Abstract
Older males tend to have a competitive advantage over younger males in sexual selection. Therefore, it is expected that signals used in sexual selection change with age. Although song repertoire size in songbirds is often mentioned as an age-related trait, many species, including the banded wren (Thryothorus pleurostictus), do not increase their repertoires after the first year. Here, we show that banded wrens reproduce the trill notes in their songs with less variability between them (i.e. more consistently) when they grow older. In a playback experiment, we also show that banded wrens discriminate between younger and older birds based on structural aspects of their song. In a second experiment, banded wrens also respond differentially to natural songs versus songs with artificially enhanced consistency. We argue that consistency in trill note reproduction may be achieved through practice. Sexual selection in the form of male–male competition may therefore operate on a phenotypic trait, the expression of which is enhanced by practice.
Keywords: sexual selection, competition, birdsong, age
1. Introduction
Older males often have a competitive advantage over younger males in sexual selection (Andersson 1994). Although the causal mechanisms are debated (Kokko & Lindstrom 1996; Brooks & Kemp 2001), two explanations are commonly put forward for age-related competitiveness. First, older individuals are thought to be, on average, of a higher quality than younger ones because they have demonstrated viability. Lower-quality individuals tend to be weeded out early, leading to a higher proportion of high-quality individuals in the older classes of the population. Second, older males have more experience, which may help them outcompete their younger opponents. It is therefore likely that male signals vary with age for rapid assessment by female receivers in mate choice and by male receivers in competition over resources.
Accordingly, the expression of secondary sexually selected traits is often related to age (Trivers 1972; Manning 1985), with older males showing a larger or more intense display (Andersson 1994). For example, long-tailed manakins (Chiroxiphia linearis) increase tail length (McDonald 1989), red deer (Cervus elaphus) stags grow longer antlers with more points (Mitchell et al. 1977) and nightingales (Luscinia megarhynchos) increase song repertoire size (Kiefer et al. 2006) with age. Song repertoire size is a known correlate of age in several oscines and it is thought to be subject to sexual selection through female mate choice (Collins 2004; Searcy & Nowicki 2005). However, there are reasons to consider other aspects of song in relation to sexual selection and age in addition to repertoire size (Byers & Kroodsma 2009).
First, many species do not increase their repertoire size after the initial learning phase early in life. Although selective attrition of low-quality individuals may link high-quality individuals with large repertoires and older age, repertoire size in these species does not reliably convey information about the age of the singer. Furthermore, in species that have large repertoires, many appear to sing less elaborate and fewer song types in male–male interactions than in other contexts (Catchpole 1983; Langmore 1997; Botero & Vehrencamp 2007; Vehrencamp et al. 2007). This reduction in repertoire use suggests that repertoire size is not the predominant target for sexual selection through male–male competition (Collins et al. 2009). Finally, estimation of the entire repertoire of an individual may be time-consuming and neurologically taxing, especially in species with large repertoires, and therefore it does not appear to be an efficient signal for mutual assessment in aggressive encounters (Botero et al. 2008).
Alternatively, song can function as a performance-based signal (Podos 1997), where the level of performance of a song type is related to the quality of the individual (ten Cate et al. 2002; Byers 2007; Hardouin et al. 2007; Janicke et al. 2007). Level of performance is based on individual capacities that may be related to general fitness measures. Several studies have shown that performance-based signals play a role in sexual selection in birds. For instance, female canaries (Serinus canariensis; Vallet & Kreutzer 1995; Draganoiu et al. 2002) and swamp sparrows (Melospiza georgiana; Ballentine et al. 2004) prefer males that produce syllables that are assumed to be difficult to produce. Male banded wrens (Thryothorus pleurostictus; Illes et al. 2006; de Kort et al. 2009) and red-winged blackbirds (Agelaius phoeniceus; Cramer & Price 2007) respond differently to high- and low-performance songs.
Furthermore, it has recently been shown that zebra finches maintain a degree of variation after song crystallization (Tumer & Brainard 2007), possibly allowing for adaptive changes in adult song (Slabbekoorn & den Boer-Visser 2006). This would imply that as individuals grow older, they could achieve higher song performance through practice. If this were true, then we would expect that structural performance-based differences exist between songs from younger and older birds (Galeotti et al. 2001; Forstmeier et al. 2006; Garamszegi et al. 2007; Botero et al. 2009), and that receivers can discriminate between such songs.
