollinated flowers of the genus Burmeistera: analysis of mechanism, process and pattern. Proc. R. Soc. B. 2007;274:2731–2737. doi: 10.1098/rspb.2007.0670. doi:10.1098/rspb.2007.0670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L.A. The evolution of flowers with deep corolla tubes. Nature. 1988;334:147–149. doi:10.1038/334147a0 [Google Scholar]
- Nilsson L.A., Jonsson L., Rason L., Randrianjohany E. Monophily and pollination mechanisms in Angraecum arachnites Schltr. (Orchidaceae) in a guild of long-tongued hawkmoths (Sphingidae) in Madagascar. Biol. J. Linn. Soc. 1985;26:1–19. doi:10.1111/j.1095-8312.1985.tb01549.x [Google Scholar]
- Pijl L. Ecological aspects of flower evolution. II. Zoophilous flower classes. Evolution. 1961;15:44–59. doi:10.2307/2405842 [Google Scholar]
- Rodríguez-Gironés M.A., Llandres A.L. Resource competition triggers the co-evolution of long tongues and deep corolla tubes. PLoS ONE. 2008;3:e2992. doi: 10.1371/journal.pone.0002992. doi:10.1371/journal.pone.0002992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Gironés M.A., Santamaría L. Resource competition, character displacement, and the evolution of deep corolla tubes. Am. Nat. 2007;170:455–464. doi: 10.1086/520121. doi:10.1086/520121 [DOI] [PubMed] [Google Scholar]
- Snow D.W., Snow B.K. Relationships between hummingbirds and flowers in the Andes of Colombia. Bull. Br. Mus. Nat. Hist. (Zool.) 1980;38:105–139. [Google Scholar]
- Stebbins G.L. Adaptive radiation of reproductive characteristics in angiosperms. I: pollination mechanisms. Annu. Rev. Ecol. Syst. 1970;1:307–326. doi:10.1146/annurev.es.01.110170.001515 [Google Scholar]
- Thompson J.N. University of Chicago Press; Chicago, IL: 1994. The coevolutionary process. [Google Scholar]
- Tripp E.A., Manos P.S. Is floral specialization an evolutionary dead-end? Pollination system transitions in Ruellia (Acanthaceae) Evolution. 2008;62:1712–1737. doi: 10.1111/j.1558-5646.2008.00398.x. doi:10.1111/j.1558-5646.2008.00398.x [DOI] [PubMed] [Google Scholar]
- von Helversen O., Winter Y. Glossophagine bats and their flowers: costs and benefits for plants and pollinators. In: Kunz T.H., Fenton M.B., editors. Bat ecology. University of Chicago Press; Chicago, IL: 2003. pp. 346–397. [Google Scholar]
- Wallace A.R. Creation by law. Q. J. Sci. 1867;S140:471–486. [Google Scholar]
- Waser N.M., Chittka L., Price M.V., Williams N.M., Ollerton J. Generalization in pollination systems, and why it matters. Ecology. 1996;77:1043–1060. doi:10.2307/2265575 [Google Scholar]
- Wasserthal L.T. The pollinators of the Malagasy star orchids Angraecum sesquipedale, A. sororium and A. compactum and the evolution of extremely long spurs by pollinator shift. Bot. Acta. 1997;110:343–359. [Google Scholar]
- Whittall J.B., Hodges S.A. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature. 2007;447:706–710. doi: 10.1038/nature05857. doi:10.1038/nature05857 [DOI] [PubMed] [Google Scholar]
- Wilson P., Wolfe A.D., Armbruster W.S., Thomson J.D. Constrained lability in floral evolution: counting convergent origins of hummingbird pollination in Penstemon and Keckiella. New Phytol. 2007;176:883–890. doi: 10.1111/j.1469-8137.2007.02219.x. doi:10.1111/j.1469-8137.2007.02219.x [DOI] [PubMed] [Google Scholar]
- Winter Y., von Helversen O. Operational tongue length in phyllostomid nectar-feeding bats. J. Mammal. 2003;84:886–896. doi:10.1644/BWG-032 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of flower length on pollen transfer by Anoura fistulata in flight cage experiments, with data further subdivided by length of donor/recipient.