We tested whether banded wrens change structural aspects of their songs (in this case trill note consistency) with age by analysing songs from the same individual at different age classes. We then tested whether territorial males discriminate between playback of songs from the same bird in its first and third breeding seasons. Finally, in a separate experiment, we tested whether territorial males discriminate between songs with artificially enhanced versus natural trill note consistency.
2. Material and methods
(a) Study area and population
The banded wren inhabits dry tropical forest on the Pacific slope of Central America from southern Mexico to northern Costa Rica. For a detailed description of habitat and population at the study site in Santa Rosa National Park in Costa Rica (10°51′ N, 85°38′ W), see Molles & Vehrencamp (1999). During the breeding season, which lasts from May until September in Costa Rica, both the male and the female of a pair defend the territory. The banded wren is a socially monogamous species with few extra-pair copulations recorded (E. Cramer & S. L. Vehrencamp 2007, unpublished data). Banded wren males have repertoires of approximately 20–25 distinct song types that consist of an introductory part followed by a series of stereotypically repeated notes, referred to as a trill (figure 1). The net repertoire size of banded wrens does not increase with age, although a few song types may be added, dropped and modified in consecutive years (S. L. Vehrencamp 2006, unpublished data).
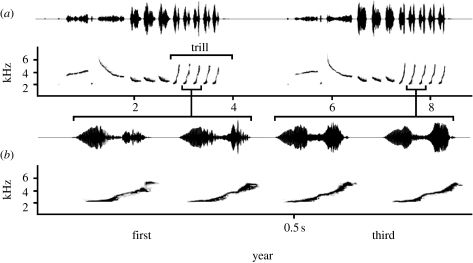
Figure 1.
(a) A waveform and spectrogram of a song stimulus pair used in the age discrimination experiment, (b) with an enlargement of two trill notes of each song. On the left a song from an individual in its first breeding season, and on the right the same song type from the same individual in its third breeding season. Spectrogram settings: fast Fourier transform (FFT) size=512 Hz, Hamming window, time resolution=2.67 ms, frequency resolution=94 Hz.
(b) Age differences in song
We compared renditions of the same song type recorded during a bird's first and second or third breeding seasons, depending on the availability of recordings. We used only the recordings of individuals that were banded as nestlings so that the absolute age was known. Recordings were made during dawn chorus, between 05.00 and 07.00, with a Sennheiser ME67 microphone connected to a Marantz PMD 690 digital recorder at a sampling rate of 48 kHz. All recordings were made between the end of May and the end of June in order to minimize potential seasonal differences in the expression of songs due to differences in breeding stage.
We assessed the consistency of a trill, i.e. the spectral similarity between consecutive trill notes within a song, by first extracting clips of individual trill notes using the template detector of XBAT release 5 (H. Figueroa, Ithaca, New York; www.xbat.org). The clips for the trill notes in a given song were then subjected to spectral cross-correlation (SPCC) using a custom Matlab routine called SoundXT v. 2.0 (K. A. Cortopassi 2005, personal communication). SPCC provides a measure of similarity between notes by comparing the spectral information in a spectrogram display (Cortopassi & Bradbury 2006). An SPCC coefficient of 0 indicates no similarity while an SPCC coefficient of 1 implies that two notes are identical. Each trill note was compared with all other trill notes within the same song, which resulted in a triangular cross-correlation matrix. The values in the correlation matrix were averaged, resulting in a consistency value for each song. This procedure was repeated and averaged for 5–10 songs for each song type per individual and age class.
(c) Age discrimination experiment
(i) Experimental design
Territory boundaries were assessed for 30 territories by monitoring the owners' movements and song posts. The playback experimental set-up was similar to that used by Illes et al. (2006) and consisted of two speakers (Anchor Mini-Vox model pb-25) 50 m apart but well within the boundaries of the territory. Each speaker formed the centre of a circle with a radius of 15 m (de Kort et al. 2009) that was indicated with coloured flagging tape. A third speaker (RadioShack mini amplifier speaker) was placed in between the two experimental speakers at a distance of 25 m from each of them. This third ‘lure’ speaker was at the centre of a lure corridor 10 m wide and 30 m long, bisecting and perpendicular to a line between the two experimental speakers (see Illes et al. 2006 for a similar set-up). The experimental speakers were each connected to one channel of a stereo iPod with a 25 m long cable. The lure speaker was connected to a second iPod. Sound files were stored on the iPod as uncompressed wav files. Speaker volume was adjusted to 90 dB, measured at 1 m from the source with a RadioShack realistic sound pressure level meter (33–2050), which approximates natural banded wren singing levels. Experiments were conducted between 20 May 2006 and 17 June 2006, from 06.00 to 10.00, after the end of the dawn chorus and before the midday decline in activity.
Before each trial, the subject was lured into the lure area by playing male calls and female banded wren song. If the subject did not come into the lure corridor within 900 s, it was considered not responsive and the trial was aborted. A second and last attempt with the same individual was made at least 3 days later. As soon as the subject entered the lure area, playback of the stimuli was started concurrently at both experimental speakers.
Playback duration was 130 s, followed by a 230 s post-playback observation period. Subsequent trials with different subjects on the same day were conducted out of acoustic range of each other to avoid interference from previous trials.
Three experimenters observed each trial. Two recorded the time of specific behaviours of the subject on Palm handheld computers using custom-made software (Sturnus; John Burt, University of Washington, Seattle, WA). The third experimenter made acoustic recordings and added spoken comments of the trial, using a digital recorder (MicroTrack 24/96, M-audio) and a microphone (Sennheiser ME67). During a trial, the following data were collected for each subject: the time spent within and on either side of the lure corridor; the time spent within the 15 m radius of one of the experimental speakers; and the number of songs sung during the trial. The observers did not know which speaker broadcasted the first- or third-year stimuli.
(ii) Stimulus preparation
From our database of banded wren recordings, we paired 15 first- and third-year recordings of the same song type for five individuals and a total of eight different song types (see figure 1 for an example). Recordings were selected based on a high signal-to-noise ratio. Each song was copied 20 times with a 6.5 s interval between the start of subsequent songs, which resulted in a playback duration of 130 s. During territorial encounters, banded wrens reduce the number of song types they sing (Vehrencamp et al. 2007) and they may sing the same song a large number of times before switching to another song type. We used Adobe Audition v. 1.0 for stimulus preparation. To circumvent the issue that birds might approach the speaker that broadcasts a song first in an alternating-speaker playback design (Naguib et al. 1999), the paired stimuli were broadcasted concurrently from the two experimental speakers at opposite sides of the lure corridor. In addition, because the sound stimuli are very similar, we anticipated a small difference in response to the two stimulus types. Therefore, we aimed to maximize the effect size of the statistical test used for the analysis of the difference. By using a concurrent playback design, we attempted to omit a factor that would reduce the effect size from the analysis, namely the correction for order of presentation. Humans are able to discriminate concurrent stimuli from different sources placed at angles of less than 30° from each other without moving their head (Perrott 1984); therefore, we believe that positioning the sound sources at an angle of 180° should allow a freely moving bird to assess each sound source independently.
(d) Consistency experiment
(i) Experimental design
While in the age discrimination experiment we tested explicitly whether birds discriminate between two stimuli, the second experiment aimed to examine the signal value of stimuli that differed in trill consistency. We therefore choose to use a within-subject design to assess the response of the same individual to both variants of the stimuli. The stimuli were presented separately and sequentially with a 3-day interval between trials from one wireless experimental speaker (Foxpro FX5) controlled by a remote control (TX200, www.gofoxpro.com). The experimental speaker formed the centre of an experimental circle with a radius of 15 m. At 35 m from the experimental speaker, we placed a lure speaker (Foxpro FX3) that formed the centre of a lure circle with a radius of 5 m. Experiments were conducted between 4 July 2007 and 9 August 2007. The order of presentation of the two types of stimuli was randomized across subjects. The observers were blind with respect to stimulus type.
(ii) Stimulus preparation
We used the first-year stimuli from the age discrimination experiment to create two sets of stimuli. The first set consisted of the first-year recordings, which were ‘sham’ treated by cutting individual elements from the trill and pasting them back into the same location. These stimuli are referred to as the inconsistent stimuli. The second set consisted of manipulated versions of the inconsistent stimuli and is referred to as consistent stimuli. In this set, we replaced all trill notes in the song with copies of the note with the median frequency bandwidth value in the trill, keeping the trill rate identical to the original song. This procedure resulted in a trill with a perfect consistency since all notes in the trill were identical. Note that the introductory parts of a pair of song stimuli are identical and the only difference lies in the trill component of the stimuli.
(e) Statistics
We report mean±s.e.m. throughout. We used SPSS v. 14 software for statistical analyses. Differences in the individual trill consistency values between age classes were assessed with a repeated-measures general linear model (GLM) with age class as a within-subject factor and song type nested within individual. Differences in response to the stimuli were assessed with a GLM in the age discrimination experiment, and with a repeated-measures GLM in the consistency experiment. All test results reported reflect two-tailed tests.
3. Results
(a) Age differences in song
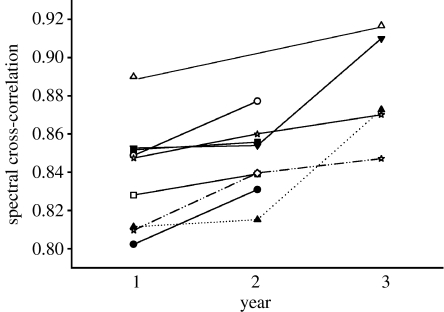
We analysed 732 songs belonging to 18 different song types of nine individuals (5.6±0.68 song types per individual). For all individuals, the average SPCC score across song types increased with age (table 1). Overall trill consistency was lower in the first year than when the birds were 2 years old or older (repeated-measures GLM, F1,33=27.8, p<0.001) and this result was not affected by song type (F17,33=1.29, p=0.25). For several individuals, one or more song types showed a decrease in SPCC value (table 1), but this pattern was not consistent across individuals for a given song type. The individuals that were recorded in all three breeding seasons show an increase in consistency (figure 2) with age at each recorded year.
Table 1.
Differences in SPCC scores of within-song trill notes per individual and song type in the banded wren for songs recorded in the first and last available breeding seasons.
| individual | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| type | AwpA | BbwE | BpwE | DadB | DOR | OwrB | WadA | WpwB | YwrP | mean |
| 6 | 0.07 | −0.03 | 0.03 | 0.02 | 0.09 | 0.037 | ||||
| 103 | 0.01 | −0.01 | 0.003 | |||||||
| 118 | 0.08 | 0.083 | ||||||||
| 193 | 0.03 | 0.04 | 0.035 | |||||||
| 201 | 0.05 | 0.055 | ||||||||
| 202 | 0.02 | 0.02 | 0.00 | 0.02 | 0.029 | |||||
| 203 | 0.01 | 0.005 | ||||||||
| 204 | 0.02 | 0.02 | 0.02 | 0.019 | ||||||
| 205 | 0.08 | 0.02 | 0.03 | 0.043 | ||||||
| 206 | −0.02 | 0.01 | 0.01 | 0.01 | 0.00 | 0.05 | 0.010 | |||
| 213 | 0.01 | 0.07 | −0.01 | 0.023 | ||||||
| 214 | 0.03 | 0.04 | 0.03 | 0.01 | 0.027 | |||||
| 217 | 0.05 | 0.02 | 0.02 | 0.03 | 0.08 | 0.029 | ||||
| 220 | 0.02 | 0.02 | 0.00 | −0.04 | 0.002 | |||||
| 222 | 0.01 | 0.00 | 0.03 | 0.013 | ||||||
| 223 | 0.02 | 0.024 | ||||||||
| 242 | 0.02 | 0.024 | ||||||||
| 420 | −0.01 | −0.011 | ||||||||
| mean | 0.029 | 0.004 | 0.028 | 0.023 | 0.011 | 0.037 | 0.020 | 0.027 | 0.035 | |
Figure 2.
Average trill consistency across song types for nine individuals (banded wrens) for the first, second and/or third breeding seasons. The lines connect the same individual at different age classes. Each individual is represented by a different symbol.
(b) Age discrimination experiment
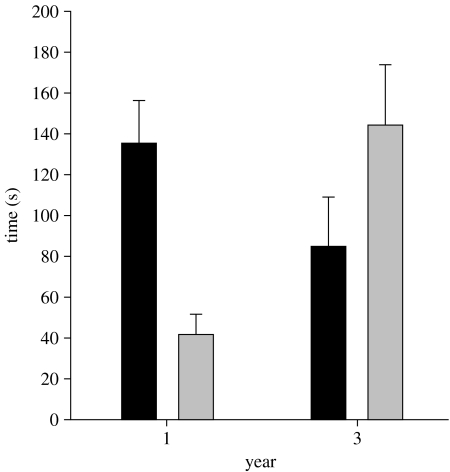
All 30 subjects entered at least one of the two experimental circles from which first- and third-year songs were broadcasted concurrently. Seventeen subjects spent more time near the speaker broadcasting first-year songs and 13 near the speaker broadcasting third-year songs (figure 3). The birds that approached the speaker broadcasting the first-year stimulus spent proportionally more time within the experimental speaker circle compared with those that approached the speaker broadcasting the third-year stimulus (F1,28=6.72, p<0.015; figure 3). Subjects demonstrated a higher song rate when approaching the speaker broadcasting first-year songs (5.18±0.68 songs min−1) than when approaching the speaker broadcasting third-year songs (2.35±0.65 songs min−1, F1,28=8.53, p<0.007).
Figure 3.
Average (+s.e.m.) time spent by territorial banded wrens inside (black bars) and outside (grey bars) the experimental circle (r=15 m) surrounding a speaker broadcasting stimuli from first-year source males (left two bars; n=17) and third-year source males (right two bars; n=13).
(c) Consistency experiment
We tested the responses of 22 subjects to playback of consistent and inconsistent stimuli in separate trials on different days. The subjects displayed a higher song rate in response to the inconsistent than to the consistent stimulus (inconsistent, 18.04±1.99; consistent, 12.23±1.94; F1,20=7.02, p<0.015). Subjects did not show a difference in time spent within the experimental circle related to the type of stimulus broadcast (GLM, F1,20=0.77, p=0.39). There was no effect of order of presentation of the stimulus types for either of the two response variables (p>0.1).
4. Discussion
Banded wrens increase the consistency of trill note repetition between their first and later breeding years. Our results further showed that banded wrens discriminated between songs from an individual in its first and third breeding seasons and between natural songs of first-year birds and the same songs with artificially enhanced consistency of trill note repetition. Since stimulus pairs were obtained from the same individual, we can exclude the possibility that individual quality differences between source birds contributed to the perceived difference between the stimuli. In addition, we used the same song type for the two stimulus types (figure 1) and thus there were no semantic differences between the stimuli (Trillo & Vehrencamp 2005). It appears therefore that the perceived difference is due to the phenotypic, age-related differences in the songs used as stimuli. We conclude that trill consistency increases with age and is used by receivers as a signal of experience in banded wrens.
Although males tend to reproduce trill notes more consistently with age, this pattern is not uniform across song types. Some individuals showed a decrease in trill consistency in certain song types between the first and later years. This suggests that the increase in consistency is not solely a by-product of longevity due to genetic quality, but rather that it may depend on a suite of life-history traits such as condition, motivation or duration of territory tenancy. Banded wrens sing only on their territories and if trill consistency is achieved through practice, then it is directly related to territory tenancy. Thus, a younger individual of high quality, which acquires a territory early, may be able to achieve a higher level of consistency than an older individual of lower quality, which became a territory owner later in life. This is illustrated by the observation that there is substantial variation between individuals in trill consistency (figure 2). Some individuals achieve the level of consistency only in their third breeding season that others achieve in their first or second season. However, since the pattern, in general, shows an increase with age, we believe that trill consistency contributes to signalling age and/or experience in the banded wren.
In the age discrimination experiment, banded wrens showed a higher song rate and a closer approach in response to the younger stimuli compared with the older stimuli. Whether a stimulus simulating a strong or a weak intruder should elicit the strongest response is a contentious issue in the bird song literature (Collins 2004). In a previous study, banded wrens showed a strong approach response to low- and average-performance songs compared with high-performance songs, while they showed a stronger vocal response to high- and average-performance songs compared with low-performance songs (de Kort et al. 2009). We think that the old stimulus in the current study corresponds to the high-performance stimulus in de Kort et al. (2009), both failing to elicit strong approach behaviour. A cautious response to a strong competitor is congruent with theoretical models of animal conflict (Enquist & Leimar 1983; Leimar & Enquist 1984), and similar interpretations were made in other playback studies (cf. Järvi et al. 1980; Cramer & Price 2007; Hardouin et al. 2007; Osiejuk et al. 2007). The young stimulus corresponds to the average stimuli from de Kort et al. (2009), eliciting stronger vocal and approach behaviour because they are more likely to be perceived by the subjects as equal competitors. These results are in line with the notion that social dominance is related to age in birds (Smith 1984; Arcese & Smith 1985; Piper & Wiley 1989; Sandell & Smith 1991).
Our analysis showed that banded wrens increase song consistency in trill note repetition with age and that territorial males discriminate between songs sung by the same individual at different age classes. The second experiment in this study complements these findings by showing that banded wrens respond differently to two versions of the same song, which were manipulated to differ only in the consistency of trill note repetition. Thus, even if other structural parameters in the song of banded wrens also vary with age, we can conclude that in male–male competition, the consistency with which certain song elements within a song (individual trill notes) are repeated conveys biologically relevant information in this species.
Consistency, like some other performance traits, differs from repertoire size in its efficiency as a signal of quality, since a receiver needs to assess the consistency of the trill only in one song but needs more time to assess repertoire size. We suggest that song consistency may represent the acoustic analogue of visual signals that rely on fluctuating asymmetry in the sense that it allows for rapid assessment of the quality of its bearer. Consistency has now been found to play a role in mate choice in chestnut-sided warblers (Dendroica pensylvanica; Byers 2007) and as an indicator of social status, age and breeding success in cooperatively breeding tropical mockingbirds (Mimus gilvus; Botero et al. 2009). A difference between these studies and the current one is that consistency was measured across renditions of a song as opposed to within the same song. Nevertheless, all three studies suggest that consistent reproduction of the same signal may indicate the quality of the signaller. Furthermore, the fact that the species involved are distantly related suggests that consistency in song parameters may be important in a variety of taxa.
Acknowledgments
We thank the Area de Conservación Guanacaste for permission to work in Santa Rosa National Park and the park staff, especially Roger Blanco, for logistical support in Costa Rica. We thank Foxpro for providing us with the wireless speakers. Margaret Kellogg and Jennifer Yantachka were excellent assistants in the field and Siddharth Khasnavis and Benjamin Rubin helped with song analysis. Two anonymous reviewers provided comments that improved the manuscript. This work was funded by NIMH grant R01-MH60461.
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Arcese P., Smith J.N.M. Phenotypic correlates and ecological consequences of dominance in song sparrows. J. Anim. Ecol. 1985;54:817–830. doi:10.2307/4380 [Google Scholar]
- Ballentine B., Hyman J., Nowicki S. Vocal performance influences female response to male bird song: an experimental test. Behav. Ecol. 2004;15:163–168. doi:10.1093/beheco/arg090 [Google Scholar]
- Botero C.A., Vehrencamp S.L. Responses of male tropical mockingbirds (Mimus gilvus) to variation in within-song and between-song versatility. Auk. 2007;124:185–196. doi: 10.1642/0004-8038(2007)124[185:ROMTMM]2.0.CO;2. doi:10.1642/0004-8038(2007)124[185:ROMTMM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botero C.A., Mudge A.E., Koltz A.M., Hochachka W.M., Vehrencamp S.L. How reliable are the methods for estimating repertoire size? Ethology. 2008;114:1227–1238. doi: 10.1111/j.1439-0310.2008.01576.x. doi:10.1111/j.1439-0310.2008.01576.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botero C.A., Rossman R.J., Caro L.M., Stenzler L.M., Lovette I.J., de Kort S.R., Vehrencamp S.L. Syllable consistency is related to age, social status, and reproductive success in the tropical mockingbird. Anim. Behav. 2009;77:701–706. doi: 10.1016/j.anbehav.2008.11.020. doi:10.1016/j.anbehav.2008.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R., Kemp D.J. Can older males deliver the good genes? Trends Ecol. Evol. 2001;16:308–313. doi: 10.1016/s0169-5347(01)02147-4. doi:10.1016/S0169-5347(01)02147-4 [DOI] [PubMed] [Google Scholar]
- Byers B.E. Extrapair paternity in chestnut-sided warblers is correlated with consistent vocal performance. Behav. Ecol. 2007;18:130–136. doi:10.1093/beheco/arl058 [Google Scholar]
- Byers B.E., Kroodsma D. Female mate choice and songbird song repertoires. Anim. Behav. 2009;77:13–22. doi:10.1016/j.anbehav.2008.10.003 [Google Scholar]
- Catchpole C.K. Variation in the song of the great reed warblers Acrocephalus arundinaceus in relation to mate attraction and territorial defense. Anim. Behav. 1983;31:1217–1225. doi:10.1016/S0003-3472(83)80028-1 [Google Scholar]
- Collins S.A. Vocal fighting and flirting: the functions of birdsong. In: Marler P., Slabbekoorn H., editors. Nature's music: the science of birdsong. Elsevier Academic Press; Amsterdam, The Netherlands: 2004. pp. 39–79. [Google Scholar]
- Collins S.A., de Kort S.R., Perez-Tris J., Telleria J.L. Migration strategy and divergent sexual selection on bird song. Proc. R. Soc. B. 2009;276:585–590. doi: 10.1098/rspb.2008.1011. doi:10.1098/rspb.2008.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortopassi K.A., Bradbury J.W. Contact call diversity in wild orange-fronted parakeet pairs, Aratinga canicularis. Anim. Behav. 2006;71:1141–1154. doi:10.1016/j.anbehav.2005.09.011 [Google Scholar]
- Cramer E.R.A., Price J.J. Red-winged blackbirds Ageliaus phoeniceus respond differently to song types with different performance levels. J. Avian Biol. 2007;38:122–127. doi:10.1111/j.2006.0908-8857.03839.x [Google Scholar]
- de Kort S.R., Eldermire E.R.B., Cramer E.R.A., Botero C.A., Vehrencamp S.L. The deterrent effect of bird song in territory defense. Behav. Ecol. 2009;20:200–206. doi: 10.1093/beheco/arn135. doi:10.1093/beheco/arn135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganoiu T.I., Nagle L., Kreutzer M. Directional female preference for an exaggerated male trait in canary (Serinus canaria) song. Proc. R. Soc. B. 2002;269:2525–2531. doi: 10.1098/rspb.2002.2192. doi:10.1098/rspb.2002.2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist M., Leimar O. Evolution of fighting behaviour: decision rules and assessment of relative strength. J. Theor. Biol. 1983;127:387–410. doi:10.1016/0022-5193(83)90376-4 [Google Scholar]
- Forstmeier W., Hasselquist D., Bensch S., Leisler B. Does song reflect age and viability? A comparison between two populations of the great reed warbler Acrocephalus arundinaceus. Behav. Ecol. Sociobiol. 2006;59:634–643. doi:10.1007/s00265-005-0090-z [Google Scholar]
- Galeotti P., Saino N., Perani E., Sacchi R., Moller A.P. Age-related song variation in male barn swallows. Ital. J. Zool. 2001;68:305–310. doi:10.1080/11250000109356423 [Google Scholar]
- Garamszegi L.Z., Torok J., Hegyi G., Szollosi E., Rosivall B., Eens M. Age-dependent expression of song in the collared flycatcher, Ficedula albicollis. Ethology. 2007;113:246–256. doi:10.1111/j.1439-0310.2007.01337.x [Google Scholar]
- Hardouin L.A., Reby D., Bavoux C., Burneleau G., Bretagnolle V. Communication of quality in owl hoots. Am. Nat. 2007;169:552–562. doi: 10.1086/512136. doi:10.1086/512136 [DOI] [PubMed] [Google Scholar]
- Illes A.E., Hall M.L., Vehrencamp S.L. Vocal performance influences male receiver response in the banded wren. Proc. R. Soc. B. 2006;273:1907–1912. doi: 10.1098/rspb.2006.3535. doi:10.1098/rspb.2006.3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke T., Hahn S., Ritz M.S., Peter H. Vocal performance reflects individual quality in a nonpasserine. Anim. Behav. 2007;75:91–98. doi:10.1016/j.anbehav.2007.04.007 [Google Scholar]
- Järvi T., Radesäter T., Jacobsen S. The song of the willow warbler Phylloscopus trochilus with special reference to singing behaviour in agonistic situations. Ornis Scand. 1980;11:236–242. doi:10.2307/3676129 [Google Scholar]
- Kiefer S., Spiess A., Kipper S., Mundry R., Sommer C., Hultsch H., Todt D. First-year common nightingales (Luscinia megarhynchos) have smaller song-type repertoire sizes than older males. Ethology. 2006;112:1217–1224. doi:10.1111/j.1439-0310.2006.01283.x [Google Scholar]
- Kokko H., Lindstrom J. Evolution of female preference for old mates. Proc. R. Soc. Lond. B. 1996;263:1533–1538. doi:10.1098/rspb.1996.0224 [Google Scholar]
- Langmore N.E. Song switching in the monandrous and polyandrous dunnocks, Prunella modularis. Anim. Behav. 1997;53:757–766. doi:10.1006/anbe.1996.0312 [Google Scholar]
- Leimar O., Enquist M. Effects of asymmetries in owner–intruder conflicts. J. Theor. Biol. 1984;111:475–491. doi:10.1016/S0022-5193(84)80235-0 [Google Scholar]
- Manning J.T. Choosy females and correlates of male age. J. Theor. Biol. 1985;116:349–354. doi:10.1016/S0022-5193(85)80273-3 [Google Scholar]
- McDonald D.B. Cooperation under sexual selection: age-graded changes in a lekking bird. Am. Nat. 1989;134:709–734. doi:10.1086/285007 [Google Scholar]
- Mitchell B., Staines B.W., Welch D. Institute for Terrestrial Ecology; Cambridge, UK: 1977. Ecology of red deer. [Google Scholar]
- Molles L.E., Vehrencamp S.L. Repertoire size, repertoire overlap, and singing modes in the banded wren (Thryothorus pleurostictus) Auk. 1999;116:677–689. [Google Scholar]
- Naguib M., Fichtel C., Todt D. Nightingales respond more strongly to vocal leaders of simulated dyadic interactions. Proc. R. Soc. Lond. B. 1999;266:537–542. doi:10.1098/rspb.1999.0669 [Google Scholar]
- Osiejuk T.S., Losak K., Dale S. Cautious response of inexperienced birds to conventional signal of stronger threat. J. Avian Biol. 2007;38:644–649. doi:10.1111/j.2007.0908-8857.04255.x [Google Scholar]
- Perrott D.R. Discrimination of the spatial distribution of concurrently active sound sources: some experiments with stereophonic arrays. J. Acoust. Soc. Am. 1984;76:1704–1712. doi: 10.1121/1.391617. doi:10.1121/1.391617 [DOI] [PubMed] [Google Scholar]
- Piper W.H., Wiley R.H. Correlates of dominance in wintering white-throated sparrows: age, sex and location. Anim. Behav. 1989;37:298–310. doi:10.1016/0003-3472(89)90119-X [Google Scholar]
- Podos J. A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes: Emberizidae) Evolution. 1997;51:537–551. doi: 10.1111/j.1558-5646.1997.tb02441.x. doi:10.2307/2411126 [DOI] [PubMed] [Google Scholar]
- Sandell M., Smith H.G. Dominance, prior occupancy, and winter residency in the great tit (Parus major) Behav. Ecol. Sociobiol. 1991;29:147–152. doi:10.1007/BF00166490 [Google Scholar]
- Searcy W.A., Nowicki S. Monographs in Behavior and Ecology. Princeton University Press; Princeton, NJ: 2005. The evolution of animal comunication: reliability and deception in signalling systems. [Google Scholar]
- Slabbekoorn H., den Boer-Visser A. Cities change the songs of birds. Curr. Biol. 2006;16:2326–2331. doi: 10.1016/j.cub.2006.10.008. doi:10.1016/j.cub.2006.10.008 [DOI] [PubMed] [Google Scholar]
- Smith S.M. Flock switching in chickadees: why be a winter floater? Am. Nat. 1984;123:81–98. doi:10.1086/284188 [Google Scholar]
- ten Cate C., Slabbekoorn H., Ballintijn M.R. Birdsong and male–male competition: causes and consequences of vocal variability in the collared dove (Streptopelia decaocto) Adv. Study Behav. 2002;31:31–75. doi:10.1016/S0065-3454(02)80005-5 [Google Scholar]
- Trillo P.A., Vehrencamp S.L. Song types and their structural features are associated with specific contexts in the banded wren. Anim. Behav. 2005;70:921–935. doi: 10.1016/j.anbehav.2005.02.004. doi:10.1016/j.anbehav.2005.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R. Parental investment and sexual selection. In: Campbell B., editor. Sexual selection and the descent of man 1871–1971. Aldine Press; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Tumer E.C., Brainard M.S. Performance variability enables adaptive plasticity of ‘crystallized’ adult birdsong. Nature. 2007;450:1240–1244. doi: 10.1038/nature06390. doi:10.1038/nature06390 [DOI] [PubMed] [Google Scholar]
- Vallet E., Kreutzer M. Female canaries are sexually responsive to special song phrases. Anim. Behav. 1995;49:1603–1610. doi:10.1016/0003-3472(95)90082-9 [Google Scholar]
- Vehrencamp S.L., Hall M.L., Bohman E.R., Depeine C.D., Dalziell H. Song matching, overlapping, and switching in the banded wren: the sender's perspective. Behav. Ecol. 2007;18:849–859. doi: 10.1093/beheco/arm054. doi:10.1093/beheco/arm054 [DOI] [PMC free article] [PubMed] [Google Scholar]